Visual acuity after intravitreal ranibizumab with and without laser therapy in the treatment of macular edema due to branch retinal vein occlusion: a 12-month retrospective analysis
Reiko Umeya, Koichi Ono, Toshimitsu Kasuga
1Department of Ophthalmology, Juntendo Tokyo Koto Geriatric Medical Center, Tokyo 136-0075, Japan
2Department of Ophthalmology, Juntendo University School of Medicine, Tokyo 113-8421, Japan
Abstract
● KEYWORDS: interaction; ranibizumab; laser; macular edema; branch retinal vein occlusion
INTRODUCTION
Since it was first described in 1984, laser photocoagulation has been used to treat macular edema (ME) associated with branch retinal vein occlusion (BRVO)[1]. More recently,the BRAVO study, which investigated the treatment of ME following BRVO[2-3], showed that intravitreal injection of the vascular endothelial growth factor (VEGF) inhibitor ranibizumab (IVR) was a safe and effective treatment for BRVO-associated ME. This treatment has also been widely used in Japan, where it was approved for coverage under the National Health Insurance system in August 2013.The BRIGHTER study showed that at six months after commencing treatment, IVR with or without laser therapy was significantly better in terms of efficacy and safety than laser therapy alone[4-5]. The multicenter investigations conducted to date point to intravitreal anti-VEGF therapy as a reasonable first-choice treatment for BRVO-associated ME. However,most prospective randomized controlled trials to date have been sponsored by the pharmaceutical industry. In the BRAVO study, patients in the 0.5 mg ranibizumab group underwent an average of 8.4 intravitreal injections per year[3]; in clinical practice, such frequent intravitreal injections would place a heavy economic burden on the patient. Another prospective study examined combined treatment with macular grid photocoagulation and anti-VEGF drugs to reduce the number of intravitreal injections per patient; however, the combination did not affect visual acuity[6]. Despite this, 14/39 (35.9%)retinal experts in clinical practice in Japan opted for anti-VEGF and laser therapy in patients with recurrent ME due to BRVO[7].This suggests that, at least in some cases, combination therapy may have some beneficial effects. Therefore, the purpose of the present study was to assess the interaction between laser therapy and IVR retrospectively.
SUBJECTS AND METHODS
Ethical Approval Each patient provided written informed consent before treatment with laser, sub-Tenon’s capsule injection of triamcinolone (STTA), or IVR at our hospital. The study protocol was reviewed and approved by the Institutional Ethics Committee. This study was conducted in accordance with the Declaration of Helsinki, and no personal information is reported.
Study Design and Patients This retrospective, observational,single-institution, case-comparison series was performed to analyze visual outcomes 1y after treatment of ME secondary to BRVO. We reviewed outpatient clinical records between June 2002 and June 2019, searching for data using the diagnostic term “retinal vascular occlusion” (International Classification of Diseases-10 code H34). Patients seen at our hospital from June 2002 to June 2019 were included if they met the following criteria: 1) a diagnosis of retinal vascular occlusion; 2) fluorescein angiography (FA) and/or optical coherence tomography (OCT)of the fundus; 3) at least 12mo of follow-up; 4) presence of ME. Exclusion criteria were 1) incorrect diagnosis or incomplete clinical record; 2) central retinal vein occlusion;3) follow-up of less than 12mo; 4) intravitreal bevacizumab injection (IVB); 5) intravitreal aflibercept injection (IVA).
Clinical Analysis and Outcome Measures BRVO-associated ME was diagnosed based on FA and/or OCT findings.All subjects underwent comprehensive ophthalmological examination, including best corrected visual acuity (BCVA),retinal structure evaluation using FA (Topcon Medical Systems, Inc., Oakland, New Jersey, USA), and/or OCT(Cirrus high-definition OCT; carl Zeiss, Dublin, CA, USA).The reported therapeutic outcome was based on change in visual acuity between initial presentation and 12-month follow-up. BCVA was measured at 5 m on a standard Japanese fractional visual acuity chart (Distant Test Chart, LANDOLT,Handaya, Tokyo, Japan). The logarithmic minimal angle of resolution (logMAR) was calculated from the decimal visual acuity. OCT was introduced in August 2008, and we quantified the fovea thickness in subsequent cases. Possible confounding factors for visual outcomes were selected in accordance with results from previous studies and included age[8], history of systemic hypertension, diabetes mellitus[9], current or past smoking history, and foveal hemorrhage[10]. A history of systemic hypertension, diabetes mellitus, and/or smoking was established from the clinical records.
Treatments After diagnosis of ME associated with BRVO,informed consent was obtained from each patient, and treatment provided at the discretion of each doctor. Treatment methods included laser therapy, IVR (Lucentis®, 0.5 mg in 0.05 mL; Genentech, San Francisco, CA, USA), sub-Tenon’s capsule injection of triamcinolone (STTA; Kenacort®, 20 mg; Bristol-Myers Squibb, Princeton, NJ, USA)[11], or a combination thereof, as well as some cases that were observed without intervention. Coagulation conditions requiring local coagulation for blood vessel dilation, and grid coagulation for diffuse leakage, were at the discretion of each doctor and considered laser treatment in the present study. Since 2013,when ranibizumab was approved for insurance in Japan, IVR has been implemented for OCT with central retinal thickness over 300 µm. Combination therapy was administered at the discretion of each doctor.
Statistical Analysis Visual acuity and central foveal thickness (CFT) were expressed as mean±standard deviation(SD). Decimal acuity was converted to logMAR units for statistical analysis. A pairedt-test was performed to evaluate the difference in visual acuity before and after treatment.Multivariate logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (CI). Age, sex, history of hypertension, diabetes mellitus, smoking history, and foveal hemorrhage were considered confounders. We examined the interaction of treatment methods that contributed to the improvement in visual acuity by using multivariate Logistic regression analysis with the interaction terms.P<0.05 was considered statistically significant. All statistical analyses were performed in STATA/SE 15.1 for windows (Stata Corp.,College Station, TX, USA).
RESULTS
Baseline Characteristics and Patient Demographics A patient selection flowchart for this study is shown in Figure 1.We included patients diagnosed with retinal vascular occlusion at our hospital from June 2002 to June 2019. From 555 records identified, we excluded records with errors in registration or documentation on clinical charts (n=60); patients with central retinal vein occlusion (n=174), late-stage BRVO (n=129),BRVO without ME (n=30), and BRVO with <12mo of followup (n=55), patients who received IVB (n=33), and patients who received IVA (n=1). Detailed patient profile and treatment history is presented in Table 1. We included 73 patients (34 men, 39 women; 73 eyes) with BRVO-associated ME, aged 33-93y (mean±SD, 69.4±12.1y). Twenty patients received laser monotherapy, 12 received IVR monotherapy, 4 received STTA monotherapy, 4 received IVR+STTA, 8 received IVR+laser,4 received STTA+laser, and 3 received IVR+STTA+laser.Eighteen patients were observed who did not receive treatment throughout the follow-up period.
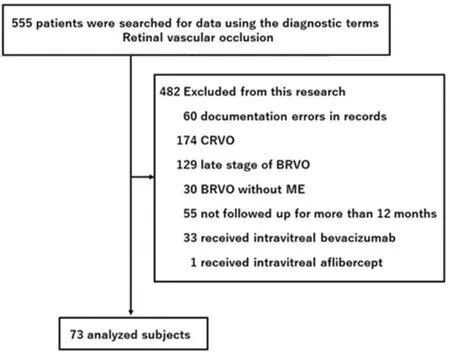
Figure 1 Subject selection flowchart Patients seen at our hospital from June 2002 to June 2019 were included if they had a diagnosis of retinal vascular occlusion. BRVO: Branch retinal vein occlusion;CRVO: Central retinal vein occlusion; ME: Macular edema.

Table 1 Clinical characteristics in patients with BRVO mean±SD
Visual Acuity and Central Foveal Thickness Table 2 shows the course of visual acuity and central retinal thickness for each treatment. Dunn’s multiple-comparison test for stochastic dominance using Bonferroni correction, showed no difference in BCVA among groups at baseline. The BCVA in the IVR monotherapy, laser monotherapy, and STTA+laser groups were significantly increased (P<0.001, <0.001, and =0.019,respectively). OCT was performed in 38 of 73 cases (52.1%)with a significant reduction in CFT at 12mo (317.2±165.1vs537.6±197.5 µm,P<0.0001). Table 2 shows visual acuity and central foveal thickness at baseline and after treatment in each group.
Factors Associated with Visual Acuity Improvement Table 3 shows the ORs of each predictor of visual acuity improvement. In simple Logistic regression models, the factor associated with visual improvement was IVR (crude OR:3.05, 95%CI: 1.12-8.30,P=0.029). After adjusting for all other variables, IVR remained the significant factor (adjusted OR:3.89, 95%CI: 1.25-12.1,P=0.019). There was no significant interaction between laser and IVR (adjusted OR: 0.07, 95%CI:0.01-0.75,P=0.029; Table 4).
DISCUSSION
Our retrospective analysis of visual outcomes after 12mo in patients treated for ME due to BRVO showed that laser and IVR improved visual acuity; however, there was no significant interaction between these treatments. Before the introduction of anti-VEGF therapy, grid laser photocoagulation was the standard treatment for ME due to BRVO[1]. Today, anti-VEGF therapy is widely accepted as the treatment of choice for BRVO-associated ME, based on prospective randomized controlled trials[2-4].
Two previous large retrospective cohort studies[12-13]have described visual improvements after anti-VEGF treatment.Within these cases, a small number (23/205[12]and 20/177[13])received laser therapy in combination with anti-VEGF treatment, but the interaction of these treatments was not analyzed statistically. The BRAVO study[3]prospectively compared visual outcomes after treatment between two groups; grid laser photocoagulation was used in 20% of the IVR group and 55% of the sham group. However, the study did not examine whether grid laser photocoagulation combined with IVR had any therapeutic effects. In another study, Tanet al[14]compared the efficacy of IVR for BRVO-associated ME for 12mo between patients who received IVR for six consecutive months then as needed for another six months, and patients who received sham injections for 12mo. Grid laser photocoagulation was performed at 13 and 25wk in 2 of 15 cases in the IVR group, and in all 21 cases of the sham group.Their results showed that, compared to the sham group, the IVR group exhibited significant and sustained improvements invisual acuity and anatomical outcomes after 12mo of treatment.However, they could not demonstrate that the combination of laser and ranibizumab therapy resulted in higher efficacy than ranibizumab monotherapy. In the RELATE study,subjects randomly assigned to treatment with 0.5 or 2.0 mg of ranibizumab every 4wk for 24wk were then re-randomized to receive only ranibizumab+laser orpro re nataranibizumab[15].Significant improvements in BCVA were reported within the first two years in the group that received additional laser treatment, but this significance disappeared in later follow-ups.The authors concluded that the addition of laser therapy neither increased edema resolution nor reduced the total number of ranibizumab injections, so there was no long-term benefit. In the BRIGHTER study[4], one group received IVR injections for at least three consecutive months initially, followed by an asneeded regimen, and was compared with an IVR+laser group and a laser monotherapy group; the IVR groups, with or without laser, showed significantly better visual results after six months than the laser monotherapy group, with no difference between the IVR and IVR+laser groups. In other words, the beneficial effects of additional laser therapy could not be demonstrated.Overall, these randomized controlled trials[3-4,14-15]indicated that adding laser treatment to IVR had no beneficial effect on BRVO edema. However, a combination of IVB and laser photocoagulation was reported to reduce the number of bevacizumab doses[16], although the interaction between IVR and laser treatment had not been studied to date.
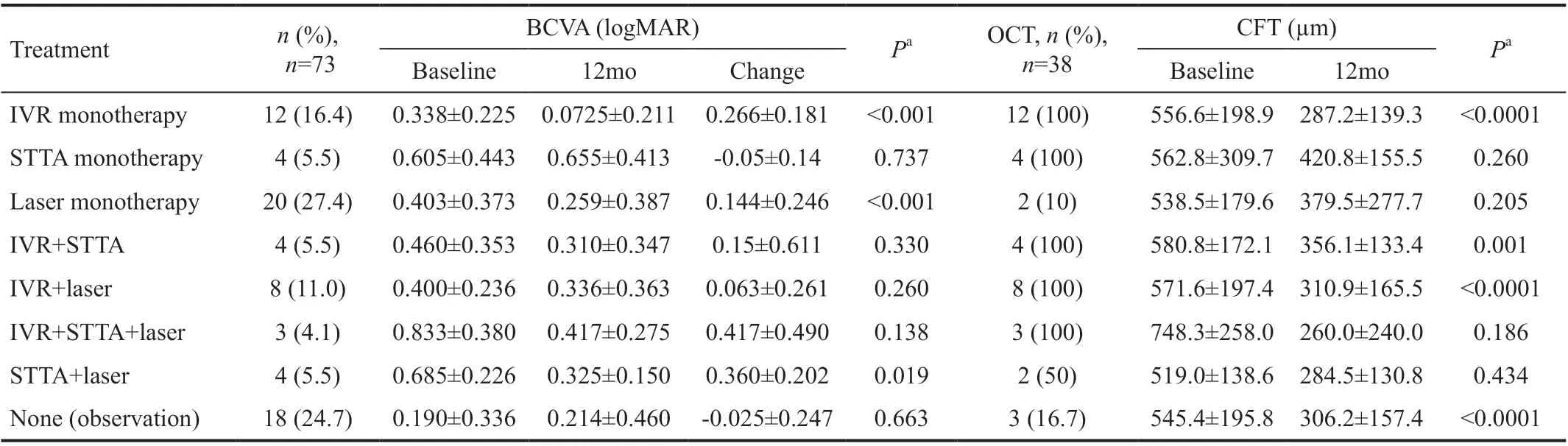
Table 2 Change in visual acuity and central retinal thickness from baseline to 12mo mean±SD
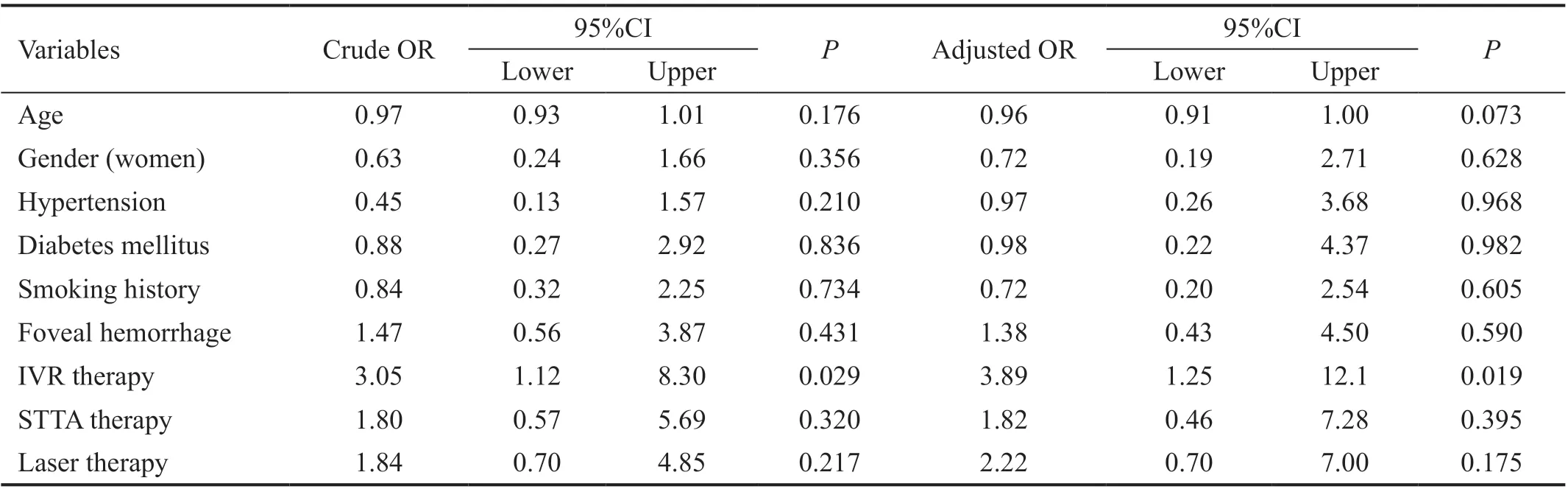
Table 3 Odds ratios of visual acuity recovery obtained by Logistic regression analysis without interaction terms
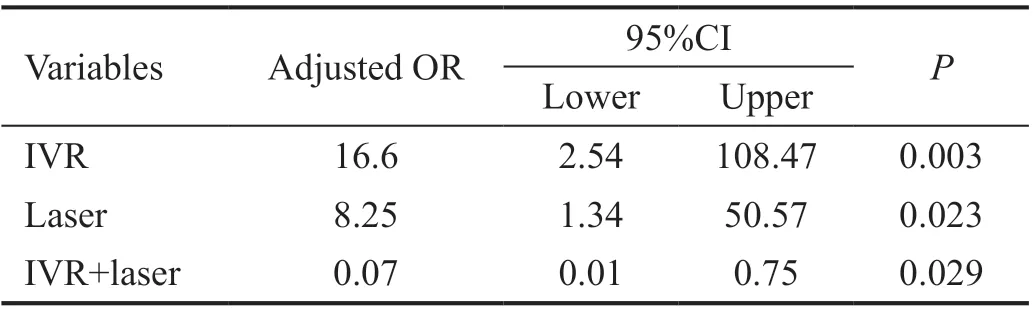
Table 4 Adjusted odds ratios using an interaction model
The strength of the present study is the inclusion of patients across the time period when laser treatment was the main treatment focus and the current time period when anti-VEGF treatment is the main focus. Therefore, by including patients treated with laser monotherapy before the introduction and approval of the anti-VEGF drug ranibizumab (Lucentis®),the likelihood of selection bias is reduced. As a result, in the Logistic analysis model without interaction terms, the factor related to visual acuity improvement was IVR, with no interaction between laser and IVR. The reason for this may be that our study included cases with long-duration ME before intervention, as well as cases of severe, refractory, and recurrent ME, reflecting real-world clinical conditions.
There are some limitations to our study, including the retrospective nature of the study. At our hospital (Juntendo Tokyo Koto Geriatric Medical Center), our patients have an older demographic, and it is difficult to conduct prospective clinical trials due to cognitive decline and physical dysfunction.Another limitation is the use of clinical records, with a strong possibility of introducing bias due to deficits in documentation and history-taking as well as patient misclassification. Also,selection bias may have been introduced as patients and ophthalmologists were not blinded to the treatment assigned,with more patients with poor response to laser monotherapy being assigned to IVR+laser. In addition, various types of laser treatment were included, and it was sometimes difficult to determine from medical records whether a grid laser,non-perfusion area laser, or both, had been used. Although a randomized controlled trial would be ideal, the doubleblind method would require the use of ethically questionable methods such as sham vitreous injection or sham laser, and unlike traditional treatments does not respect the patient’s self-determination. Last, the sample size was small (n=73).This is because older patients are difficult to follow for more than 12mo, and this study does not include patients treated with IVB or IVA. Due to the small sample size, we had to reduce the number of confounding factors. Previous studies included ethnicity, high hematocrit[17-18], and the presence of hyperlipidemia, cardiovascular disease, apoplexy, renal disease, sleep apnea syndrome[19], and primary open-angle glaucoma[20]. Other studies showed that factors related to poor visual outcome were age, time to disease onset, visual acuity at initiation of treatment[5], and the presence of sub-foveal hemorrhage[9]. The small sample size in the present study was likely insufficient to test the effects of these all potential confounding factors.
In conclusion, IVR therapy significantly improved the visual acuity of patients with BRVO-associated ME, and no interaction was observed between laser and IVR therapies.However, in routine clinical situations, ME can recur after IVR therapy, and in some cases requires laser photocoagulation.In future studies, it would be worth identifying factors associated with lower doses and shorter duration of anti-VEGF administration, and even those associated with ultimate treatment cessation.
ACKNOWLEDGEMENTS
Conflicts of Interest:Umeya R, None; Ono K, None;Kasuga T, None.
 International Journal of Ophthalmology2021年10期
International Journal of Ophthalmology2021年10期
- International Journal of Ophthalmology的其它文章
- Exosomal miR-29b found in aqueous humour mediates calcium signaling in diabetic patients with cataract
- Intraluminal stenting versus external ligation of Ahmed glaucoma valve in prevention of postoperative hypotony
- Dexamethasone intravitreal implant (Ozurdex) in diabetic macular edema: real-world data versus clinical trials outcomes
- Comparative analysis of the clinical outcomes between wavefront-guided and conventional femtosecond LASlK in myopia and myopia astigmatism
- Reliability of the ocular trauma score for the predictability of traumatic and post-traumatic retinal detachment after open globe injury
- Vitreous function and intervention of it with vitrectomy and other modalities
