ADAM10基因多态性与阿尔茨海默病的相关性研究
司君增 王秀芹 杨艳红 邢晓玲 郑立峰 亓勤德 朱峻岭

[关键词] ADAM10;基因多态性;阿尔茨海默病;整合素
[中图分类号] R749.1 [文献标识码] A [文章编号] 1673-9701(2021)17-0001-03
Study on correlation between ADAM10 gene polymorphism and Alzheimer′s disease
SI Junzeng WANG Xiuqin YANG Yanhong XING Xiaoling ZHENG Lifeng QI Qinde ZHU Junling
Department of Neurology, Ji′nan City People′s Hospital, Ji′nan People′s Hospital Affiliated to Shandong First Medical University, Ji′nan 271199, China
[Abstract] Objective To explore the correlation between a disintegrin and metalloproteinase 10 gene (ADAM10) rs2305421 and rs653765 polymorphisms and the genetic susceptibility of northern Chinese Han population to Alzheimer′s disease (AD). Methods A total of 96 AD patients (the case group) and 102 healthy people (the control group) admitted to our hospital from January 2013 to May 2020 were matched in age and gender. The ADAM10 rs2305421 and rs653765 loci were genotyped by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The distribution of genotype frequency and allele frequency between the case group and the control group was compared by chi-square test. The intensity analysis of single gene nucleotide polymorphism and AD risk was expressed by odds ratio (OR) and 95% confidence interval (95%CI). Results The genotype of ADAM10 rs2305421 locus in the case group was [46(47.92)%], which was higher than that in the control group [(45 (44.12)%], with no significant difference(P>0.05). The allele frequency of ADAM10 rs2305421 in the case group was(62.50%), which was higher/lower than that in the control group(72.06%), with no significant difference(P>0.05). However, the frequency of AA genotype at ADAM10 rs653765 locus was significantly different from that of the control group(P=0.042), and the risk of AD was increased compared with GG genotype(OR=2.99, 95%CI: 1.04-8.59). In addition, the proportion of allele A in AD patients was significantly higher than that in the control group(OR=1.55, 95%CI: 1.01-2.36, P=0.043). Conclusion The polymorphism of rs653765 locus of ADAM10 gene may be related to AD in Han nationality in northern China, but it has nothing to do with rs2305421 locus.
[Key words] ADAM10; Gene polymorphism; Alzheimer′s disease; Integrin
阿爾茨海默病(Alzheimer′s disease,AD)是以进行性记忆力减退和认知功能障碍为主的神经退行性疾病,是痴呆最常见的类型,约占痴呆总数的60%~80%[1-2]。整合素和金属蛋白酶(A disintegrin and metalloproteinase with thrombospondin motifs,ADAMs)是一种结合细胞膜的糖蛋白,其参与细胞信号转导、细胞粘附、细胞外基质的降解。研究表明,ADAMs参与了细胞的增殖、分化及细胞外基质的重建、血管生成、细胞迁移,涉及炎症等疾病的病理过程[3]。ADAM10的表达可以通过启动子区域的多态性调节,这已被证实同AD、行为障碍和其他疾病相关[4]。因此,本研究分析两种单核苷酸多态性(Single nucleotide polymorphisms,SNPs)即rs2305421,rs653765同AD的发病敏感性之间的相关性预测,现报道如下。
1 资料与方法
1.1 一般资料
本研究采用病例对照设计。所有受试者均无血缘关系,本研究经济南市人民医院医学伦理委员会批准。按照国家伦理标准,对所有受试者进行样本采集,并签署知情同意书。阿尔茨海默病诊断参照NINCDS-ADRDA诊断标准[3],96例AD患者为2013年1月至2020年5月在济南市人民医院神经内科门诊及住院患者,包括男36例,女60例,年龄43~86岁,平均(66.48±3.11)岁。结合家族史及相应的临床检查,排除糖尿病、严重精神疾病、肿瘤等内科疾病。同时,102例对照组为门诊健康体检者,其中男35例,女67例,平均年龄(67.31±9.47)岁。
1.2 血液标本采集及基因组DNA提取
早晨空腹采集外周静脉血2 mL,收集后保存在EDTA抗凝的离心管。48 h内通过北京天根生化提供的DNA提取试剂盒提取基因组DNA,-80℃保存。
1.3 ADAM10基因多态性分型
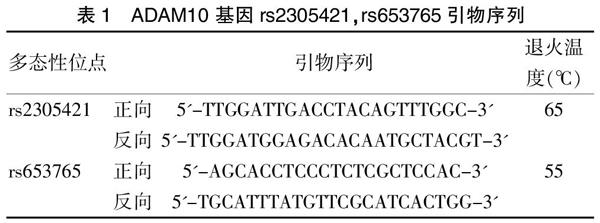
提取ADAM10基因序列,通过聚合酶链反应-限制性片段长度多态性(PCR-RFLP)进行基因多态性分型。PCR引物按照表1设计,在上海生工生物技术有限公司合成。选择20 μL PCR体系,含2 μL 10×缓冲溶液,0.5 μL dNTPs、1 μL DNA模板,各0.5 μL的正向和反向引物,2 μL氯化镁,0.5 μL Taq酶,13 μL ddH2O。PCR扩增条件如下:95℃,2 min的预变性;然后,在94℃,20 s变性、退火65℃ 40 s,72℃ 90 s延伸,共35个周期;最后,在72℃,2 min延伸。此后在20 μL酶反应体系进行,2 μL酶(rs2305421位点的EcoR I、rs653765位点的XhoI),10 μL PCR产物,2 μL 10×缓冲溶液,6 μL灭菌去离子水。该混合物被放在37.0℃水浴箱水浴一晚。10 μL酶切产物通过2%琼脂糖凝胶电泳进行分离。
1.4 统计学方法
用HWE程序检测Hardy-Weinberg平衡。采用SPSS 22.0统计学软件等位基因和基因型频率在患者和对照组中的分布差别采用χ2检验。等位基因和基因型与疾病的关联强度用比值比(Odds Ratio,OR)和95%可信区间(95% confidence intervals,95%CI)表示,P<0.05为差异有统计学意义。
2 结果
2.1 研究对象一般情况
本研究包含96例患者和102例健康对照者。AD患者和对照组的男、女比例分别为1∶1.67和1∶1.91,两组年龄、性别比较,差异无统计学意义(P>0.05),这表明选择的对象匹配。对rs2305421和rs653765基因型分布均符合Hardy-Weinberg平衡,可以进行候选基因的关联分析。
2.2 ADAM10基因rs2305421,rs653765位点的基因型和等位基因分布
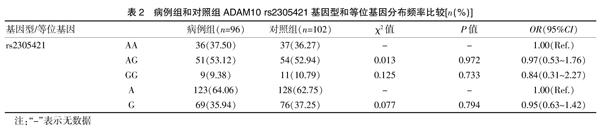
rs2305421位点AA、AG和GG基因型的频率分别为37.50%、53.12%、9.38%,而对照组分别为36.27%、52.94%、10.79%;等位基因A频率在病例组和对照组分别为64.06%、62.75%,等位基因G频率在病例组和对照组分别为35.94%、37.25%。基因型和等位基因频率在不同的患者和对照组之间比较,差异无统计学意义(P>0.05)。rs653765位点GG、GA、AA基因型頻率在病例组和对照组分别为38.54%、47.92%、13.54%和50.00%、44.12%,5.88%;G和A等位基因频率在两组分别为62.50%,37.50%,72.06%,27.94%;在rs653765位点AA基因型频率在两组之间差异显著,增加AD发展的风险(OR=2.99,95%CI=1.04~8.59,P=0.042)。此外,在病例组的A等位基因频率显著高于对照组(P=0.04),表明携带A等位基因的个体AD发病概率明显增加(OR=1.55,95%CI:1.01~2.36)。见表2~3。
3 讨论
阿尔茨海默病的特征性临床表现为进行性记忆力减退和认知功能障碍,并可伴有精神症状和行为异常,严重影响患者的日常生活和工作。目前,对于AD的致病机制有多种学说,其中Aβ的细胞毒性作用是AD发病的关键这个观点得到大家的广泛认同。如何使Aβ分泌减少、Aβ降解增加成为治疗AD的新靶点。淀粉样前体蛋白(APP)经过Aβ分泌酶和γ分泌酶的作用产生Aβ,而APP经过α分泌酶和γ分泌酶在Aβ内部被水解,从而阻止Aβ的生成,同时产生具有神经营养及神经保护作用的sAPPα。因此,增加α分泌酶的分泌是治疗AD更为有效的一条途径。Gibb等[5-7]研究发现ADAML10具有α分泌酶活性,在许多生理和病理过程中起着重要的作用。
研究表明,ADAM10涉及神经系统的发育过程,包括细胞增殖、迁移、分化、存活和轴突生长及髓鞘形成[8]。Kuhn等[9]发现敲除小鼠ADAM10基因,由于神经系统、体节和血管系统的发育缺陷,小鼠在胚胎期9.5 d死亡。有研究证明ADAM10是构成α-分泌酶的重要组成,其与神经细胞生理学相关。Postina等[10]研究表明,ADAM10转基因小鼠与AD小鼠杂交,发现杂交小鼠的淀粉样斑块明显减少,因α分泌酶到APP的可溶性片段的增多,学习记忆能力同时增加,这正和ADAM10的突变小鼠的条件相反。这些结果表明,ADAM10是通过功能性分泌抑制淀粉样斑块的形成,通过修改应用程序提高学习记忆能力。
ADAM10基因多态性已经在许多神经系统疾病中得到研究[11]。rs653765和rs514049多态性被认为能调节AD患者淀粉样前体蛋白(APP)的表达。基姆等检测ADAM10已经发现九个SNPs,发现rs2305421与AD的发病风险相关。此外,阿尔茨海默病的基因库中的AD家庭样本的检测仍然得到相同的观点[12]。然而,对白种人研究ADAM10 27个SNP对AD的影响,仍然找不到AD的生物标志物[13]。这些研究结果的不一致性,进一步表明ADAM10基因多态性的异质性。本研究探讨中国北方AD汉族人群的ADAM10基因多态性,在rs2305421位点,基因型和等位基因频率差异在AD和对照组比较,差异无统计学意义。而在rs653765位点,对照组AA基因型频率明显低于AD患者,在病例组的A等位基因频率显著高于对照组,表明携带A等位基因的个体AD发病概率明显增加。
综上所述,本研究支持ADAM10基因多态性与中国北方汉族阿尔茨海默病人群之间存在相关性。掌握这些研究成果将有助于深入了解ADAM10基因的功能,筛选高危人群,并在基因水平上为疾病发生提供一定的理论依据,从而达到早期诊断、及时治疗的目的。但本实验样本较少,因此结论有一定局限性。未来需要更大样本量的研究对研究结果进行验证,以确定ADAM10基因变异在阿尔茨海默病发病中的作用。
[参考文献]
[1] Khalsa DS.Stress,meditation,and Alzheimer′s disease prevention:Where the evidence stands[J].J Alzheimers Dis,2015,48(3):1-12.
[2] Deng JF,Deng YY,Li W,et al.Association between angiotensin-converting enzyme gene polymorphism and Alzheimer′s disease[J]. Nan Fang Yi Ke Da Xue Xue Bao,2015,35(1):1325-1330.
[3] Bekris LM,Lutz F,Li G,et al.ADAM10 expression and promoter haplotype in Alzheimer′s disease[J]. Neurobiol Aging,2012,33(12):2229 e2221-2229 e2229.
[4] Jian XQ,Wang KS,Wu TJ,et al.Association of ADAM10 and CAMK2A polymorphisms with conduct disorder:Evidence from family-based studies[J]. J Abnorm Child Psychol,2011,39(2):773-782.
[5] Gibb DR,El Shikh M,Kang DJ,et al.ADAM10 is essential for Notch2-dependent marginal zone B cell development and CD23 cleavage in vivo[J].J Exp Med,2010,207(1):623-635.
[6] Peng M,Guo S,Yin N,et al. Ectodomain shedding of Fcalpha receptor is mediated by ADAM10 and ADAM17[J].Immunology,2010,130(23):83-91.
[7] Nagara Y,Hagiyama M,Hatano N,et al.Tumor suppressor cell adhesion molecule 1 (CADM1) is cleaved by a disintegrin and metalloprotease 10 (ADAM10) and subsequently cleaved by gamma-secretase complex[J]. Biochem Biophys Res Commun,2012,417(23):462-467.
[8] Yang P,Baker KA,Hagg T.The ADAMs family:Coordinators of nervous system development,plasticity and repair[J].Prog Neurobiol,2006,79(11):73-94.
[9] Kuhn PH,Wang H,Dislich B,et al.ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons[J]. EMBO J,2010,29(22):3020-3032.
[10] Postina R,Schroeder A,Dewachter I,et al.A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model[J].J Clin Invest,2004,113(12):1456-1464.
[11] Song JH,Yu JT,Liu M,et al.Genetic association between ADAM10 gene polymorphism and Alzheimer′s disease in a Northern Han Chinese population[J]. Brain Res,2011, 1421(34):78-81.
[12] Bertram L,Hiltunen M,Parkinson M,et al.Family-based association between Alzheimer′s disease and variants in UBQLN1[J]. N Engl J Med,2005,352(25):884-894.
[13] Laws SM,Eckart K,Friedrich P,et al.Lack of evidence to support the association of polymorphisms within the alpha- and beta-secretase genes (ADAM10/BACE1) with Alzheimer′s disease[J]. Neurobiol Aging,2011,32(11):541-543.
[14] 陈煜森,许志恩,赵斌,等.ApoE基因多态性与阿尔茨海默病的相关性研究[J].神经疾病与精神卫生,2006,6(2):89-91.
[15] 骆小梅.新疆维、汉两民族阿尔茨海默病ApoE基因多态性研究[D].乌鲁木齐:新疆醫科大学,2008.
(收稿日期:2020-12-08)

