Bicarbonate use and carbon dioxide concentrating mechanisms in photosynthetic organisms
Yanyou Wu
Abstract Photosynthesis is crucial to the reduction of carbon dioxide in the atmosphere.The key enzyme of photosynthesis,Ribulose-1,5-bisphosphate carboxylase/oxygenase(Rubisco),has two mutably competing substrates,CO2 and O2.It has features of carboxylase and oxygenase.Rubisco performs the function of carboxylase to reduce inorganic carbon to form organic substances,which precondition is that more carbon dioxide accumulates around it.Carbon dioxide concentrating mechanisms(CCMs)are vital to cope with the limit of carbon dioxide.Various bicarbonate use pathway has a differential contribution to inorganic carbon assimilation.Bicarbonate transport,extracellular bicarbonate dehydration,or H+-ATPase-driven bicarbonate uptake,which induced CCMs,can support a considerable share of photosynthesis in photosynthetic organisms.However,CCMs in thylakoid membranes may be the most important.The CCMs occurred in the plasma membrane were secondary,evolutionary,and inducible,while CCMs coupled with photosynthetic oxygen evolution in thylakoid membranes,were primitive,major,and indispensable.A hypothetical schematic model of CCMs occurred in the plasma membrane and thylakoid membranes being proposed.
Keywords Bicarbonate photolysis·Inorganic carbon utilization·Plasmamembrane·Photosynthesis·Thylakoid membranes
1 Forms of inorganic carbon and photosynthetic availability in water medium
In a natural water medium,there are four forms,such as CO2,HCO3-,H2CO3,and CO32-.The constituent of dissolved inorganic carbon is dependent on the pH and temperature in the water environment.At pH 6.0,the concentration of dissolved carbon dioxide,bicarbonate,and carbonate were 1.2,1.0,and 0 mM,respectively.The dissolved inorganic carbon in the water medium at pH 7.0 is constituted by 0.21 mM of dissolved carbon dioxide,and 1.9 mM of bicarbonate(Inverset al.2001).Today’soceans contain less than 0.02 mM of dissolved carbon dioxide,approximately 1.9 mM of bicarbonate,and 0.17 mM of carbonateat pH approximately 8.07 and 15°C(Roledaand Hurd 2012;Zweng et al.2018).
Carbon dioxide and bicarbonate can be utilized by photosynthetic cells,and the other two forms of inorganic carbon in water medium can only be transformed into CO2or HCO3-,which can be utilized by plants.Carbon dioxide,as an electrically neutral,linear nonpolar molecule,diffuse freely into the bilayer lipid membrane of cellsto be used by photosynthetic cellsfor photosynthesis,but 10,000 times slower in water compared to air.However,bicarbonate(HCO3-)is charged not permeable to lipid bilayers.Bicarbonate enters the cell through the process of direct or indirect transport,and then can be used by photosynthetic cells.Passive CO2uptake relative to bicarbonate pathways could provide energy savings as the process of bicarbonate entering the cell is energetically costly(Burnell et al.2014).Although bicarbonatepathwayshad low aff inity and high cost(Hussner et al.2016),plants still assimilate inorganic carbon through this pathway under the limited of carbon dioxide.
2 Critical importance of carbon dioxide concentrating mechanisms(CCMs)in aquatic photosynthetic plants
The aquatic ecosystem contributes to approximately 50%of the net primary productivity on Earth,which is the largest ecosystem.Some submerged aquatic plants,such as macroalgae,microalgae,and cyanobacteria,which live in water environment,usebicarbonateasan alternativesource of dissolved inorganic carbon for photosynthesis due to the limiting of carbon dioxide with the low diffusivity in water(Maberly 1990;Axelsson et al.1999;Larsson and Axelsson 1999;Huertaset al.2000;Gao and Zou 2001;Spalding 2008;Ferna´ndez et al.2014).When the terrestrial plants encounter such as drought and salt adversity,the stomata are closed,and CO2is diff icult to enter the photosynthetic cells.At this time,the plants use root-derived bicarbonate to avoid thedamageof photosynthetic organscaused by the uncoupling between electron transport and photophosphorylation.
The key enzyme for inorganic carbon reduction in photosynthetic organisms is Rubisco(ribulose-1,5-bisphosphate carboxylase/oxygenase),which has two features,one iscarboxylase,the other isoxygenase.The enzyme has two mutably competing substrates,CO2and O2.Plants reduce inorganic carbon to form organic substances,as Rubisco performs the function of carboxylase.When Rubisco carries out the function of oxygenase,plants perform photorespiration to oxidize organic carbon into CO2under illumination.Therefore,CCMs are essential for photosynthetic inorganic carbon assimilation.In addition,C4-plants and CAM-plants can concentrate carbon dioxide to reduce photorespiration by using phosphoenolpyruvate carboxylase(PEPC)incorporating bicarbonate.
3 Diversified bicarbonate use pathways in photosynthetic organisms
Many species of submerged aquatic photosynthetic plants,macroalgae,and microalgae utilize both CO2and bicarbonateassubstratesfor photosynthetic carbon assimilation.The proportion of CO2and bicarbonate use are different among species under different habitats.Meanwhile,the mode of inorganic carbon utilization is species-specif ic(Invers et al.2001;Campbell and Fourqurean 2013).The diversified photosynthetic carbon assimilation is the response to the diversity of the water environment.
3.1 Bicarbonate transport,extracellular bicarbonate dehydration,and H+-ATPasedriven bicarbonate uptake
The giant kelp,Macrocystis pyrifera,can acquire and utilize bicarbonate to support an average of 40%of photosynthesis,dissolved CO2,and an unknown proportion of active bicarbonate uptake to support the remaining 60%of photosynthesis.The proportion is affected by irradiance and f low velocity in Monterey Bay(Drobnitch et al.2016).However,another experiment demonstrated thatMacrocystis pyriferaacquires and utilize bicarbonate to support up to 99%of itsphotosynthesisvia anion exchange protein and external bicarbonate dehydration mediated by the surface-bound enzyme carbonic anhydrase(CA).The direct bicarbonate uptake via an anion exchange protein is the main mechanism of bicarbonate use,not be affected by CO2concentrations and pH in the water environment(Ferna´ndez et al.2014).
In contrast toMacrocystis pyrifera,the mode of bicarbonate utilization in many species is dependent on pH in the water environment.The bicarbonate dehydration catalyzed by CA isthe main mechanism of bicarbonateuse ofUlva lactuca,and be affected by the concentrations of inorganic carbon,external O2and pH(Axelsson et al.1999).Enteromorpha intestinalisuse bicarbonate as the mechanism of both bicarbonate dehydration via surfacebound CA and bicarbonate transport.Bicarbonate transport rather than extracellular bicarbonate dehydration is dominated mechanism at high pH values(9.5).It is important to note that the CA inhibitor,acetazolamide,increased net photosynthetic O2evolution ofEnteromorpha intestinalisat pH 8.4 and 9.5 in rockpool water(Larsson et al.1997).
The terrestrial cyanobacterium,Nostoc f lagelliforme,usually f ix most of theinorganic carbon in theform of CO2,it uses bicarbonate as the external inorganic carbon source mainly via direct bicarbonate uptake associated with Na+/HCO3-symport rather than Cl-/HCO3-exchange system when submerged.The inorganic carbon utilization as bicarbonate dehydration via external CA had little contribution to photosynthetic oxygen evolution when the pH of the medium was alkaline(Gao and Zou 2001).In contrast toNostoc f lagelliforme,Hizikia fusiformeuses bicarbonate as the external inorganic carbon source mainly via the external CA-mediated bicarbonate dehydration,do not operate active bicarbonate uptake via anion exchange at alkaline pH(Zou et al.2003).
Except for the mechanisms of bicarbonate transport and extracellular bicarbonate dehydration,the third mechanism,H+-ATPase-driven bicarbonate uptake has been identified in a number of species(Kaplan et al.1982;Thielmann et al.1990;Snoeijs et al.2002;Choo et al.2002;Zou and Gao 2010).LaminariadigitataandLaminaria saccharinafrom the Swedish west coast possess all of the three mechanisms,bicarbonate dehydration by external CA,direct bicarbonate transport via anion exchange,and the active uptake of carbon dependent on P-type H+-ATPase activity.The external CA-mediated bicarbonate dehydration rather than direct bicarbonate transport via anion exchange is dominated mechanism above pH 9.5(Klenell et al.2004).
Endarachne binghamiaeof natural seawater use bicarbonate two pathways of bicarbonate dehydration and P-type H+-ATPase-driven bicarbonate uptake,and external CA-mediated bicarbonate dehydration mechanism was much more important than P-type H+-ATPase-driven bicarbonate uptake during the period of photosynthesis(Zou and Gao 2010).Similarly,inCoccotylus truncatus,external CA as well as P-type H+-ATPase(proton pump),are involved in inorganic carbon uptake(Snoeijs et al.2002).
Two tropical seagrassspecies,Cymodocea serrulataandHalodule uninervis,had different bicarbonate use modes in response to the same environment.InCymodocea serrulate,bicarbonate uptake via H+-HCO3-co-transport is dominant mechanism,and that via the external CA-mediated bicarbonate dehydration hasan important contribution to photosynthesis under the light irradiance of 40μmol m-2s-1or 600μmol m-2s-1.InHalodule uninervis,bicarbonate uptake via external CA-mediated bicarbonate dehydration is dominant mechanism,and that via H+-HCO3-co-transport has less contribution to photosynthesis under the light irradiance of 40μmol m-2s-1.However,external CA-mediated bicarbonate dehydration does not affect photosynthesis,and bicarbonate uptake via H+-HCO3-co-transport has a great contribution to photosynthesisunder thelight irradianceof 600μmol m-2s-1(Ow et al.2016).
Bicarbonate uptake via transporters,which is found in many photosynthetic organisms,is another direct bicarbonate use mode.However,no bicarbonate transporter has so far been characterized in higher terrestrial plants.Itsrole will not be discussed here,relevant information can be obtained from the references(Spalding 2008;Wang and Spalding 2014;Yamano et al.2015;Zoccola et al.2015;Uehara et al.2016;Charlotte et al.2018).
3.2 Plasticity in the mode of inorganic carbon use
Many aquatic plants have been shown to express plasticity in the mode of inorganic carbon use(Jones 2005;Holland et al.2012;Hussner et al.2016).Elodea nuttalliif lexibly switch between CO2and bicarbonate use.When plants weregrown under CO2-limiting conditionsthat favored the switch from CO2to bicarbonate use.Although reduced CO2availability brought out an increasein bicarbonateuse,no differences were found in the growth of the plants(Jones 2005).However,when plants were grown under supersaturated CO2conditions that favored the switch from bicarbonate to CO2use.A species of Potamogetonaceae(Stuckenia pectinata)and Ceratophyllaceae(Ceratophyllum demersum),six species of Hydrocharitaceae(Elodea canadensis,Elodea nuttallii,Egeria densa,Egeria najas,Hydrilla verticillata,andVallisneria spiralis),and two species of Haloragaceae(Myriophyllum heterophyllum,
Myriophyllum spicatum)are all able to use bicarbonate,acclimated to air saturated and supersaturated CO2concentrations.The photosynthetic aff inity for bicarbonatewas lower in species acclimated to supersaturated CO2rather than air saturated.Net photosynthesis was more stimulated by supersaturated CO2concentrations due to their depressed bicarbonate use capacity(Hussner et al.2016).
Cylindrospermopsis raciborskiiswitch from CO2and bicarbonate use when it has grown under high light.Photosynthetic quantum eff iciency did not vary signif icantly with light intensity,pH,bicarbonate concentration,or CO2concentration.The growth rate in 6 mM bicarbonate was twice what it was in 0.6 mM bicarbonate,which indicated high concentration of bicarbonate is another swift from CO2and bicarbonate use at low light irradiance(20μmol m-2s-1).This effect was much less than the effect of thelight treatment.That isto say,light isthemain switch,bicarbonate is a secondary switch from CO2and bicarbonate use(Holland et al.2012).
4 Differential contribution of various bicarbonate use pathways
Diversified modesof bicarbonateusearedetermined by the stoichiometric relationships among protons,electrons,and inorganic carbon.Proton and bicarbonate are thesubstrates of photosynthetic oxygen evolution(Wu 2021).Therefore,direct bicarbonate uptake including via anion exchanger and transporters,bicarbonate dehydration via external CA and P-type H+-ATPase,which inf luenced theconcentration of proton and bicarbonate,all affect the assimilation of photosynthetic oxygen evolution and inorganic carbon assimilation.The above-mentioned bicarbonate uses pathways determined only by applying specif ic inhibitors are qualitative,and it is diff icult to quantify the contribution of each bicarbonate use pathway.Using bidirectional isotope labeling tracer technology combined with applying specif ic inhibitors,the contribution of different bicarbonate use pathways can be quantified(Wu et al.2015).
4.1 Contribution of CCMs induced by bicarbonate dehydration
Many of the above work about the contribution of inorganic carbon use via bicarbonate dehydration are considered to be specif ic external CA based on the application of water-soluble CA inhibitor,acetazolamide.Although acetazolamide is water-soluble,there is no evidence that it cannot enter the chloroplast.In fact,the extrinsic CA of photosystem IIis also affected by acetazolamide due to CA in chloroplast.The response of acetazolamide increasing net photosynthetic O2evolution inEnteromorpha intestinalisissimilar to that in theextrinsic CA of photosystem II(Larsson et al.1997;Ignatova et al.2006;Shitov et al.2009,2011).Meanwhile,inhibiting one of bicarbonate use pathways would affect others.Therefore,quantifying the contribution of the bicarbonate use pathway only via applying specif ic inhibitorsisinaccurate and unreasonable.
Bidirectional isotope labeling tracer technology combined with applying specif ic inhibitors was used to study that the dose-effect of bicarbonate and CA inf luencing inorganic carbon use(Wu et al.2015).Table 1 shows the dose-effect of the inorganic carbon use via bicarbonate dehydration catalyzed by CA.Whether in the present or absence of acetazolamide,the growth,and the proportion of bicarbonateadded ascarbon sourcesto thewholecarbon sources,as well as bicarbonate utilization pathway to the whole carbon utilization pathway inChlamydomonas reinhardtiiandChlorella pyrenoidosa,was affected by the bicarbonate added.
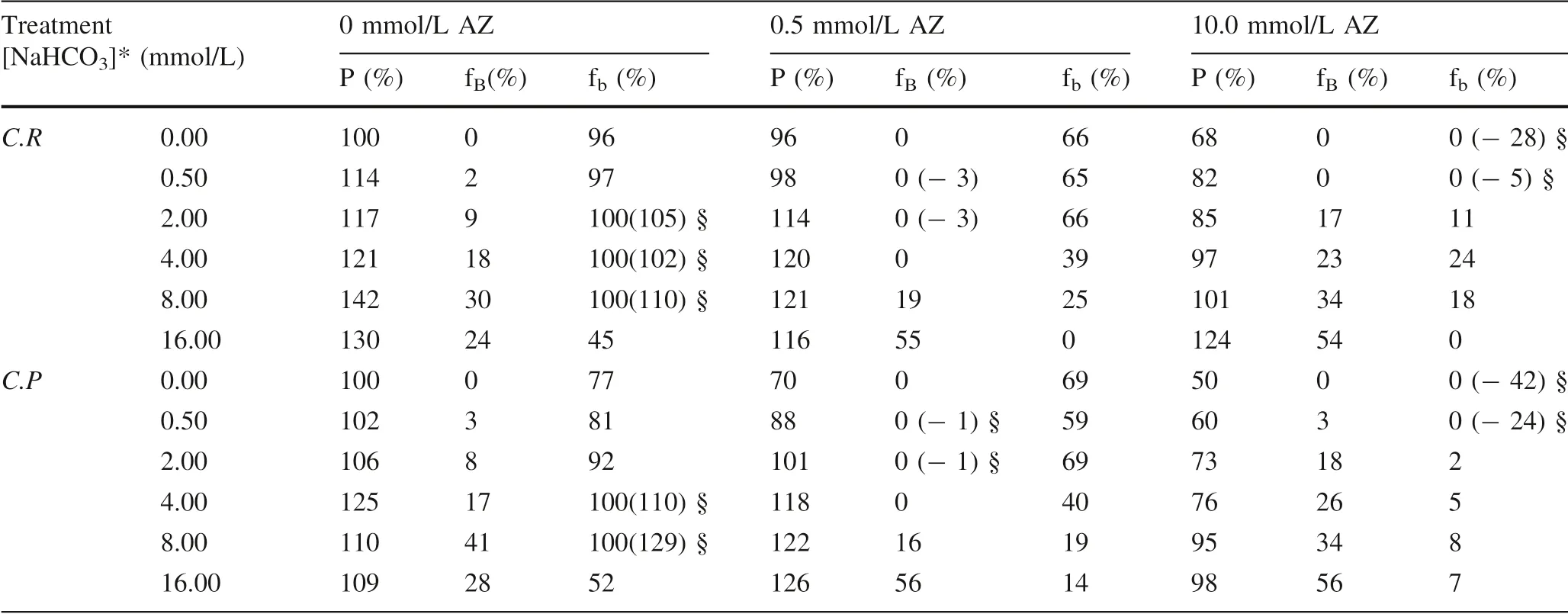
Table 1 Algal growth and variation in the carbon utilization pathway and carbon sources in C.reinhardtii and C.pyrenoidosa treated with different concentrations of bicarbonate added and acetaolamide(AZ)(Wu et al.2015)
Bicarbonate increases the growth ofC.reinhardtiiandC.pyrenoidosa.C.reinhardtiireached the maximum growth in the absence of AZ when 8.0 mmol/L sodium bicarbonate was added,andC.pyrenoidosareached the maximum growth when 4.0 mmol/L sodium bicarbonate was added without AZ.It may be explained that the high concentration of sodium bicarbonate inhibited the extracellular CA,and f inally restricted the growth of microalgae(Moroney and Tolbert 1985;Wu et al.2015).Algal biomass increases with the increase of the concentration of bicarbonateadded to the culture medium in thepresence of 10 mmol/L AZ.However,algal growth obviously decreased with the increasing concentrations of AZ added exceptC.pyrenoidosacultured in high concentrations of bicarbonate(8,and 16 mmol/L).When the same concentration of NaHCO3was added,the growth of the microalgae was severely restricted by AZ.A low concentration of AZ(0.5 mmol/L)can promote the growth ofC.pyrenoidosaunder the high concentration of sodium bicarbonate added(8.0-16.0 mmol/L),which issimilar to thef inding inEnteromorpha intestinalis(Larsson et al.1997).It shows that the high concentration of sodium bicarbonate can promote the growth ofC.pyrenoidosamore than inhibit under low concentration of AZ.
Bicarbonate increases the proportion of bicarbonate added ascarbon sourcesby microalgae.BothC.reinhardtiiandC.pyrenoidosautilize the maximum proportion of bicarbonate added in the absent of AZ when 8.0 mmol/L sodium bicarbonate was added.The proportion of bicarbonate added as carbon sources used by microalgae increases with the increase of the concentration of bicarbonate added to the culture medium in the presence of AZ.However,the proportion of bicarbonate added as carbon sources used by microalgae is the independence of AZ.
AZ signif icantly inhibits the proportion of bicarbonate use via bicarbonate dehydration catalyzed by CA.The proportion of bicarbonate use pathway decreased with increasing amounts of AZ added.When the same concentration of NaHCO3was added,the proportion of bicarbonate use pathway in the microalgae was severely decreased by AZ.
An interesting phenomenon is that increasing the concentration of bicarbonate can compensate for the CA’s function.The growth ofC.reinhardtiicultured in the medium adding 16 mmol/L sodium bicarbonate in the presence of 10 mmol/L AZ,or adding 4 mmol/L sodium bicarbonate in the presence of 0.5 mmol/L AZ is close to that in the medium adding 4 mmol/L sodium bicarbonate in the absent AZ.Similarly,the growth ofC.pyrenoidosacultured in the medium adding 16 mmol/L sodium bicarbonate in thepresence of 0.5 mmol/L AZ iscloseto that in the medium adding 4 mmol/L sodium bicarbonate in the absent AZ.It deduced that direct bicarbonate uptake increases under the high concentration of bicarbonate,and the importance of carbon dioxide concentrating mechanisms(CCMs)induced by CA in microalgae(See review by Wang et al.2011)should be turned down a little.
4.2 Contribution of CCMs via anion exchange
An anion channel-specif ic inhibitor, 4,4-diisothiocyanatostilbene-2,2-disulfonate(DIDS),which can inhibit the process of anion entering cells(Cabantchik and Greger 1992;Huertas et al.2000;Young et al.2001),was applied to study the dose-effect of direct bicarbonate use via anion exchange by adding different concentration of DIDSto the culture medium(pH 8.2).Table 2 showsthe dose-effect of direct bicarbonate use via anion exchange.
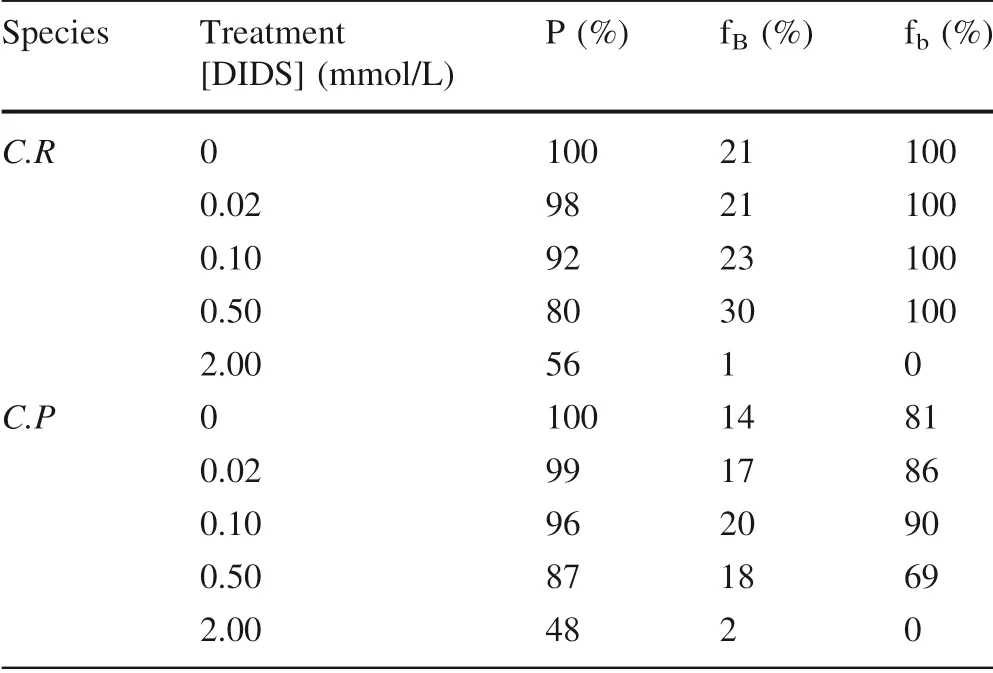
Table 2 Algal growth and variation in the carbon utilization pathway and carbon sources in C.reinhardtii and C.pyrenoidosa treated with different concentrations of DIDSand 2 mmol/L bicarbonate added

Table 3 Algal growth and variation in the carbon utilization pathway and carbon sources in C.reinhardtii and C.pyrenoidosa treated with different concentrations of AZ and DIDS
Thegrowth ofC.reinhardtiiandC.pyrenoidosaslightly decrease under a low concentration of DIDS(lower than 0.5 mmol/L),and a signif icant decrease under a high concentration of DIDS(2 mmol/L).DIDS slightly affects the proportion of bicarbonate added as carbon sources by the two microalgal species under low concentration of DIDS(lower than 0.5 mmol/L),and tremendousrestrict the use of bicarbonate added to the culture medium under high concentration of DIDS(2 mmol/L).DIDS does not inf luence the proportion of bicarbonate utilization pathway to the whole carbon utilization pathway of microalgae under low concentration of DIDS(lower than 0.5 mmol/L),and bicarbonate use completely inhibits under high concentration of DIDS(2 mmol/L).It is suggested that microalgal bicarbonate use via anion exchange can be fully inhibited under high concentrations of DIDS,and f inally microalgae can only use carbon dioxide from the atmosphere.Even if there is no bicarbonate use inCreinhardtiiandC pyrenoidosa,the two species of microalgal biomass reach 1.8 times,or 2.1 times after 4 days culture than before the culture,respectively(Wu et al.2015).Similarly,the important of carbon dioxide concentrating mechanisms(CCMs)via anion exchange(See review by Wang et al.2011)should be also turned down a little.
4.3 Jointly contribution of CCMs via bicarbonate dehydration and anion exchange
AZ and DIDS have an effect on the superimposed inhibition of microalgal growth,bicarbonate utilization pathway,and carbon sources(See Table 3).The microalgal growth signif icantly changed under the interaction of AZ and DIDS.Asa whole,the growth rate ofC.reinhardtiiandC.pyrenoidosadecreased with the increase of the concentration of AZ or DIDS.Especially,the microalgal growth was signif icantly reduced under the combined action of 1.0 mmol/L of AZ and 0.5 mmol/L of DIDS.
WhetherC.reinhardtiiorC.pyrenoidosa,the proportion of bicarbonate added as carbon sources to the whole carbon sources used by microalgae varies with the concentration of AZ and DIDS.Specif ically,DIDS increased theproportion of bicarbonate added ascarbon sourcesused by both microalgae species.
The microalgae utilize inorganic carbon mainly via the bicarbonateusepathway,97%in C.reinhardtiiand 77%inC.pyrenoidosawithout AZ and DIDSadded to the culture medium.AZ and DIDS signif icantly decreased the bicarbonate use pathway in the two microalgal species.Especially,the microalgal bicarbonate use pathway was signif icantly reduced under the combined action of 1.0 mmol/L of AZ and 0.5 mmol/L of DIDS.
Thesensitivity of microalgal bicarbonateusepathway to specif ic inhibitors is different.Specif ically,C.reinhardtiiutilizing the inorganic carbon pathway is more sensitive to AZ thanC.pyrenoidosa,which resulted from the great activity of extracellular CA inC.reinhardtii.Oppositely,C.pyrenoidosautilizing the inorganic carbon pathway is moresensitive to DIDSthanC.reinhardtii,which ref lected a more active anion exchange inC.pyrenoidosa.
4.4 Capital contribution of CCMs in thylakoid membranes
It is worth noting that little proportion of the bicarbonate utilization pathway to thewholecarbon utilization pathway does not necessarily bring the small proportion of bicarbonate added as carbon sources to the whole carbon sources used by microalgae.The microalgal growth reached more than 70%that of control when the bicarbonate utilization pathway was inhibited by 90%.It demonstrated that CCMs in the plasma membrane are not the most important.
CCMs in thylakoid membranes may be the most important.So far,it hasnot been found thebicarbonate can transport across the thylakoid membranes into the thylakoid lumen(Wang et al.2011).However,bicarbonate photolysis in thylakoid membranes provides protons,electrons,and carbon dioxide,which would concentrate and elevate the CO2concentration at the site of ribulose-1,5-bisphosphate carboxylase/oxygenase(Rubisco)(Wu 2021).Therefore,even if there is no direct bicarbonate use dependent on CCMs in plasma membrane,the carbon dioxide from the atmosphere continuously enters the cell membrane due to great concentration difference,which resulted from the indirect removal of inorganic carbon within the plasma membrane via bicarbonate photolysis in thylakoid.This situation is similar to that the vast majority of carbon sources used by microalgae are carbon dioxide from the atmosphere in the presence of CA.
5 Conclusions
It can be concluded that two sites would function CCMs,one at the plasma membrane(Su¨ltemeyer 1998;Wang et al.2011),the other at the thylakoid membranes.CCMs occurred in thylakoid membranes were primitive,major,and indispensable,coupled with photosynthetic oxygen evolution.CCMs in the plasma membrane were secondary,evolutionary,and inducible.As illustrated in Fig.1,two putative carbon dioxide concentrating processes take place in the plasma membrane and chloroplast.
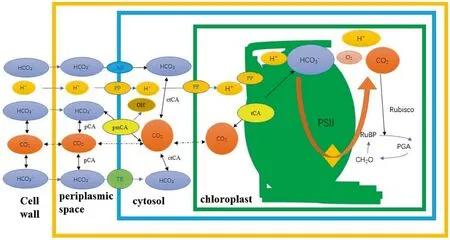
Fig.1 Hypothetical schematic model of carbon dioxide concentrating mechanism occurred in the plasma membrane and thylakoid membranes.PS II,Photosystem II pCA,Periplasmic CA,pmCA,Cytoplasmic membrane CA,ctCA,Cytosolic CA,tCA,Thylakoid CA(the extrinsic CA of photosystem II?),AE,Anion exchanger,PP P-type H+-ATPase,TR,transporter(Yamano et al.2015;Ueharaet al.2016);Rubisco,ribulose-1,5-bisphosphate carboxylase/oxygenase,RuBP,ribulose-1,5-bisphosphate,CH2O,Carbohydrate,PGA,3-phosphoglycerate
AcknowledgementsThe author thanks the foundations of the National Natural Science Foundation of China[No.U1612441-2],the National Key Research and Development Program of China[2016YFC0502602],and Support Plan Projects of Science and Technology Department of Guizhou Province[No.(2021)YB453].
Declarations
Conf lict of interestThe author declares that they have no conf lict of interest.
- Acta Geochimica的其它文章
- Correction to:Variations of methane stable isotopic values from an Alpine peatland on the eastern Qinghai-Tibetan Plateau
- The possible source of uranium mineralization in felsic volcanic rocks,Eastern Desert,Egypt of the Arabian-Nubian Shield:Constraints from whole-rock geochemistry and spectrometric prospection
- Dissolved organic carbon concentration and its seasonal variation in the Huguangyan Maar Lake of Southern China
- Geochemistry and provenance of the lower-middle pliocene cheleken formation,Iran
- Geochemistry,geochronology,and zircon Hf isotopic compositions of felsite porphyry in Xiangshan uranium oref ield and its geological implication
- Geochemistry of platinum-group elements in the podiform chromitites and associated peridotites of the Nain ophiolites,Central Iran:Implications for geotectonic setting

