Nitrogen-doped porous carbon supported nickel nanoparticles as catalyst for catalytic hydroconversion of high-temperature coal tar
XIE Rui-lun ,ZHANG Xia ,TIAN Yu-jiao ,LEI Zhao ,CAO En-de
(School of Chemistry & Chemical Engineering, Anhui Province Key Laboratory of Coal Clean Conversion & High Valued Utilization, Anhui University of Technology, Ma’anshan 243002, China)
Abstract: A novel and highly active nitrogen-doped porous carbon-supported nickel catalyst Ni@N-PC was successfully developed by a thermolysis of nickel-based zeolitic imidazolate frameworks growing on both sides of graphitic carbon nitride and used for catalyzing hydroconversion of isopropanol soluble portion from ultrasonic extraction of high-temperature coal tar(ISPHTCT). The active nickel nanoparticles were mainly encapsulated on the top of carbon nanotubes and partially dispersed on the surface of carbon nanosheets. Naphthalen-1-ol was used as a model compound to investigate the catalytic hydroconversion activity under different reaction conditions and reveal the mechanism for catalytic hydroconversion. The ISPHTCT and catalytic hydroconversion products of ISPHTCT (ISPCHCP) were analyzed with gas chromatograph/mass spectrometer. The results show that 70% of naphthalen-1-ol was converted at 160 °C and completely converted at 200 °C for 120 min, and the ISPHTCT was greatly upgraded. A total of 180 organic compounds including 33 nitrogen-containing organic compounds, 11 sulfurcontaining organic compounds and 39 oxygenates were identified in ISPHTCT, while no obvious nitrogen-containing organic compounds, sulfur-containing organic compounds and oxygenates were detected in ISPCHCP, indicating the excellent performance of Ni@N-PC for heteroatom removal. All the alkenes, cyclenes and alkynes were saturated and the majority of arenes were converted to cyclanes by catalytic hydroconversion over Ni@N-PC, which exhibited high catalytic hydrogenation activity.
Key words: high-temperature coal tar;catalytic hydroconversion;hydrocarbons;catalysts
High-temperature coal tar (HTCT), a by-product from coal carbonization, contains more than thousands of organic compounds[1,2], mainly composed of arenes(especially condensed arenes), nitrogen-containing organic compounds, sulfur-containing organic compounds, oxygenates and cyclic hydrocarbons with high molecular weight[3]. Because of its own characteristics, HTCT has become a major alternative to petroleum resources, which can be used to produce high-quality clean liquid fuels and high value-added chemicals. However, the production of clean liquid fuel by catalytic hydroconversion of HTCT is still facing great challenges due to the disadvantages of the low activity and deactivation of catalysts[4−6]. Therefore,many efforts have been made to develop hydroconversion catalysts with high dispersion, high activity and easy recovery.
The catalytic hydroconversion reaction process of HTCT includes the catalytic hydrogenation of unsaturated hydrocarbons and arenes and the removal of heteroatoms[7,8]. In the process, the catalysts play a key role in catalytic hydroconversion and account for the majority of the production cost. Precious metals,such as Pt, Pd, La, etc.[9−13], as the main active components of hydroconversion catalysts, have significant catalytic activities and high selectivities.However, the high price, sensitivity to heteroatoms,toxicity and deactivation limit their application in industry. Nickel-based catalysts with non-precious metal nickel as the main active component have been intensively applied in catalytic hydroconversion of coal tar, due to their advantages of high activity, excellent stability and low price, which effectively overcome the disadvantages of precious metals. For example, nickelloaded mesoporous Y zeolite catalysts exhibited excellent catalytic upgrading performance in light tar yield[14]. NiW/Al2O3-SiO2doped with F could effectively improve the dynamic viscosity, density,distillation range and viscosity of liquid products[15].Moreover, most of arenes in HTCT and lowtemperature coal tar were converted to cyclanes over Ni/ZSM, and about 67.1% of hydroaromatics and cycloalkanes were obtained by catalytic hydrogenation over NiMo-C[16,17]. Nickel-based catalysts not only have high catalytic activity for aromatics hydrogenation, but also show excellent performance for heteroatom removal[18]. No heteroatoms were detected in catalytic hydroconversion products[8], and the contents of nitrogen and sulfur in medium-temperature coal tar were reduced from 1.69% and 0.98% to less than 0.001% and 0.01%, respectively, by catalytic hydroconversion[19].
Nickel-based catalysts are composed of active component nickel and catalyst support. The commonly used nickel-based catalyst supports such as γ-Al2O3,molecular sieve, carbon support and diatomite have their own advantages and disadvantages[20−23]. Among them, one-dimensional carbon nanotubes (CNTs) are the ideal supports for nickel-based catalysts due to their large specific surface area, excellent electronic conductivity, special adsorption properties and high thermal stability, which have been studied by many researchers. Moreover, CNT-supported nickel catalysts exhibited excellent performance in catalytic hydroconversion of furfural[24−26], methyl levulinate[27],benzene[28]and aromatic nitro compounds[29]. In addition, the metal nanoparticles encapsulated inside the CNT channel showed higher activity and selectivity than those dispersed outside the channel[29]. Particularly,a novel carbon-based catalyst with nitrogen-doped CNTs encapsulated metal nanoparticles was successfully designed and constructed with zeolitic imidazolate frameworks (ZIFs), one of the metal organic frameworks, as precursors, and showed excellent oxygen reduction reaction activities[30].Inspired by these studies, developing special nickelbased catalysts with high activity for catalytic hydroconversion of coal tar is particularly important.
In this work, a novel nickel-based catalyst with nickel nanoparticles mainly encapsulated inside CNTs and partially dispersed on the surface of carbon nanosheets (CNSs) was prepared with graphitic carbon nitride (g-C3N4) nanosheets as nitrogen source and nickel-based zeolitic imidazolate frameworks (Ni-ZIF)as metal source and used for catalytic hydroconversion of isopropanol soluble portion from ultrasonic extraction of HTCT (ISPHTCT). The catalytic hydroconversion products of ISPHTCT(ISPCHCP) were analyzed with a gas chromatograph/mass spectrometer(GC/MS) and the catalytic mechanism of the catalytic hydroconversion using naphthalen-1-ol as a model compound under different reaction conditions was also discussed.
1 Experimental
1.1 Materials
HTCT was collected from Magang (Group)Holding Co., Ltd., China. Urea, NiCl2·6H2O and 2-methylimidazole used in the experiments were purchased from Shanghai Aladdin Bio-Chem Technology Co., LTD., China. All the organic solvents were purchased from Sinopharm Chemical Reagent Co., Ltd., China. All the chemicals are analytical reagents and were used without any treatment.
1.2 Catalyst preparation
10.00 g urea was put into a crucible with a lid, and then the crucible was placed in a tubular furnace and heated to 550 °C for 4 h with a heating rate of 4 °C/min under air atmosphere. After reaction, the g-C3N4nanosheets were collected.
The nickel-based catalyst was synthesized according to the following steps: 3.24 g NiCl2·6H2O and 7.94 g 2-methylimidazole were magnetically stirred in 80 mL methanol for 12 h at room temperature. At the same time, 1.00 g g-C3N4was dispersed into another 40 mL methanol and magnetically stirred for 12 h. Afterwards, the former solution was successively poured into the latter solution and stirred for 2 h. After centrifugation,washing, and drying in a vacuum at 80 °C for 12 h, the yellow green precursor Ni-ZIF@g-C3N4was obtained.Subsequently, the precursor Ni-ZIF@g-C3N4was put into a tubular furnace and heated to 650 °C for 2 h with a heating rate of 2 °C/min under nitrogen atmosphere to remove g-C3N4. The black materials containing metal nickel, CNTs and CNSs were eventually collected, which are designated as Ni@N-PC.
1.3 Catalyst characterizations
The morphology and microstructure of the samples were characterized by a FEI NANO SEM430 scanning electron microscopy (SEM) and a FEI Tecnai G2 F30 high-resolution transmission electron microscopy (HRTEM) equipped with selected-area electron diffraction. X-ray diffraction (XRD)characterization was carried out on a Bruker D8 Advance diffractometer with CuKα irradiation. N2isothermal adsorption and desorption characterization was performed on a V-Sorb 2800TP instrument at 77 K. The specific surface area and pore size distribution were obtained by Brunauer-Emmett-Teller method and the Barrett-Joyner-Halenda method,respectively. X-ray photoelectron spectroscopy (XPS)was recorded on a Thermo ESCALAB 250XI photoelectron spectrometer. NH3temperature programmed desorption (NH3-TPD) was performed on a FineSorb3010 instrument.
1.4 Catalytic hydroconversion of naphthalen-1-ol and ISPHTCT
0.1 g Ni@N-PC, 1 mmol naphthalen-1-ol and 20 mLn-hexane were successively put into a 50 mL stainless steel autoclave. After replacing the air in the autoclave with N2for three times, the autoclave was pressurized to 5.0 MPa with H2at room temperature,and then the autoclave was quickly heated to a set temperature (160–240 °C) within 30 min, and maintained at the temperature for a prescribed period of time(0–120 min) under magnetic stirring.
ISPHTCTwas prepared by ultrasonic extraction of 10 g HTCT with 200 mL isopropanol at 50 °C for 2 h.For catalytic hydroconversion of ISPHTCT, 0.1 g ISPHTCT, 0.1 g Ni@N-PC and 20 mLn-hexane were added into a 50 mL stainless steel. After replacing the air in the autoclave with N2for three times, the autoclave was pressurized to 5.0 MPa with H2and heated to 300 °C for 20 h. After reaction, ISPCHCPwas centrifuged and analyzed with a GC/MS.
2 Results and discussion
2.1 Ni@N-PC characterizations
Figure 1 illustrates the synthesis approach of Ni@N-PC with Ni-ZIF as metal source and g-C3N4nanosheets as sacrificial templates and nitrogen source.Ni-ZIF was firstly synthesized by the reaction of Ni2+with 2-methylimidazole, and then the synthesized Ni-ZIF further grew on both sides of g-C3N4to form a homogeneous Ni-ZIF@g-C3N4. During carbonization,2-methylimidazole in Ni-ZIF and g-C3N4underwent thermolysis to afford the unique nitrogen-doped porous carbon containing bamboo-like CNTs and CNSs.Meanwhile, the Ni2+species were reduced to Ni0during thermolysis.
Figure 2 shows the morphology and microstructure of the Ni@N-PC characterized by SEM and HRTEM.As displayed in Figures 2(a)-2(c), the Ni@N-PC shows a three-dimensional cotton-like shape with interconnected structure. There are many pores with different sizes on the surface of porous carbon, which are beneficial to the loading of nickel. As can be seen from Figures 2(d) and 2(e), the Ni@N-PC is composed of a large number of CNSs with a thickness of several nanometers to tens of nanometers and bamboo-like CNTs with a diameter in the range of 10 nm, which may be formed by the thermolysis of g-C3N4and the evaporation of nickel. The CNSs closely connected with CNTs are large in size and thin in thickness, and have many folds on the surface, which are similar to the multilayer graphene nanosheets. Nickel nanoparticles are mainly encapsulated on the top of CNTs and partially dispersed on the surface of CNSs, which can effectively prevent the agglomeration of nickel nanoparticles and make them uniformly dispersed. As shown in Figure 2(f), the lattice fringes with the interplanar spacing of 0.2 nm in the particles are assigned to the Ni (111) plane, while the lattice fringes with the inter-planar spacing of 0.34 nm correspond to the C(002) plane. EDS mappings shown in Figures 2(h)-2(j)display that nickel nanoparticles are uniformly distributed on the surface of the catalyst. Besides carbon and nickel, the well dispersed nitrogen was also detected in the catalyst.
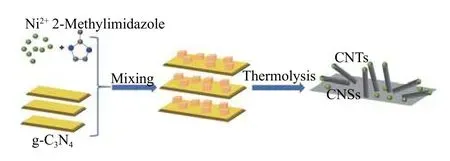
Figure 1 Schematic illustration of the Ni@N-PC preparation
XRD was employed to analyze the crystal structure of the catalyst. As Figure 3 displays, Ni@NPC shows a wide C (002) diffraction peak at 26.21°,which can be considered as the characteristic peak of carbon, while the diffraction peaks at 44°, 52° and 76°are indexed as the (111), (200) and (220) crystal planes of pure nickel with face centered cubic structure[31],respectively. Thus, the results also indicate that the g-C3N4nanosheets in the initial mixture were completely decomposed and there are no other crystals in the catalyst except pure nickel and carbon. The structural characteristics are consistent with the results of SEM analysis.

Figure 2 SEM images ((a)-(c)), HRTEM images ((d)-(f)) and EDS mappings ((g)-(j)) of Ni@N-PC
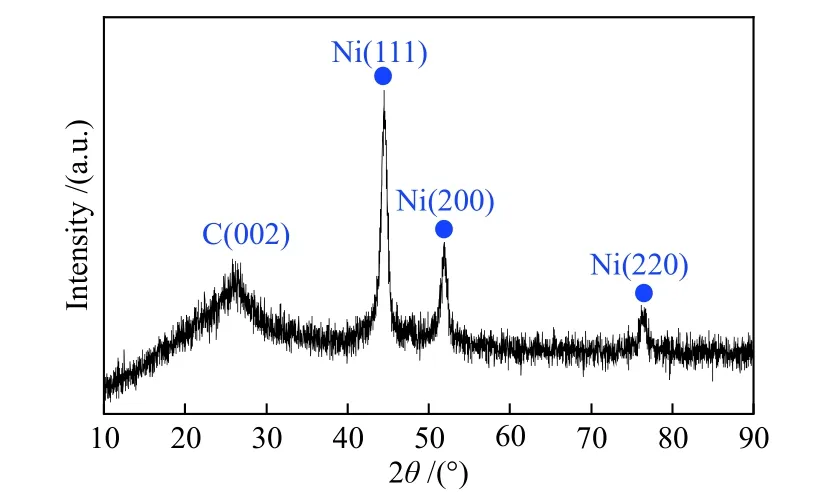
Figure 3 XRD pattern of Ni@N-PC
The chemical composition of the catalyst was further investigated by XPS. As shown in Figure 4,Ni@N-PC consists of nickel, carbon, nitrogen and a small amount of oxygen. The nitrogen content mainly stemmed from the nitrogen-containing functional groups formed during the thermolysis of g-C3N4nanosheets and 2-methylimidazole in the raw materials,while the oxygen content may originate from the water/oxygen in the air. The strong and sharp C 1speak and the weak N 1speak, O 1speak and Ni 2ppeak appearing in Figure 4(a) suggest that Ni@N-PC contains carbon, nitrogen, oxygen and nickel, but no other elements. The high-resolution C 1s, N 1sand Ni 2pspectra can be divided into different components.As Figure 4(b) shows, the high and strong peak centered at 284.7 eV is assigned tosp2carbon, whereas the peaks centered at 285.7 and 287.2 eV correspond to the bonds betweensp2andsp3carbon atoms and nitrogen[32], respectively, which confirms the successful doping of nitrogen atoms into the catalyst. As Figure 4(c) exhibits, the N 1sspectra can be deconvoluted into four distinct peaks due to the different bonding configurations of nitrogen atoms. The peaks centered at the bond energies of 398.4, 399.4, 401.1 and 402.6 eV are assigned to pyridinic N, pyrrolic N, graphitic N and oxidized N, respectively. According to XPS analysis,the nitrogen content of Ni@N-PC is up to 10.24% and the pyridinic N is predominant. Researches show that high pyridine N content can effectively increase the charge density, which is conducive to anchoring more metals and forming metal–N–C active centers[30,33]. For Ni 2pspectra shown in Figure 4(d), the peaks centered at the bond energies of 853.8 and 871.1 eV, 854.6 and 872.1 eV, 862.5 and 878.9 eV can be assigned to the metallic nickel, Ni –N and Ni 2psatellite peaks,respectively. Different nickel states can influence the surface properties and then improve the activity of catalyst[34].

Figure 4 (a) General XPS spectra, (b) high-resolution C 1s spectra, (c) high-resolution N 1s spectra and(d) high-resolution Ni 2p of Ni@N-PC
As the surface area, pore distribution and pore size can directly affect the catalytic activity, the synthesized catalyst was further characterized by N2adsorption/desorption. As Figure 5(a) illustrates, the N2adsorption/desorption isotherm of Ni@N-PC shows a characteristic IV-type isotherm and a typical H1 hysteresis loop appearing at the high pressure of 0.7–1.0, confirming the presence of abundant cylindrical pores with uniform size and regular shape in the catalyst. Meanwhile, the rapid adsorption of N2at the high pressure also suggests the abundant mesopores in the structure of the catalyst. In addition, Ni@N-PC shows a large specific surface area of 899 m2/g and a mesopore diameter of ca. 14 nm, as shown in Figure 5(b). The results are in agreement with those of HRTEM and SEM.
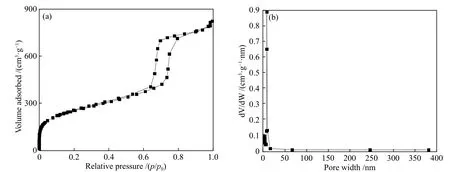
Figure 5 (a) N2 adsorption-desorption isotherm and (b) pore size distribution of Ni@N-PC
The surface acidity and basicity were studied by NH3-TPD. As shown in Figure 6, three desorption peaks with different intensities are observed in low temperature region (< 300 °C) and high temperature region (300–500 °C). A broad desorption peak of NH3appears at 421.3 °C in high temperature region, which proves the strong acidic sites on the surface of Ni@NPC, while the peaks at 103.8 and 200.1 °C in low temperature region correspond to the weak acid sites.Liu et al[35]reported that with fluorine doping into porous carbon, fluorine and carbon atoms are bonded to form Lewis acid sites due to the different electronegativity between fluorine and carbon atoms.Thus, the acidic sites in Ni@N-PC may be formed by the sites containing electron holes in porous carbon or the unsaturated nickel centers with nitrogen doping into porous carbon.
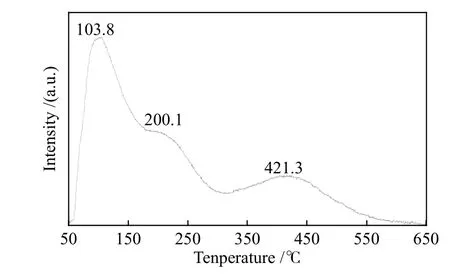
Figure 6 NH3-TPD profile of Ni@N-PC
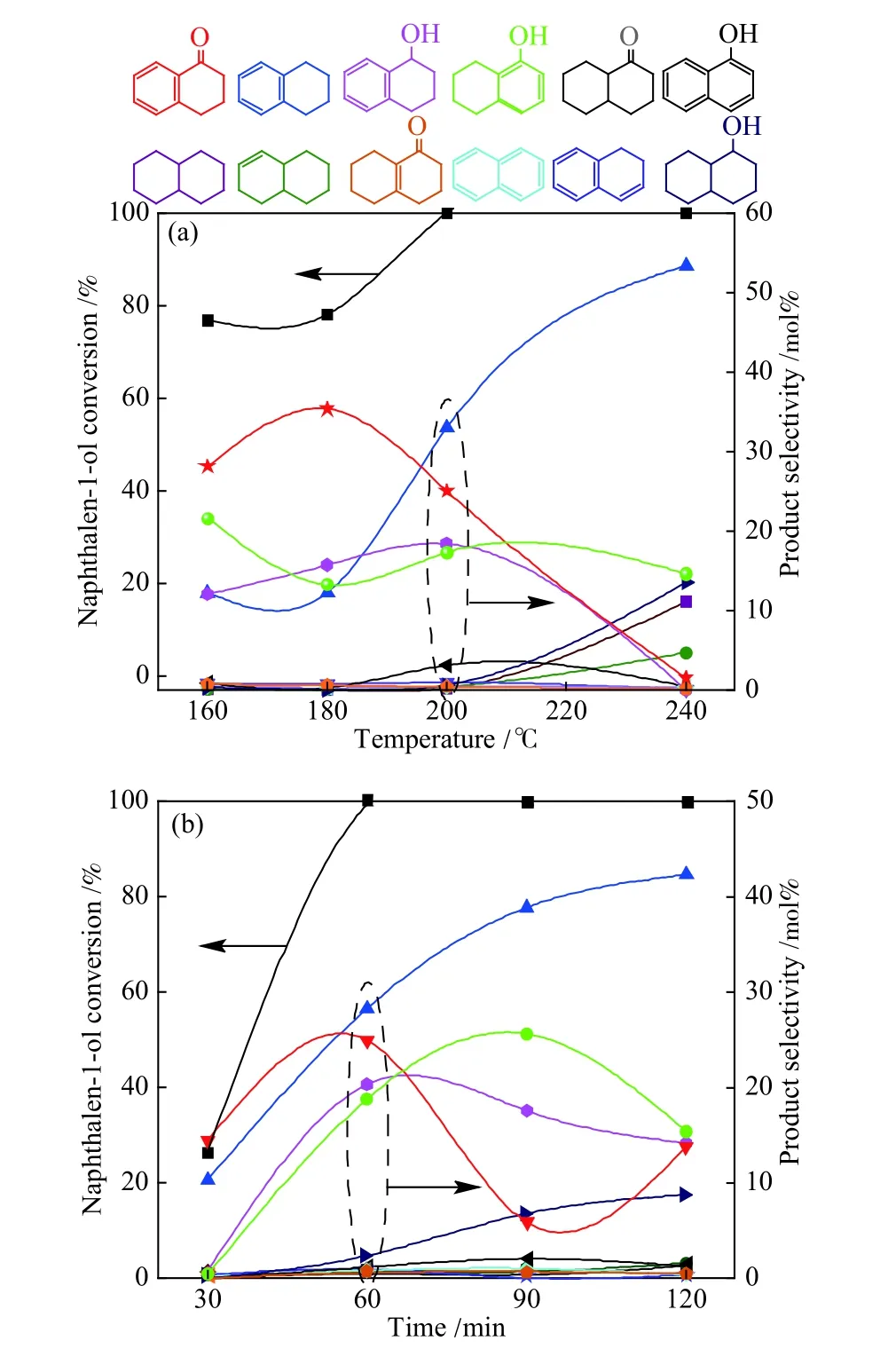
Figure 7 Effects of temperature and time on the catalytic hydroconversion of naphthalen-1-ol over Ni@N-PC
2.2 Catalytic hydroconversion of naphthalen-1-ol
The catalytic conversion and product distribution of main products vary with the reaction conditions. As shown in Figure 7, naphthalen-1-ol conversion increases significantly with raising the reaction temperature and prolonging the reaction time. 70% naphthalen-1-ol was converted at 160 °C and completely converted at 200 °C.10 products including 1,2,3,4-tetrahydronaphthalene,1,2,3,4-tetrahydronaphthalen-1-ol, 3,4-dihydronaphthalen-1(2H)-one, and 5,6,7,8-tetrahydronaphthalen-1-ol were detected from catalytic hydroconversion of naphthalen-1-ol over Ni@N-PC. With the reaction temperature raises, the selectivities of 1,2,3,4-tetrahydronaphthalene, 1,2,3,4-tetrahydronaphthalen-1-ol and 5,6,7,8-tetrahydronaphthalen-1-ol decrease, but the others increase. Thus, 200 °C was used as the optimal temperature to investigate the conversion of naphthalen-1-ol at different time. With prolonging the reaction time to 120 min, the selectivity of 1,2,3,4-tetrahydronaphthalene reaches maximum of 42.4%,while those of 1,2,3,4-tetrahydronaphthalen-1-ol, 3,4-dihydronaphthalen-1(2H)-one, and 5,6,7,8-tetrahydronaphthalen-1-ol decrease to ca. 10%, indicating the high selectivity and excellent catalytic performance of Ni@N-PC.
2.3 Possible pathways for the catalytic hydroconversion of naphthalen-1-ol
As Figure 8 depicts, Ni@N-PC can effectively promote the formation and transfer of H···H, and then H···H is further split into a mobile H+and a H−adhered to the surface of Ni@N-PC[36]. According to the different intermediate and final products depicted in Figure 9, there may be three possible pathways. First,the O atom with strong electronegativity and hydrogen receptivity in naphthalen-1-ol is preferentially attacked by H+followed by H2O leaving and H−attacking to form naphthalene. Subsequently, naphthalene can be partially hydrogenated to 1, 2-dihydronaphthalene, 1, 2,3, 4-tetrahydronaphthalene and 1, 2, 3, 4, 4a, 5, 6, 8aoctahydronaphthalene, and completely converted to(4as,8as)-decahydronaphthalene. On the other hand,naphthalen-1-ol is first hydrogenated to 3,4-dihy dronaphthalen-1(2H)-one, and then to 3, 4, 5, 6, 7, 8-hexahydronaphthalen-1(2H)-one, (4aR, 8aS)-octahydronaphthalen-1(2H)-one and decahydronaphthalen-1-olviaH···H transfer. The decahydronaphthalen-1-ol abstracts H+from the Ni@N-PC surface followed by H2O leaving to produce (4as, 8as)-decahydronaphthalene. Alternatively, 3, 4-dihydronaphthalen-1(2H)-one can also be hydrogenated to 1, 2, 3, 4-tetrahydronaphthalen-1-olviaH···H transfer and subsequent abstracting H+, H2O leaving and H−attacking to produce 1, 2, 3, 4-tetrahydronaphthalene. In addition, a part of naphthalen-1-ol can be converted to 5, 6, 7, 8-tetrahydro-naphthalen-1-ol and decahydro-naphthalen-1-ol, and finally to (4as, 8as)-decahydronaphthalene by catalytic hydroconversion.
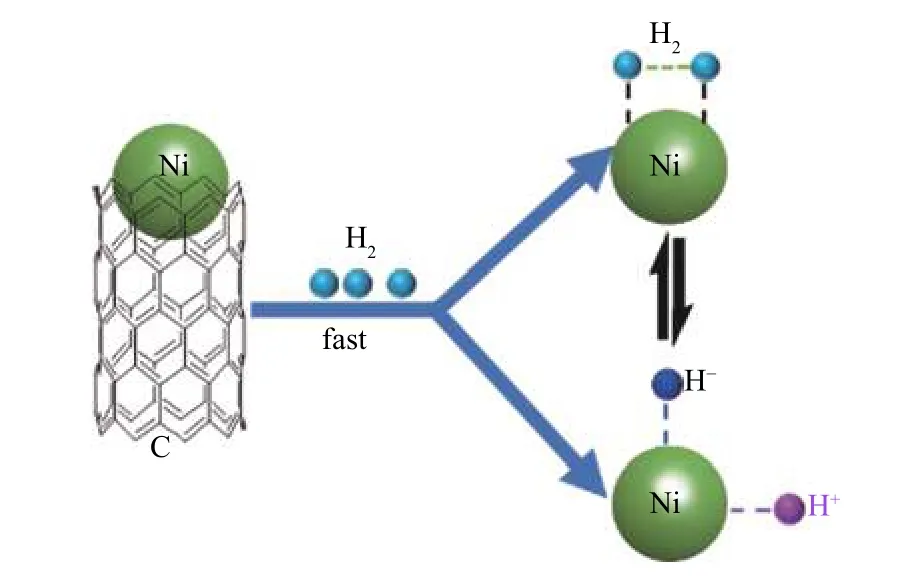
Figure 8 Proposed pathways for the formation of H···H and H+ over Ni@N-PC
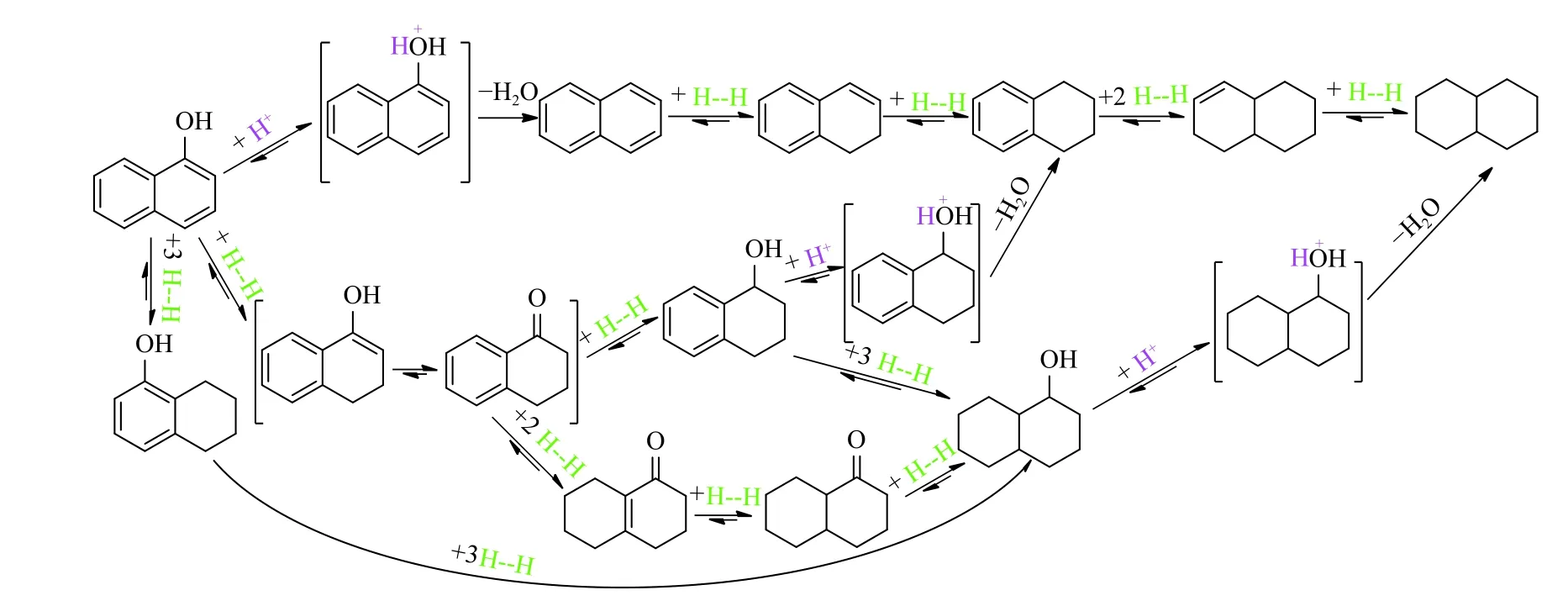
Figure 9 Possible pathways for the catalytic hydroconversion of naphthalen-1-ol over Ni@N-PC
2.4 Catalytic hydroconversion of ISPHTCT
As shown in Figures S1 and S2 and Tables S1−S5, a total of 180 organic compounds were detected in ISPHTCTby GC/MS, which contain alkanes,cyclanes, cyclenes, alkenes, alkynes, arenes, nitrogencontaining organic compounds, sulfur-containing organic compounds and oxygenates. Among them, the heteroatom compounds include 33 nitrogen-containing organic compounds, 11 sulfur-containing organic compounds and 39 oxygenates, accounting for more than ca. 16.4% of the total detectable compounds,which would seriously poison the catalysts. Due to the complex components of HTCT, total hydrogenation faces enormous difficulties. Nevertheless, all the alkenes, cyclenes and alkynes were saturated by catalytic hydroconversion over Ni@N-PC. Most of arenes were converted to cyclanes, such as (4ar, 8ar)-decahydronaphthalene, decahydronaphthalene, 1-methyldecahydronaphthalene, tetradecahydrophenanthrene and dodecahydroacenaphthylene, and others were also deeply hydrogenated to hydroarenes, such as 1, 2, 3, 4, 5, 6, 7, 8-octahydrophenanthrene, 1, 2, 3, 6b, 7,8, 9, 10, 10a, 10b-decahydrofluoranthene, 1, 2, 3, 3a,3a1, 4, 5, 9, 10, 10a-decahydropyrene and (4as, 8as)-4a-phenyldecahydronaphthalene. Moreover, no obvious nitrogen-containing organic compounds,sulfur-containing organic compounds and oxygenates were detected in ISPCHC, which shows that the oxygen,nitrogen, sulfur and other heteroatoms in HTCT can be effectively removed by catalytic hydrogenation over Ni@N-PC. As Figure. S3 displays, the brownish black ISPHTCTwas converted into colorless ISPCHCPby catalytic hydroconversion, during which the catalyst can be recovered rapidly by magnetism.
2.5 Effect of catalytic hydroconversion on Ni@NPC
In order to investigate the state of active components of the catalyst, the used Ni@N-PC was characterized by XRD and XPS.
As shown in Figure 10, there are no other characteristic peaks except carbon and nickel. The characteristic peaks of nickel have no obvious change.The C (002) diffraction peak of carbon broadens and weakens, which may be due to the increase of defects in CNTs and CNSs during catalytic hydroconversion.
Compared with the spectra of fresh catalyst, the high-resolution N 1sand Ni 2pspectra in Figure 11 are slightly different. According to XPS analysis, as shown in Figure 4(c), the increase of the relative content of pyrrolic N and the decrease of the relative content of pyridinic N show that nitrogen doped porous carbon may be involved in the catalytic hydroconversion,which is consistent with the results of XRD analysis.As Figure 11(d) shows, the relative content of Ni–N increases slightly, while the relative content of metallic nickel decreases indicating the loss of active components of the catalyst.
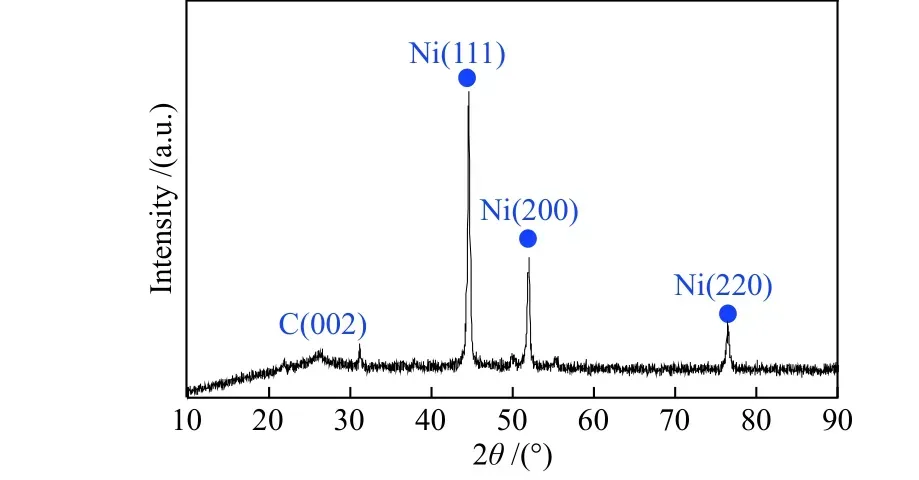
Figure 10 XRD pattern of Ni@N-PC after catalytic hydroconversion of ISPHTCT
3 Conclusions
In conclusion, a novel Ni@N-PC catalyst composed of CNSs and bamboo-like CNTs was successfully synthesized with g-C3N4nanosheets as sacrificial templates and nitrogen source and Ni-ZIFs as precursors. The nickel nanoparticles are encapsulated inside the channel of CNTs and uniformly dispersed on the surface of CNSs. The catalyst exhibits high activities for the catalytic hydroconversion of naphthalen-1-ol and ISPHTCT. All the alkenes, cyclenes and alkynes were saturated and the arenes were converted to cyclanes or deeply hydrogenated to hydroarenes over Ni@N-PC. In particular, the catalyst shows the excellent performance for removing the sulfur, nitrogen, oxygen and other heteroatoms from coal tar. This work might provide a feasible and effective way for the preparation of clean fuel oil or high value-added chemicals from coal tar.
Nomenclature
HTCT: High-temperature coal tar;
g-C3N4: graphitic carbon nitride;
ISPHTCT: isopropanol soluble portion from ultrasonic extraction of high-temperature coal tar;
ISPCHCP: catalytic hydroconversion products of ISPHTCT;
ZIFs: zeolitic imidazolate frameworks;
Ni-ZIF: nickel-based zeolitic imidazolate frameworks;
GC/MS: gas chromatograph/mass spectrometer;
CNTs: carbon nanotubes;
CNSs: carbon nanosheets;
SEM: scanning electron microscopy;
HRTEM: high-resolution transmission electron microscopy;
XRD: X-ray diffraction;
XPS: X-ray photoelectron spectroscopy;
NH3-TPD: NH3temperature programmed desorption
- 燃料化学学报的其它文章
- 生物质气再燃脱除流化床N2O 的机理研究
- Catalytic pyrolysis of sugarcane bagasse by zeolite catalyst for the production of multi-walled carbon nanotubes
- Probing into the crystal plane effect on the reduction of α-Fe2O3 in CO by Operando Raman spectroscopy
- CoSOH/Co(OH)2 复合纳米片的制备及其氧析出催化性能
- Effects of promoters on carburized fused iron catalysts in Fischer-Tropsch synthesis
- Effects of Ca content on the activity of HZSM-5 nanoparticles in the conversion of methanol to olefins and coke formation

