钩藤的生物碱构成及其抗炎活性研究
龚 爽,熊 凤,张兴杰,张芮菡,李晓莉,肖伟烈
(云南大学教育部自然资源药物化学重点实验室,云南省天然产物转化与应用重点实验室,化学科学与工程学院,云南 昆明 650500)
根据《中国药典》2015版[1]的记载,茜草科植物钩藤U.rhynchophylla的干燥带钩茎的茎叶作为其正品来源之一,具有息风定惊、清热平肝的功效,可用于治疗肝风内动、惊痫抽搐、高热惊厥、感冒夹惊、小儿惊啼、妊娠子痫、头痛眩晕等.其植物主要分布在在云南、贵州、福建、广西、广东等地.先前研究表明钩藤属植物中含有生物碱[2]、黄酮[3]和三萜[4-5]等化学成分.为了更好地研究钩藤的生物碱成分,并研究其中具有潜力的抗炎活性成分,本实验对钩藤甲醇提取物氯仿相的化学成分进行了系统研究,从中分离并鉴定了18个化合物,分别为dihyrdocorynantheine(1),isocorynantheidine(2),毛钩藤碱(3),柯楠因碱(4),去氢毛钩藤碱(5),蓬籽嗪甲醚(6),villocarine A(7),16R-E-isositsirikine(8),sitsirikine(9),19,20-dihydroisositsirikine(10),去氢毛钩藤N-氧化物(11),geissoschizine N-oxide methyl ether(12),柯诺辛碱(13),异钩藤酸(14),异柯诺辛因碱(15),柯诺辛碱B(16),去氢钩藤碱(17),阿枯阿米精(18),化合物结构见图1.化合物2,7,8和10为首次在该植物中分离得到.通过炎症小体的实验,对分离得到的化合物的抗炎活性进行了筛选,结果表明化合物13具有一定的抗炎活性.
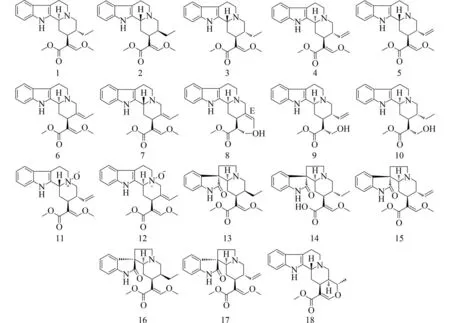
图1 钩藤生物碱1~18的结构Fig.1 The structure of alkaloid constituents 1-18 from Uncaria rhynchophylla
1 仪器与材料
AV-400、AV-III-600 HD核磁共振仪(德国Bruker公司);旋转蒸发仪(东京理化公司);薄层层析分析GF254型硅胶板,0.054~0.077 mm(200~300目)、0.172~0.216 mm(80~100目)柱色谱硅胶(青岛海洋化工有限公司);75~150μm MCI填料(日本三菱化工公司);Sephadex LH-20(瑞士Pharmacia公司);0.077~0.172 mm(100~200目)层析用氧化铝(上海五四试剂化学有限公司);Agilent1260分析型高效液相色谱、G7161A制备型高效液相色谱(美国Agilent公司);Zorbax SBC18(φ9.4 mm×250 mm)半 制 备 柱、Zorbax SBC18(φ4.6 mm×250 mm)分析柱(美国Agilent公司);XSelect CSH C18 OBD(φ30 mm X 100 mm)半制备柱(美国Waters公司);色谱纯乙腈溶剂(CINC公司);UPLC-IF-TOF质谱仪(美国Agilent公司);所用溶剂均为分析纯(昆明腾科科技有限公司).
钩藤带钩茎的茎叶于2019年采自云南省文山市坝心彝族乡,由昆明采智生物有限公司采集及鉴定,标本保存于云南大学教育部自然资源药物化学重点实验室.
2 提取与分离
钩藤干燥带钩茎的茎叶21 kg,粉碎后用甲醇常温浸泡提取3次,每次24 h,提取液浓缩去除甲醇后以适量水混悬,加入酒石酸调节pH值至pH=2~3,用乙酸乙酯萃取3次,将乙酸乙酯部分浓缩.剩余水相用氨水调节pH至9~10,然后用氯仿萃取3次得到钩藤总生物碱部分,浓缩氯仿相,得到浸膏450 g.氯仿相浸膏经硅胶柱色谱,以二氯甲烷-甲醇(体积比100∶1→0∶100,下同)进行梯度洗脱,得到组分Fr.1~7.
组分Fr.3段经氧化铝柱经石油醚-乙酸乙酯(4∶1→0∶1)洗脱,得到4个组分.Fr.3-2经过正相硅胶柱色谱多次分离纯化,最后通过HPLC分离得到化合物11(15.3 mg),洗脱条件为乙腈-水(45∶55,含5‰二乙胺),流速为3.0 mL/min,检测波长230、254、280 nm.
组分Fr.4段经氧化铝柱用石油醚-乙酸乙酯(2∶1→0∶1)洗脱,得到7个组分,Fr.4-1通过氧化铝柱色谱用石油醚-乙酸乙酯(4∶1→0∶1)洗脱,得到9个组分.经过多次硅胶柱色谱、凝胶柱色谱、氧化铝柱色谱分段富集,得到化合物6(1 156.4 mg),化合物7(13.5 mg),化合物12(7.5 mg). 通过半制备HPLC,分离得到化合物1(132.6 mg),化合物2(7.7 mg),化合物4(22.1 mg),化合物5(18.5 mg),化合物18(7.0 mg),洗脱条件为乙腈-水(60∶40,含5‰二乙胺),流速为3.0 mL/min,检测波长分别为230、254、280 nm.
组分Fr.4-5通过氧化铝柱色谱用石油醚-丙酮(4∶1→0∶1)洗脱,得到5个组分.通过多次硅胶柱色谱,从组分Fr.4-5-1中得到化合物13(90.7 mg).组分Fr.4-5-3、Fr.4-5-4和Fr.4-5-7使用半制备HPLC分离得到化合物3(56.2 mg),化合物8(6.1 mg),化合物9(12.9 mg),化合物10(38.9 mg),化合物14(7.7 mg),化合物15(4.0 mg),化合物16(5.5 mg),化合物17(3.4 mg),洗脱条件为乙腈-水(59∶41,含5‰二乙胺),流速为3.0 mL/min,检测波长分别为230、254、280 nm.
3 结构鉴定
化合物1棕色固体,C22H28N2O3.1H NMR(400 MHz,CDCl3)δ:7.58(1H,d,J=7.4 Hz,H-9),7.38(1H,d,J=7.6 Hz,H-12),7.20(2H,m,J=6.9 Hz,H-10,H-11),3.86(3H,s,17-OMe),3.80(3H,s,22-OMe);13C NMR(100 MHz,CDCl3)δ:169.8(C-22),160.2(C-17),136.2(C-13),134.9(C-20),127.2(C-8),120.9(C-10),119.0(C-11),117.9(C-9),111.5(C-16),110.8(C-12),107.4(C-7),61.6(17-OMe),60.7(C-21),60.3(C-3),53.0(C-5),51.5(22-OMe),39.9(C-20),38.3(C-15),33.3(C-14),24.3(C-19),21.5(C-6),11.1(C-18).以上数据与文献报道基本一致[6],故鉴定化合物1为dihyrdocorynantheine.
化合物2棕色固体,C22H28N2O3.1H NMR(600 MHz,CDCl3)δ:7.45(1H,d,J=7.7 Hz,H-9),7.34(1H,d,J=8.3 Hz,H-12),7.28(1H,s,H-17),7.10(1H,m,H-11),7.05(1H,t,J=7.4 Hz,H-10),3.82(3H,s,17-OMe),3.74(3H,s,22-OMe),1.14(3H,t,J=7.4 Hz,H-18);13C NMR(150 MHz,CDCl3)δ:172.8(C-22),159.7(C-17),136.3(C-13),135.3(C-2),127.4(C-8),121.3(C-11),119.4(C-10),118.2(C-9),112.7(C-16),110.9(C-12),108.6(C-7),62.1(C-21),61.6(17-OMe),56.3(C-3),51.5(C-5),51.5(22-OMe),36.4(C-20),31.3(C-15),29.8(C-14),21.7(C-6),18.3(C-19),12.9(C-18).以上数据与文献报道基本一致[7],故鉴定化合物2为isocorynantheidine.
化合物3淡黄色固体,C22H28N2O3.1H NMR(600 MHz,CDCl3)δ:8.16(1H,s,1-NH),7.50(1H,d,J=7.7 Hz,H-9),7.36(1H,d,J=8.0 Hz,H-12),7.32(1H,s,H-17),7.15(1H,t,J=7.4Hz,H-11),7.11(1H,t,J=7.4Hz,H-10),4.46(1H,s,H-3),3.75(3H,s,17-OMe),3.68(3H,s,22-OMe),3.31(2H,t,J=5.3Hz,H-5),3.03(1H,m,H-6α),2.78(1H,dd,J=11.2,3.3 Hz,H-21α),2.57(1H,dd,J=15.8,4.5 Hz,H-6β),2.45(1H,m,H-14α),1.99(1H,d,J=13.4 Hz,H-14β),1.29(1H,m,H-19α),0.76(3H,d,J=4.9 Hz,H-18),0.75(1H,m,H-19β);13C NMR(150 MHz,CDCl3)δ:169.1(C-22),159.8(C-17),136.0(C-13),133.5(C-2),128.0(C-8),121.3(C-10),119.3(C-11),118.0(C-9),112.0(C-16),111.2(C-12),107.9(C-7),61.5(17-OMe),54.3(C-3),51.5(C-5),51.3(22-OMe),50.8(C-21),39.2(C-20),35.1(C-15),31.9(C-14),24.4(C-19),17.1(C-6),11.4(C-18).以上数据与文献报道基本一致[8],故鉴定化合物3为毛钩藤碱.
化合物4棕色固体,C22H26N2O3.1H NMR(400 MHz,CDCl3)δ:7.84(1H,s,1-NH),7.39(1H,d,J=7.4 Hz,H-9),7.20(1H,d,J=7.5 Hz,H-12),7.19(1H,s,H-17),7.02(2H,m,H-10,H-11),5.49(1H,dd,J=17.5,8.9 Hz,H-19),4.91(2H,dd,J=18.8,14.1 Hz,H-18),3.68(3H,s,17-OMe),3.62(3H,s,22-OMe),3.38(1H,s,H-3);13C NMR(100 MHz,CDCl3)δ:169.0(C-22),160.0(C-17),139.5(C-19),136.2(C-13),135.0(C-2),127.5(C-8),121.3(C-11),119.4(C-10),118.2(C-9),115.6(C-18),111.7(C-16),110.9(C-12),108.1(C-7),61.7(17-OMe),61.5(C-21),60.2(C-3),53.0(C-5),51.4(22-OMe),42.9(C-20),38.9(C-15),33.5(C-14),21.9(C-6).以上数据与文献报道基本一致[9],故鉴定化合物4为柯楠因碱.
化合物5棕色固体,C22H26N2O3.1H NMR(600 MHz,CDCl3)δ:7.25(1H,s,H-17),7.15(1H,t,J=7.5 Hz,H-11),7.10(1H,t,J=7.7 Hz,H-10),5.34(1H,m,H-19),4.91(1H,dd,J=17.2,1.5 Hz,H-18α),4.83(1H,dd,J=10.3,2.0 Hz,H-18β),4.45(1H,dt,J=5.0,2.6 Hz,H-3),3.74(3H,s,17-OMe),3.66(3H,s,22-OMe),3.29(2H,d,J=6.2 Hz,H-5),3.02(1H,ddt,J=15.4,8.1,2.5 Hz,H-6α),2.95(1H,ddd,J=11.4,8.4,4.1 Hz,H-6β);13C NMR(150 MHz,CDCl3)δ:168.9(C-22),159.7(C-17),139.6(C-19),136.1(C-13),133.2(C-2),128.1(C-8),121.4(C-10),119.4(C-11),118.1(C-9),115.3(C-18),111.9(C-16),111.3(C-12),108.1(C-7),61.5(17-OMe),54.2(C-3),51.4(C-5,C-21,22-OMe),43.2(C-20),34.3(C-15),31.3(C-14),17.1(C-6).以上数据与文献报道基本一致[8],故鉴定化合物5为去氢毛钩藤碱.
化合物6棕色固体,C22H26N2O3.1H NMR(400 MHz,CDCl3)δ:7.41(1H,d,J=7.7 Hz,H-9),7.32(1H,d,J=7.6 Hz,H-12),7.33(1H,s,H-17),7.10(1H,t,J=7.2Hz,H-11),7.04(1H,t,J=7.3 Hz,H-10),5.47(1H,d,J=7.0 Hz,H-19),3.83(3H,s,17-OMe),3.73(2H,m,H-3,15),3.69(3H,s,22-OMe),3.63(1H,m,H-21α),3.37(1H,d,J=12.1 Hz,H-21β),3.10(1H,m,H-5α),2.88(3H,m,H-5β,6α,6β),2.28(1H,m,H-14α),1.99(1H,m,H-14β),1.47(3H,d,J=7.0 Hz,H-18);13C NMR(100 MHz,CDCl3)δ:168.5(C-22),159.8(C-17),136.4(C-13),133.3(C-2),132.6(C-20),126.9(C-8),123.6(C-19),121.6(C-11),119.4(C-10),118.2(C-9),112.0(C-16),111.2(C-12),107.8(C-7),63.0(C-21),61.9(17-OMe),57.5(C-3),51.5(22-OMe),50.6(C-5),35.3(C-15),33.1(C-14),20.8(C-6),13.2(C-18).以上数据与文献报道基本一致[10],故鉴定化合物6为蓬籽嗪甲醚.
化合物7棕色固体,C22H26N2O3.1H NMR(600 MHz,CDCl3)δ:7.45(1H,d,J=7.7 Hz,H-9),7.34(1H,d,J=8.3 Hz,H-12),7.28(1H,s,H-17),7.10(H,m,1H-11),7.05(1H,t,J=7.4 Hz,H-10),5.43(1H,q,J=7.0 Hz,H-19),3.86(3H,s,17-OMe),3.70(3H,s,22-OMe),1.54(3H,d,J=7.0 Hz,H-18);13C NMR(150 MHz,CDCl3)δ:172.2(C-22),158.9(C-17),136.3(C-13),135.3(C-2),134.6(C-20),127.6(C-8),122.4(C-19),121.4(C-11),119.4(C-10),118.2(C-9),112.7(C-16),110.9(C-12),108.3(C-7),62.1(C-21),61.9(17-OMe),56.3(C-3),52.8(C-5),51.5(22-OMe),35.9(C-14),31.3(C-15),21.5(C-6),13.2(C-18).以上数据与文献报道基本一致[11],故鉴定化合物7为villocarine A.
化合物8黄色固体,C21H26N2O3.1H NMR(600 MHz,CDCl3)δ:8.68(1H,s,1-NH),7.47(1H,d,J=7.7 Hz,H-9),7.38(1H,d,J=8.0 Hz,H-12),7.16(1H,t,J=7.6 Hz,H-11),7.10(1H,t,J=7.5 Hz,H-10),5.63(1H,q,J=6.7 Hz,H-19),3.81(3H,s,22-OMe),3.50(1H,d,J=4.8 Hz,H-21α),3.26(1H,dd,J=13.0,5.0 Hz,H-5α),3.13(2H,m,H-6),2.92(1H,d,J=12.1 Hz,H-21β),2.63(1H,dd,J=15.7,4.8 Hz,H-5β);13C NMR(150 MHz,CDCl3)δ:175.6(C-22),136.4(C-13),134.2(C-20),133.7(C-2),127.8(C-8),123.6(C-19),121.7(C-11),119.7(C-10),118.1(C-9),111.4(C-12),108.0(C-7),62.3(C-17),52.9(C-3),52.6(C-21),52.4(22-OMe),51.6(C-5),49.8(C-16),32.8(C-15),30.5(C-14),17.9(C-6),13.4(C-18).以上数据与文献报道基本一致[12],故鉴定化合物8为16R-E-isositsirikine.
化合物9黄色固体,C21H26N2O3.1H NMR(600 MHz,CDCl3)δ:8.35(1H,s,1-NH),7.45(1H,d,J=7.7 Hz,H-9),7.29(1H,d,J=8.0 Hz,H-12),7.12(1H,d,J=7.5 Hz,H-11),7.07(1H,t,J=7.4 Hz,H-10),5.53(1H,dt,J=18.3,9.6 Hz,H-19),5.21(1H,dd,J=17.3,1.9 Hz,H-18α),5.17(1H,dd,J=10.2,1.9 Hz,H-18β),3.93(1H,dd,J=11.1,7.3 Hz,H-17α),3.73(1H,dd,J=11.2,5.8 Hz,H-17β),3.63(3H,s,22-OMe);13C NMR(150 MHz,CDCl3)δ:174.5(C-22),138.5(C-19),136.3(C-13),134.4(C-2),127.4(C-8),121.6(C-11),119.5(C-10),118.3(C-18),118.3(C-9),111.1(C-12),108.1(C-7),62.3(C-17),61.2(C-21),59.8(C-3),52.8(C-5),51.9(22-OMe),48.3(C-16),44.8(C-20),40.6(C-15),32.0(C-14),21.7(C-6).以上数据与文献报道基本一致[13],故鉴定化合物9为sitsirikine.
化合物10棕色固体,C21H28N2O3.1H NMR(600 MHz,MeOD)δ:7.40(1H,d,J=7.8 Hz,H-9),7.32(1H,d,J=8.1 Hz,H-12),7.07(1H,m,H-11),7.00(1H,m,H-10),4.07(1H,dd,J=10.8,8.5 Hz,H-17α),3.73(1H,dd,J=10.8,6.1 Hz,H-17β),3.67(3H,s,22-OMe),0.96(3H,t,J=7.5 Hz,H-18);13C NMR(150 MHz,MeOD)δ:174.9(C-22),138.1(C-13),135.6(C-2),128.3(C-8),122.0(C-11),119.8(C-10),118.6(C-9),112.0(C-12),107.8(C-7),62.6(C-17),61.3(C-3),61.1(C-21),54.2(C-5),51.9(22-OMe),49.4(C-16),40.9(C-15),40.3(C-20),31.5(C-14),24.0(C-19),22.3(C-6),10.9(C-18).以上波谱数据与文献报道基本一致[14],故鉴定化合物10为19,20-dihydroisositsirikine.
化合物11棕色固体,C22H26N2O4.1H NMR(600 MHz,CDCl3)δ:7.59(1H,s,H-12),7.42(1H,d,J=7.8 Hz,H-9),7.26(1H,s,H-17),7.22(1H,d,J=7.8 Hz,H-10),7.12(1H,t,J=7.4 Hz,H-11),4.99(1H,d,J=17.2 Hz,H-19),4.93(1H,d,J=11.2 Hz,H-18α),4.80(1H,s,H-18β),3.76(3H,s,17-OMe),3.62(3H,s,22-OMe);13C NMR(150 MHz,CDCl3)δ:168.3(C-22),160.8(C-17),137.8(C-13),136.5(C-19),129.7(C-2),126.4(C-8),122.6(C-10),119.8(C-11),118.0(C-9),117.8(C-18),112.6(C-12),109.6(C-16),105.1(C-7),70.4(C-3),67.9(C-5),62.4(C-21),62.0(17-OMe),51.3(22-OMe),37.3(C-20),26.8(C-14),19.9(C-6).以上数据与文献报道基本一致[15],故鉴定化合物11为去氢毛钩藤碱N-氧化物.
化合物12棕色固体,C22H26N2O4.1H NMR(600 MHz,CDCl3)δ:7.40(1H,s,H-9),7.25(1H,s,H-17),7.01(2H,m,H-10,H-11),4.76(1H,d,J=12.9 Hz,H-21α),4.50(1H,d,J=12.6 Hz,H-3),3.94(1H,d,J=12.9 Hz,H-21β),3.81(3H,s,17-OMe)),3.61(3H,s,22-OMe);13C NMR(150 MHz,CDCl3)δ:167.7(C-22),159.8(C-17),137.2(C-13),130.8(C-2),130.1(C-20),129.8(C-19),126.1(C-8),121.8(C-11),119.3(C-10),118.0(C-9),112.0(C-12),110.7(C-16),76.7(C-21),72.0(C-3),62.0(17-OMe),61.2(C-5),51.3(22-OMe),33.2(C-14),32.7(C-15),18.0(C-6),13.3(C-18).以上数据与文献报道基本一致[16],故鉴定化合物12为geissoschizine N-oxide methyl ether.
化合物13白色固体,C22H28N2O4.1H NMR(400 MHz,CDCl3)δ:9.56(1H,s,1-NH),7.47(1H,d,J=7.4 Hz,H-9),7.18(1H,t,J=7.6 Hz,H-11),7.06(1H,d,J=7.5 Hz,H-10),6.94(1H,d,J=7.7 Hz,H-12),3.61(3H,s,22-OMe),3.52(3H,s,17-OMe),2.79(1H,d,J=13.2 Hz,H-15),0.89(3H,t,J=7.3 Hz,H-18);13C NMR(100 MHz,CDCl3)δ:182.7(C-2),169.2(C-21),160.4(C-17),140.5(C-13),134.5(C-8),127.3(C-11),124.8(C-9),122.2(C-10),111.6(C-16),109.7(C-12),73.1(C-3),61.1(17-OMe),57.4(C-7),54.6(C-21),53.9(C-5),51.2(22-OMe),40.2(C-20),38.9(C-15),34.8(C-6),25.4(C-14),19.3(C-19),12.9(C-18).以上波谱数据与文献报道基本一致[17],故鉴定化合物13为柯诺辛碱.
化合物14 淡黄色固体,C21H26N2O4.1H NMR(600 Hz,CDCl3)δ:7.45(1H,m,H-9),7.16(1H,t,J=7.6 Hz,H-11),7.03(1H,m,H-10),6.86(1H,d,J=7.3 Hz,H-12),3.71(3H,s,17-OMe),3.61(1H,s,H-5α),0.82(3H,s,H-18);13C NMR(150 MHz,CDCl3)δ:182.1(C-2),171.6(C-22),159.7(C-17),140.3(C-13),134.2(C-8),127.6(C-11),125.4(C-9),123.4(C-10),112.5(C-16),109.4(C-12),72.5(C-3),61.3(17-OMe),58.3(C-21),56.3(C-7),54.4(C-5),38.3(C-15),35.7(C-6),29.5(C-14),24.4(C-19),11.5(C-18).以上数据与文献报道基本一致[18],故鉴定化合物14为异钩藤酸.
化合物15淡黄色固体,C22H26N2O4.1H NMR(600 MHz,CDCl3)δ:8.20(1H,s,1-NH),7.45(1H,d,J=7.4 Hz,H-9),7.17(1H,m,H-11),7.03(1H,t,J=7.6 Hz,H-10),6.85(1H,d,J=7.7 Hz,H-12),4.93(2H,m,H-18),3.68(3H,s,17-OMe),3.57(3H,s,22-OMe),3.29(1H,t,J=9.4 Hz,H-3),3.19(1H,dd,J=10.9,4.1 Hz,H-15);13C NMR(150 MHz,CDCl3)δ:182.0(C-2),168.4(C-22),159.6(C-17),139.8(C-19,C-13),134.1(C-8),127.6(C-11),125.4(C-9),122.5(C-10),115.4(C-18),112.2(C-16),109.4(C-12),72.2(C-3),61.4(17-OMe),58.8(C-21),56.9(C-7),54.1(C-21),51.1(22-OMe),42.6(C-20),41.0(C-15),35.6(C-6),27.4(C-14).以上数据与文献报道基本一致[19],故鉴定化合物15为异柯诺辛因碱.
化合物16淡黄色固体,C22H28N2O4.1H NMR(600 MHz,MeOD)δ:7.41(1H,m,H-9),7.33(1H,s,H-17),7.18(1H,d,J=7.5 Hz,H-12),7.04(1H,t,J=7.1 Hz,H-11),6.88(1H,d,J=7.9 Hz,H-10),3.59(3H,s,22-OMe),3.53(3H,s,17-OMe),2.72(1H,dt,J=13.3,3.6 Hz,H-15),2.42(1H,d,J=8.8 Hz,H-3),0.88(3H,s,H-18);13C NMR(150 MHz,MeOD)δ:181.0(C-2),170.7(C-22),162.1(C-17),135.9(C-8),128.6(C-11),125.7(C-9),123.2(C-10),112.4(C-16),110.5(C-12),74.6(C-3),61.7(17-OMe),58.8(C-7),55.8(C-5),54.9(C-21),51.6(22-OMe),41.8(C-20),40.5(C-15),35.7(C-6),26.7(C-14),20.5(C-19),13.2(C-18).以上数据与文献报道基本一致[20],故鉴定化合物16为柯诺辛碱B.
化合物17棕色固体,C22H26N2O4.1H NMR(600 MHz,CDCl3)δ:7.23(1H,s,H-17),7.19(1H,d,J=7.5 Hz,H-9),7.18(1H,t,J=7.6 Hz,H-11),7.04(1H,t,J=7.5 Hz,H-10),6.84(1H,d,J=7.6 Hz,H-12),5.50(1H,dt,J=17.8,11.8 Hz,H-19),4.95(1H,d,J=17.8 Hz,H-18a),4.90(1H,d,J=11.8 Hz,H-18b),3.73(3H,s,17-OMe),3.61(3H,s,22-OMe),3.39(1H,t,J=8.1 Hz,H-3),3.27(1H,dd,J=10.8,4.1 Hz,H-15);13C NMR(150 MHz,CDCl3)δ:181.2(C-2),159.8(C-17),139.6(C-13,19),133.8(C-8),128.0(C-11),123.4(C-9),122.7(C-10),115.5(C-18),109.3(C-9),75.2(C-3),60.2(17-OMe),58.9(C-21),56.2(C-7),55.0(C-5),51.3(22-OMe),42.2(C-20),39.8(C-15),34.9(C-6),27.4(C-14).以上数据与文献报道基本一致[19],故鉴定化合物17为去氢钩藤碱.
化合物18棕色固体,C21H23N2O3.1H NMR(600 MHz,CDCl3)δ:7.55(1H,s,H-17),7.53(1H,d,J=7.3 Hz,H-9),7.46(1H,d,J=8.0 Hz,H-12),7.14(1H,t,J=7.5 Hz,H -11),7.08(1H,t,J=7.4 Hz,H-10),3.74(3H,s,17-OMe)1.26(3H,d,J=7.2 Hz,H-18);13C NMR(151 MHz,CDCl3)δ:167.8(C-22),155.2(C-17),137.9(C-13),135.9(C-2),127.4(C-8),121.4(C-11),119.3(C-10),117.9(C-9),111.0(C-12),109.4(C-16),72.6(C-19),54.6(C-3),52.9(C-5),51.0(22-OMe),37.4(C-20),30.7(C-14),27.2(C-15),22.6(C-6),18.5(C-18).以上数据与文献报道基本一致[21],故鉴定化合物18为阿枯阿米精.
4 抗炎活性
研究表明,炎症小体作为炎症反应的中心环节,其与多数疾病密切相关,因此可成为治疗炎症性疾病的重要靶点,其中,与NLRP3炎症小体相关疾病较多,如痛风、糖尿病、类风湿关节炎等.钩藤虽具有息风定惊,清热平肝的功效,但近年来,对于钩藤中生物总碱的抗炎活性的研究较少,为了寻找其中具有抗炎活性的成分,对分离鉴定的部分生物碱进行了NLRP3炎症小体活性的初筛,NLRP3炎症小体激活会导致巨噬细胞中的LDH释放,通过对小鼠巨噬细胞J774A.1细胞中NLRP3炎症小体的抑制作用,筛选出钩藤生物碱中具有抗炎活性的化合物.实验中用Nigericin对NLRP3炎症小体进行激活,使用LDH分析试剂盒进行测试,测定了分离鉴定的生物碱在20μmol/L浓度下对细胞中LDH释放的抑制率.以MCC950(0.1μmol/L)为阳性对照,化合物的LDH释放率如表1所示.实验结果显示,除化合物13外,其余化合物对于抑制LDH释放反应抑制活性较差,释放率大多大于50%,几乎没有抗炎活性.其中,化合物13抑制LDH的释放率为29.9%,能在一定程度上抑制LDH的释放,表明该化合物可能具有一定抗炎活性.

表1 化合物对于J774A.1细胞中LDH释放率Tab.1 LDH release rate by some compounds in J774A.1 cells
5 讨论
本实验从钩藤带钩茎的茎叶中分离鉴定了18个生物碱,包括10个柯楠因型生物碱,2个柯楠因型N-氧化物,5个柯诺辛型生物碱,1个阿马里新型生物碱.化合物2,7,8和10首次在该植物中分离得到,丰富了钩藤生物碱成分.根据近年来相关文献报道,关于钩藤提取物的活性报道大多集中在神经退行疾病方面,其中,蓬籽嗪甲醚[22]和geissoschizine N-oxide methyl ether[23]具有非常不错的抑制乙酰胆碱酯酶活性,起到预防或治疗的目的.在抗炎活性中,化合物13可能抑制NLRP3炎症小体活化从而抑制细胞凋亡,但其具体信号通路机制仍需探索,其余化合物的抗炎活性较弱,因此,化合物13有可能成为治疗免疫和炎症相关疾病的候选药物.

