Coronavirus and cardiovascular manifestations- getting to the heart of the matter
Monika Bhandari,Akshyaya Pradhan,Pravesh Vishwakarma,Rishi Sethi
Monika Bhandari,Akshyaya Pradhan,Pravesh Vishwakarma,Rishi Sethi,Department of Cardiology,King George's Medical University,Lucknow 226003,Uttar Pradesh,India
Abstract Coronavirus disease has unarguably been the largest pandemic of recent times.Over 150 million cases have occurred worldwide,and more than 3 million have succumbed to the disease.Cardiac manifestations can have varied presentations from an asymptomatic troponin rise to fulminant myocarditis.The pathogenesis of myocardial damage could be direct or indirect,including inflammation,coronary spasm,plaque rupture,and cytokine storm.Thromboembolism is also an important feature of cardiovascular affliction with both arterial and venous systems being affected.Hence,anticoagulation has also been a matter of debate.Fulminant myocarditis is the most severe form and can lead to circulatory shock with a high mortality.Management of cardiac patients with coronavirus disease 2019 (COVID-19) infection is not considerably different from non-COVID-19 cardiovascular disease,but interaction between cardiovascular drugs and anti-COVID-19 therapy requires careful attention.More recently,vaccines have emerged as a ray of hope for the disease.But simultaneously,there have been reports of thromboembolism following vaccination.In this review,we discuss the various aspects of coronavirus disease affecting of heart and its management.
Key Words:Myocarditis;Cytokine storm;Angiotensin-converting enzymes-2;Acute coronary syndrome;Hypercoagulable state;Vaccine
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).The disease has evolved into a global pandemic and has affected more than 150 million people worldwide.Unfortunately,more than 3.5 million people have succumbed to the disease till now[1].
Infection with the SARS-CoV-2 virus mainly causes fever (77%-98% of cases),fatigue (52%-75%),and cough (62%-81%).It primarily affects the respiratory system,but its effects on the cardiovascular (CV) system have also been noted.While those with pre-existing CV disease are at an increased risk of mortality,the disease also contributes to CV complications.These may be acute coronary syndrome (ACS),including myocardial infarction (MI),arrhythmias,myocarditis,acute heart failure(HF),cardiogenic shock,and even death[2-4].
CARDIAC INVOLVEMENT IN COVID-19
Cardiac injury is described by the presence of raised cardiac troponins (cTn) in COVID-19.Cardiac biomarkers are elevated in 7%-28% of COVID-19 patients.It can be either due to MI or due to myocardial injury from myocarditis and hemodynamic shock[5-8].
According to the American College of Cardiology (ACC),patients with cTn elevation (myocardial injury) can be classified as (1) Chronic myocardial injury;(2)Acute nonischemic myocardial injury;or (3) Acute myocardial infarction (MI)[9].
An association between ACS is already described for influenza virus and other respiratory viruses.Thus,it is of extreme importance to understand the pathophysiological mechanism of ACS in COVID-19[10,11].
Many patients with COVID-19 present with symptoms mimicking ACS.According to the study of Huanget al[4],prevalence of myocardial injury in SARS-CoV-2 is 12%.In another study,cardiac injury is observed in 7.2% overall and 22.2% of intensive care unit (ICU) patients infected with the SARS-COV-2 virus[2].
From a retrospective analysis of 191 COVID-19 pneumonia patients by Zhouet al[12],it can inferred that those who develop acute myocardial injury are more likely to die (odd ratio of 21.4,P< 0.0001).Thus,myocardial injury is an independent predictor of mortality.
MECHANISM OF CARDIOVASCULAR INVOLVEMENT IN COVID-19
Several mechanisms are postulated to cause CV events in SARS-COV-2 infection(Figure 1).These include a pro-inflammatory state and cytokine storm,which leads to plaque instability in patients with pre-existing CAD.In addition to this,COVID-19 infection induces a prothrombotic state as evidenced by raised D-dimer levels and hypoxemia-related myocardial ischemia due to respiratory failure.All these mechanisms cause myocardial ischemia even in patients without pre-existing CV disease[13].

Figure 1 Various mechanisms of myocardial injury associated with coronavirus disease 2019 disease.
Pro-inflammatory state
In a recently published case of series of COVID-19 patients,Vargaet al[14]demonstrated evidence of direct viral infection of the endothelial cells.This leads to endothelitis along with micro- and macro-thrombosis in both the arterial and venous circulation.This in addition to the hypercoagulability predisposes to ACS[15].
Cytokine storm
An excessive immune response to SARS-COV 2 virus has been demonstrated in certain patients,which leads to a cytokine storm[4].In a study of 53 patients with clinically moderate to severe COVID-19 disease,14 types of cytokines were elevated.Of them,interferon gamma-induced protein 10 (IP-10),monocyte chemotactic protein 3 (MCP-3),and interleukin-1 receptor antagonist (IL-1ra),are independently associated with hypoxaemia,disease progression,and death[16].Cytokine production damages the healthy cells initially in the lungs.Subsequently,it involves other organs,such as the kidney,heart blood vessels,and brain.Due to this hypercytokinemia,there is an increase in hypercoagulability contributing to a 31% increase in incidence of thrombosis.This eventually manifests as ischemic stroke,deep vein thrombosis,acute pulmonary embolism,MI,and systemic arterial embolism[14].
Angiotensin-converting enzymes
The pathophysiology of COVID-19 infection involves binding of the SARS-COV2 virus to the host ACE-2 receptor,which mediates virus entry into the cell.Angiotensinconverting enzymes (ACE)-2 receptors are present in the epithelial cells of the lungs,kidneys,heart,intestines,and blood vessels.ACE is also an important component in the pathophysiology of CAD[17,18].
In COVID-19 infection,there is dysregulation of the rennin-angiotensin-aldosterone system (RAAS)/ ACE-2viathe SARS-COV-2 virus,leading to CV involvement[19].This may be a primary manifestation of COVID-19 or may be secondary to lung involvement,leading to hypoxemia-induced myocardial damage.Patients with preexisting cardiac disease are especially prone for this.Additionally,the downregulation of ACE-2 with concomitant upregulation of angiotensin II results in RAS overactivation.The ultimate result is loss of the beneficial effects of angiotensin (1-7),aggravating and perpetuating cardiac injury.
Relationship between COVID-19,ACE-2,and hypertension
ACE inhibitors (ACE-I) lead to upregulation of ACE-2 receptors;thus causative role in severe COVID-19 disease is postulated.However,in the study of Guoet al[8] in patients with COVID-19,mortality is not impacted by use of ACE-I.In contrast,a retrospective analysis of 1128 hypertensive patients with COVID-19 the inpatient use of ACE-I reduced the all-cause mortality[20].Thus,patients with pre-existing hypertension who are on ACE-I should continue to take it.
ACS
Decrease in ACS cases
Although COVID-19 has led to an increase in hospitalizations,the amount of ACS admissions has substantially decreased.This decline is more for ST elevation myocardial infarction (STEMI) than for non-ST elevation myocardial infarction(NSTEMI).This finding is observed in multiple countries and is believed to be due to multiple factors.These include hesitance of patients to visit hospitals despite initial symptoms for fear of contracting the COVID-19 infection and confusion regarding symptoms.Other factors include better medication adherence,lower pollution levels,less smoking,and less physical strain[21,22].
In one study,40% decline in hospital admission for ACS is reported.Another finding of interest is the late presentation for STEMI compared to the pre-COVID-19 era.Fortunately enough,there is no difference in door to balloon time.Mortality rates are also high for STEMI during the COVID-19 pandemic (P< 0.05)[23].
Presentation of ACS during COVID-19
In one case series,of 18 patients of ACS with COVID-19,56% of the patients presented with STEMI and the remaining developed it during the course of illness.ST elevations could be diffuse or focal and there was high prevalence of LV dysfunction in latter.However,obstructive CAD is seen only in a minority of patients,as seen in 6 (33%) out of 9 patients in the study.Of note,all 18 patients had elevated D-dimer levels[24].In a retrospective single center study by Shiet al[25],of 416 patients with COVID-19,prevalence of ACS was 3.6%,and all were NSTEMI.
Management of ACS in COVID-19
According to the latest European Society of Cardiology (ESC) guidelines for management of CAD during the COVID-19 pandemic,primary PCI is the norm if they are in the window period (< 12 h from symptom onset).The primary PCI should be performed with a door to balloon time of 120 min.However,owing to the delay in transfer to a catheterization lab-facilitated hospital during the COVID-19 pandemic,a delay of up to 60 min can be accepted due in many instances.If the delay is more than this and there is no contraindication for thrombolysis,then patient can be thrombolyzed.In any circumstance,treatment should not be delayed ensuring necessary COVID-19 safety precautions[26].
Patients presenting with NSTEMI,should be risk stratified initially depending on the presence of recurrent chest pain,elevation of biomarkers,recurrent ST-T changes,heart failure,and LV dysfunction.COVID-19 testing should be performed as soon as possible regardless of treatment strategy.Patients having very high risk features should undergo immediate invasive management as for STEMI with COVID safety norms.Patients at high risk should be managed in a separate ICU while waiting for the COVID-19 results,and invasive management should be done within 24 h.Patients with intermediate and low risk can undergo non-invasive testing,such as CT coronary angiography,to rule out obstructive CAD.If patients with high risk and intermediate risk NSTEMI are COVID-19 positive,they should be transferred to equipped COVID-19 hospitals for cardiac intervention so that that along with cardiac illness they can also be treated for COVID[26].
MYOCARDITIS
The exact mechanism of myocarditis in COVID-19 is not clear.Some studies suggest high viral load as the possible mechanism.The cytokine storm and inflammatory state in COVID-19 may lead to myocarditis.It is suspected if a COVID-19 patient without pre-existing cardiac disease develops fulminant HF.Echocardiography shows marked depression in LVEF with global hypokinesia,LV dilatation,and pericardial effusion.Patients respond to anti-inflammatory therapy including parenteral glucocorticoids and immunoglobins[26,27].
STRESS-INDUCED CARDIOMYOPATHY
There is a strong relationship between psychosocial stress and cardiomyopathy.In a study published in JAMA,a significant rise in the incidence of stress cardiomyopathy is observed during the COVID-19 pandemic (incidence proportion:7.8% of total ACS patients).However,in comparison to the pre-pandemic time,the incidence proportion range is only 1.5%-1.8%.Nevertheless,there is no difference in mortality as compared to that of the pre-pandemic era[28].
CARDIOGENIC SHOCK
All patients presenting with cardiogenic shock or out of hospital arrest,must be considered COVID-19 positive until results are available.Those who are COVID-19 negative should be managed according to the latest guidelines.Patients should be admitted to the ICU,and mechanical circulatory support (MCS) should be provided.For COVID-19-positive patients,ICU admission should be given to those who require ventilatory support,use of MCS should be more restrictive,and health care personnel protection should be the priority[26,29].
HEART FAILURE
Patients with pre-existing cardiovascular disease (CVD) are at increased risk of severe COVID-19 infection.Hypertension and CVD is seen in approximately 17.1% and 16.4%of hospitalized COVID-19 patients as suggested in a meta-analysis of 6 studies and contributes to a 2-3 fold increased risk of mortality[30] (Table 1).
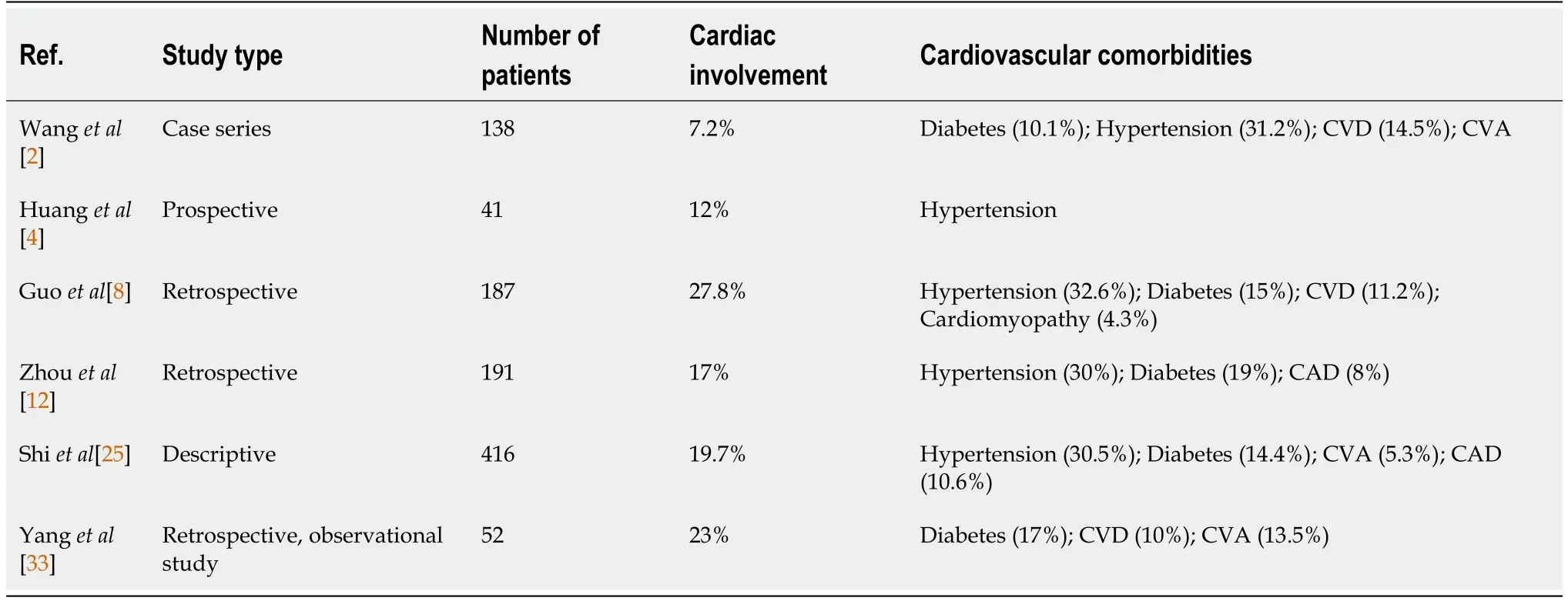
Table 1 Cardiac involvement in coronavirus disease 2019 patients among various studies
Acute heart failure (HF) can complicate COVID-19 cases.It is diagnosed by the classical signs and symptoms like chest rales,increased jugular venous pressure cardiomegaly,and bilateral pleural effusion.Significant elevation of brain natriuretic peptide (BNP) and NT-pro BNP also suggest HF.
There are various mechanisms responsible for HF in COVID-19,including myocardial ischemia,myocarditis,acute respiratory distress syndrome,acute kidney injury,stress induced cardiomyopathy and arrhythmias.Treatment should be administered according to the standard HF treatment guidelines.
ARRHYTHMIAS
The incidence of cardiac arrhythmias in COVID-19 patients was found to be approximately 16.7% in a single center study of 138 patients.The incidence is higher in seriously ill patients admitted to the ICU[2].The management of arrhythmias in COVID-19 patients is essentially similar to as in COVID-19-negative patients.
Follow-up and monitoring of patients with implantable intracardiac devices should be done remotely.In COVID patients presenting with atrial arrhythmias,ratecontrolling agents should be used in place of anti-arrhythmic therapy.This is because there may be need for the concomitant use of hydroxychloroquine/azithromycin which can lead to QT-c prolongation and torsades de pointes.
In patients presenting with VT/VF,amiodarone is the drug of choice.The implantation of an intracardiac device should be postponed for as long as possible.
For patients with bradyarrhythmias,if pacemaker implantation is required,all the necessary precautions should be taken for protection of health care personnel and to prevent nosocomial infections[26].
PULMONARY EMBOLISM
Strong evidence suggesting the increased risk of pulmonary embolism (PE) in COVID-19 is lacking.Some case reports have suggested that the incidence of pulmonary embolism may be high in hospitalized patients with COVID-19[31,32].Owing to the high inflammatory state,enhanced hypercoagulability and immobilization,prophylactic anticoagulation should be given to hospitalized patients.
Because patients with COVID-19 pneumonia also have respiratory distress and chest pain,it is possible to miss PE.However,in the case of any unexpected tachycardia,deterioration of respiratory symptoms,and a decrease in blood pressure,PE should be suspected.This is specially important if there are new ECG changes suggestive of PE or there is deep vein thrombosis.Although the D-dimer is not reliable in COVID-19,it can still be used to rule out PE.In case of high suspicion and normal CT chest despite respiratory worsening,CT pulmonary angiography should be performed.The treatment protocol is similar to as in COVID-19-negative patients[32,33].
INDICATIONS OF ANTICOAGULATION WITH COVID-19 INFECTION
Parenteral anticoagulants are indicated in all acutely ill hospitalized patients[34].
Dosing of anticoagulation:(1) Moderate disease (standard risk patients):standard weight-adjusted prophylactic dose (e.g.,enoxaparin at 40 mg once daily for a 70-kg adult with CrCl > 30 mL/min);(2) Severe and critical disease (high-risk patients requiring invasive ventilation/continuous positive airway pressure (CPAP)/noninvasive ventilation (NIV)/high-flow nasal oxygen):intermediate dose Low Molecular Weight Heparin (enoxaparin at 40 mg two times per day for a 70-kg adult with CrCl >30 mL/min);(3) Diagnosed/highly suspected macro-thrombosis (PE/DVT):therapeutic dose (enoxaparin at 1 mg/kg with 12 h subcutaneous or 1.5 mg/kg subcutaneously once daily);and (4) Renal insufficiency:Enoxaparin with dose reduction is the preference over other LMWH drugs/fondaparinux.UFH with aPTT monitoring indicated at eGFR < 15 mL/min.
Decisions regarding post discharge prophylactic anticoagulation should be individualized.Based on past and ongoing trials regarding usage of anticoagulants,it can be concluded that patients with moderate to severe disease and fulfilling any one of the below criteria should receive post discharge thromboprophylaxis (Table 2)[34]:(1) Modified IMPROVE venous thromboembolism (VTE) (MIV) score ≥ 4;(2) MIV ≥ 2 with a D-dimer value > 2 times the upper limit of the normal range;(3) Age ≥ 75 years;(4) Age > 60 years with a D-dimer value > 2 times the upper limit of the normal range(ULN);and (5) Age 40-60 years with a D-dimer value > 2 times (ULN) and a history of VTE or with diagnosed malignancy.

Table 2 Modified venous thrombo-embolism score for risk stratification in pulmonary embolism/deep vein thrombosis
PROGNOSTIC IMPLICATION
Many serum biomarkers can be utilized for both diagnosis and prognosis in COVID-19 with cardiovascular affliction (Figure 2).In a study by Liuet al[35],C-reactive protein(CRP),lactate dehydrogenase,and D-dimer were elevated in 85.5%,65.2%,and 65.2%of patients,respectively,in the severe category.In another study,the proportions of patients with increased IL-6,CRP,and procalcitonin levels were significantly higher in the severe COVID-19 group than in mild COVID-19 patients.The Cox proportional hazard model showed that IL-6 and CRP are independent factors in predicting the severity of COVID-19.Based on their analysis,patients with IL-6 > 32.1 pg/mL or CRP> 41.8 mg/L are more likely to have severe complications[36].

Figure 2 Biomarkers of inflammation/injury commonly utilized for prognostic implications in coronavirus disease 2019 infection.
Troponin elevation during a hospital stay enhances the risk of arrhythmias,mechanical ventilation,and indicated a subgroup poised for high mortality[36].
Deranged coagulation parameters (D-dimer,fibrinogen,activated partial thromboplastin time,and prothrombin time) are also predictors of poor prognosis as evidenced in a study from Wuhan[37].Critically ill patients and deceased patients demonstrate more frequently elevated levels of D-dimer,PT/INR,and lower fibrinogen levels.Interestingly,D-dimer levels also correlated with the CT findings.
COVID-19 VACCINE AND THROMBOEMBOLISM
Vaccination is thought to be the most promising approach for containing or ending the COVID-19 pandemic.The efficacy of the COVID-19 vaccine in preventing COVID-19 infection varies from 50% to 70%,while the efficacy in preventing serious disease is 70-90%.No major safety warnings,other than rare cases of anaphylaxis,are reported in large trials.In February 2021,the first case of prothrombotic syndrome appeared with the AstraZeneca vaccine (COVISHIELD),which is an adenoviral vector-based vaccine.Subsequently,Ad26.COV2.S vaccine (Janssen;Johnson and Johnson) also reported similar issues[38].Both arterial and VTE are noted with COVID-19 vaccines.However,the distribution is symmetrical for COVISHIELD,while it is skewed in favor of arterial thromboembolism with mRNA vaccines (Pfizer-BioNTech and Moderna)[39].The underlying mechanism discovered is an immune-mediated thrombotic thrombocytopenia and is similar to that observed with heparin-induced thrombocytopenia.The condition starts usually within 1-2 wk after vaccination and is common in young females[40].
The incidence of VTE is 1 case per 100000 exposure.The frequency of COVID-19-related VTE is 14.7% to 17.6%,and the frequency rates of overall arterial thromboembolism is approximately 3.9%[41].Thus,the chances of thromboembolic episodes are far lower as compared to COVID-19 infection itself[.Thus,the vaccines available are safe and effective.
COVID-19 VACCINE AND ACCELERATED HYPERTENSION
Meylanet al[42] reported a case series of 9 patients who developed accelerated hypertension (Stage III) shortly after their vaccinations.However,the majority were being treated for hypertension with drugs beforehand.Anxiety and allergic reactions to vaccine components have been proposed,but in the absence of tachycardia,the former is less likely.
CONCLUSION
The COVID-19 pandemic is producing an adverse impact on health care systems.Studies have demonstrated that this disease not only involves the respiratory system but also multiple organs,including the heart,brain,and kidneys.Cardiovascular involvement is quite common and can be a source of mortality.Thus,there should be a high index of suspicion for cardiac involvement in severe COVID-19 cases,and appropriate measures should be taken.Because the acuteness of pandemic,there is dearth of randomized studies.For patients with primary cardiac presentation,such as ACS,first preference should be given for cardiac management pending a COVID testing.STEMI patients should undergo revascularization either by primary PCI or by thrombolysis according to the window periods and time delays.For NSTEMI patients,treatment should be according to the risk category.For heart failure,arrhythmias,and myocarditis,the standard treatment protocol should be followed.
 World Journal of Cardiology2021年10期
World Journal of Cardiology2021年10期
- World Journal of Cardiology的其它文章
- Cardiac involvement in hydrocarbon inhalant toxicity—role of cardiac magnetic resonance imaging:A case report
- Cardiovascular efficacy and safety of dipeptidyl peptidase-4 inhibitors:A meta-analysis of cardiovascular outcome trials
- Patent hemostasis of radial artery:Comparison of two methods
- Elderly patients with non-cardiac admissions and elevated highsensitivity troponin:the prognostic value of renal function
- Artificial intelligence and machine learning in cardiovascular computed tomography
- Electrocardiographic changes in Emphysema
