FXR信号通路介导何首乌导致脂质沉积的作用及机理研究
韩宗萍 卓飞霞 李芳 夏瑾瑜 黄明星
【摘要】目的 探索何首烏对肝细胞脂质沉积的影响及其机制。方法 利用永生化肝细胞系LO2,采用1 mmol/L游离脂肪酸(FFA)处理,建立脂肪肝沉积细胞模型。同时加入不同浓度的何首乌提取物处理(0、2.5、5、10 μg/mL),采用油红O染色以观察何首乌对LO2细胞脂肪沉积的作用;采用实时荧光定量PCR进行实验组LO2细胞内法尼酯X受体(Fxr)基因及其下游通路胆盐输出泵(Bsep)、小分子异源二聚体伴侣(Shp)、胆固醇7α-羟化酶1(Cyp7a1)关键基因的检测;并采用蛋白免疫印迹法检测FXR及其下游通路关键蛋白的表达水平。结果 何首乌处理后的LO2细胞中,油红O的染色量会随着药物浓度增加而增加;Fxr的mRNA表达下降,而FXR蛋白诱导促进的下游基因Bsep、Shp的表达相应下降,而其下游抑制基因Cyp7a1的表达则上升(P均<0.05)。何首乌处理的LO2肝细胞中,FXR的蛋白水平明显降低,蛋白激酶C (PKC)蛋白磷酸化水平明显降低,而PKC总蛋白表达水平无明显改变,SHP的蛋白水平也明显下降。结论 何首乌可通过抑制FXR-PKC/SHP信号转导通路,从而促进LO2肝细胞内的脂质沉积。为何首乌导致胆汁淤积所引起的肝损伤的治疗提供新的理论依据。
【关键词】何首乌;药物性肝损伤;脂质沉积;法尼酯X受体信号通路
The effect and mechanism of FXR signaling pathway on lipid accumulation induced by Polygonum multiflorum thumb Han Zongping, Zhuo Feixia, Li Fang, Xia Jinyu, Huang Mingxing. Department of Clinical Nutrition, the Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai 519000, China
Corresponding author,Huang Mingxing, E-mail: huangmx5@mail.sysu.edu.cn
【Abstract】Objective To investigate the effect and mechanism of Polygonum multiflorum thumb (PMT) on lipid accumulation in hepatic cells. Methods Immortalized hepatocyte LO2 cell line was treated with non free fatty acids (FFA) to establish the lipid accumulation cell model. Following treatment with 0, 2.5, 5 and 10 μg/mL PMT for 24 h, the LO2 cells were assessed for lipid accumulation by Oil red O staining. The expression levels of Fxr, Bsep, Shp and Cyp7a1 were quantitatively measured by real-time quantitative fluorescence PCR. The expression levels of FXR and key proteins in the downstream signaling pathway were detected by Western blot. Results In LO2 cells treated with PMT, the intensity of Oil red O staining was increased with the increasing concentration of PMT. The expression level of Fxr mRNA was significantly down-regulated, those of Bsep and Shp mRNA in the downstream signaling pathway were down-regulated accordingly, whereas that of Cyp7a1 mRNA in LO2 cells was significantly up-regulated (all P < 0.05). In PMT-treated LO2 cells, the expression level of FXR protein was significantly down-regulated, the phosphorylated level of PKC was considerably decreased, the expression level of total PKC was not significantly changed and that of SHP protein was significantly down-regulated. Conclusions PMT may aggravate lipid accumulation via suppressing the FXR-PKC/SHP signaling pathway. These findings provide novel theoretical evidence for the treatment of liver injury caused by PMT induced-intrahepatic cholestasis.
【Key words】Polygonum multiflorum thumb; Drug-induced liver injury; Lipid accumulation;
FXR signaling pathway
近年来,药物性肝损伤的发生率随着人均药物处方的增加而大幅升高,引起国内外的广泛关注,特别是保健食品添加剂和中草药导致的肝损伤占药物性肝损伤中的比例高达16.1%[1-3]。何首乌是国内中医常用的药物,为蓼科植物何首乌的干燥块根[4-7]。目前常用于补肝肾中药类保健品和护发制剂,以及大众日常餐饮、食疗保健等。但英国药品与健康产品管理局曾于2006年通报过何首乌制剂的肝损伤案例,其后国内陆续出现了大量关于何首乌导致肝损伤报道,甚至致死或肝移植案例[4-7]。欧洲和美国等药品监管部门均出台了对何首乌及含何首乌的制剂进行监管的相关政策[3]。目前认为何首乌引起的肝损伤中36.0%为胆汁淤积的表现和症状[4-7]。
然而,目前何首乌导致的肝内胆汁淤积的确切机制尚不清楚。目前药物性肝内胆汁淤积的主要机制是胆汁合成和转运受损,这个过程受膜受体、核受体及其转录因子转录等一系列调控,其中法尼酯X受体(FXR)是常见的胆汁酸合成的生物感受器和受体[8-10]。FXR调节胆汁酸代谢的胆盐输出泵(Bsep)基因介导肝内一价胆汁酸向胆管内转运,FXR也调控肝脏Mrp2、Ntcp等胆汁相关基因表达,调控肝细胞和胆小管的胆汁酸盐及其有机阴离子的摄取和排泄。我们推测,FXR信号通路的抑制和功能下调导致胆汁淤积的发生[8-10]。
本研究通过LO2肝细胞脂质沉积模型,初步发现了何首乌可通过调节FXR介导的信号通路而引发脂质沉积。从而为明确何首乌药物性胆汁淤积肝病的发病机制提供了重要的研究基础,为治疗何首乌导致药物胆汁淤积的作用靶点提供重要的理论参考价值。
材料与方法
一、细胞系及实验试剂
人永生化肝细胞系LO2购自中国医学科学院细胞库,冻存于实验室液氮罐。何首乌水提取物粉末购自南京泽朗医药有限公司。试验开展前将何首乌粉末均匀混悬于0.5%羧甲基纤维素钠盐(CMC-Na)溶液。胎牛血清(FBS)、RPMI-1640培养基、0.25%胰蛋白酶、磷酸盐缓冲液(PBS)均购自Gibco公司;油红O及游离脂肪酸(FFA)购自Sigma公司;抗FXR抗体、抗PKC抗体、抗磷酸化PKC抗体、抗小分子异源二聚体伴侣(SHP)抗体、Actin抗体购自Cell Signaling Technology公司,抗SHP抗体购自Abcam公司。总RNA提取试剂TRIzol Reagent购自Life公司。
二、实时荧光定量PCR(RT-PCR)
采用TRIzol法提取各組细胞总RNA,使用HiScript Ⅱ qRT SuperMix Ⅱ(Vazyme)逆转录获取cDNA。按实时荧光定量TapMan 探针法试剂盒LightCycler 480 Probe Master (Roche, Indianapolis, IN)说明书,依次加入试剂:Probe Master,cDNA,Primer Forward/Reverse,Probe,ddH2O,混匀。95℃反应10 min,95℃反应15 s,60℃反应60 s,40个循环,PCR仪器自动收集荧光信息,获取相关基因的Ct值,计算得出目的基因的相对含量。
三、蛋白免疫印迹法
根据何首乌不同浓度的处理设为实验组,未加何首乌处理为对照组。收集各实验组及对照组细胞,采用裂解液消化细胞获得总蛋白裂解液样品,各组蛋白BCA法定量后,进行SDS-PAGE;电泳完成后,将3张3M滤纸和1张PVDF膜,浸泡在甲醇去离子水中5 min后,与专用滤纸和纤维垫浸泡于1×转膜缓冲液中;剥下凝胶,去掉浓缩胶部分,并把滤纸和PVDF膜裁成凝胶大小;按照三明治夹心法进行转膜,300 mA转膜2 h;立刻取出PVDF膜,然后用蛋白封闭液封闭1 h;采用待测蛋白的一抗室温孵育PVDF膜3 h后,TBST洗膜3次,每次15 min;采用相对应的二抗孵育PVDF膜1 h后,TBST洗膜3次,每次15 min;于暗房,避光采用ECL显色液显色底片,并采用X线片压片发光显影定影而获得数据片。
四、统计学处理
采用SPSS 25.0进行统计学分析,正态分布计量资料以 表示,比较采用成组t检验(双侧检验),P < 0.05为差异有统计学意义。
结果
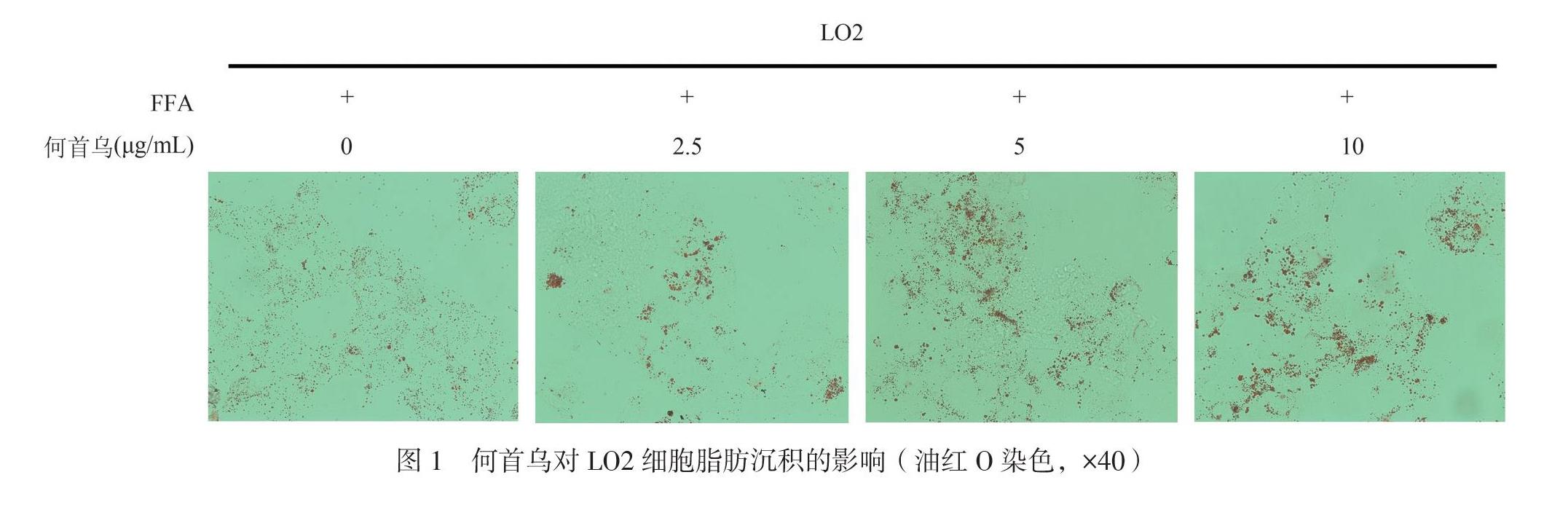
一、何首乌对LO2肝细胞脂肪沉积的促进作用
利用永生化肝细胞系LO2,采用FFA(1 mmol/L)处理24 h,建立脂肪肝沉积细胞模型,同时加入不同浓度的何首乌提取物进行处理(0、2.5、5、10 μg/mL),24 h后用油红O染色以观察何首乌对LO2细胞脂肪沉积的作用。结果显示,与对照组相比,何首乌处理后的LO2细胞中,油红O的染色量会随着药物浓度增加而增加(图1),提示何首乌可促进LO2细胞中脂肪的沉积。
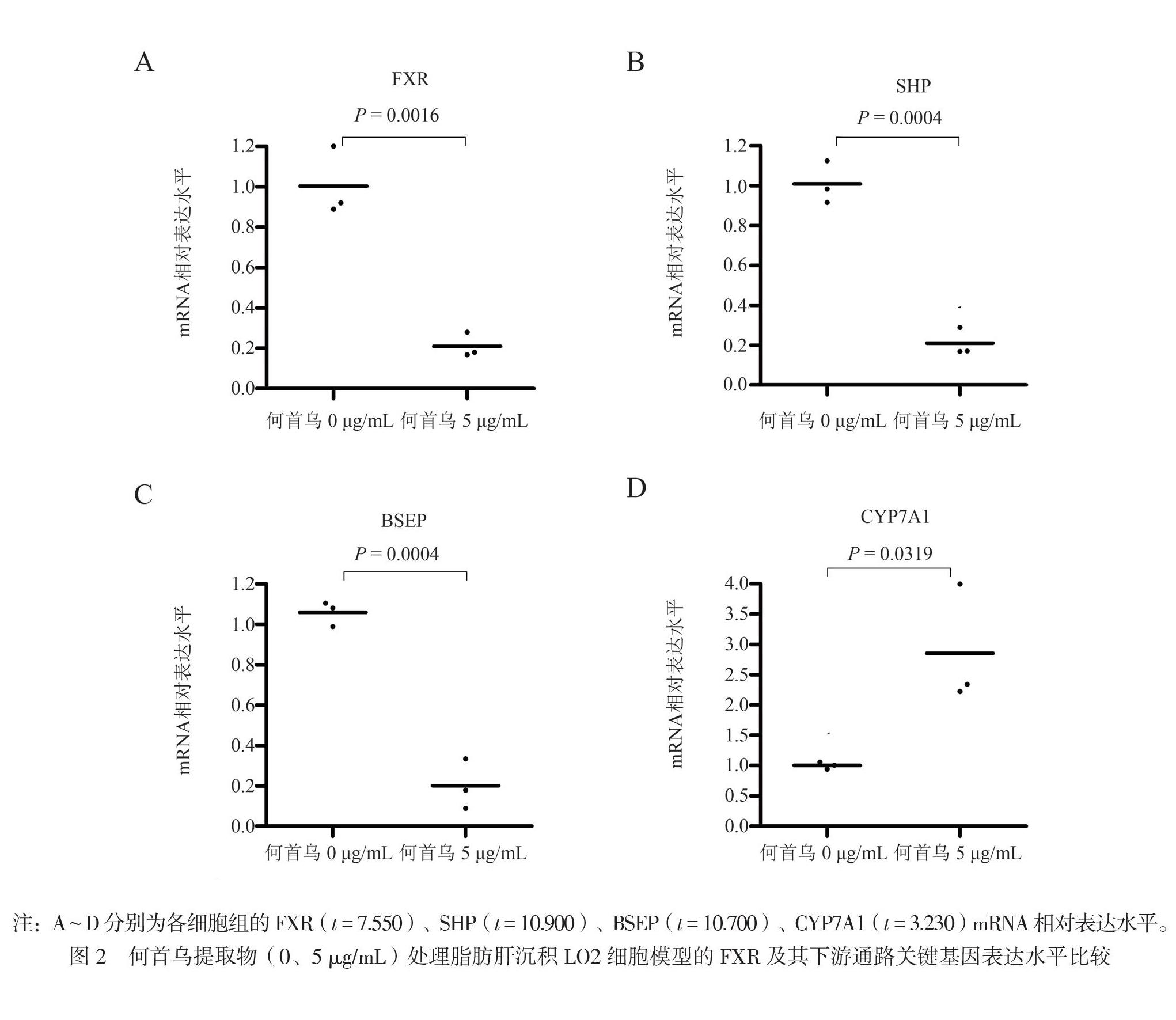
二、何首乌导致脂肪肝沉积细胞模型中Fxr及Fxr下游基因的mRNA表达情况
研究中收集2组何首乌提取物(0、5 μg/ml)
处理过的脂肪肝沉积LO2细胞模型(1 mmol/L FFA处理24 h)进行了mRNA芯片检测,发现FXR通路的相关基因变化比较明显,进而采用RT-PCR进行Fxr及其下游基因的验证检测。结果显示,Fxr的mRNA表达明显下降,而FXR蛋白诱导促进的下游基因Bsep、Shp的表达相应下降,而其下游抑制基因胆固醇7α-羟化酶1(Cyp7a1)的表达则上升(图2)。由此可见LO2细胞中何首乌诱导的脂质积累,可能是通过抑制FXR信号通路相关基因的表达所致。
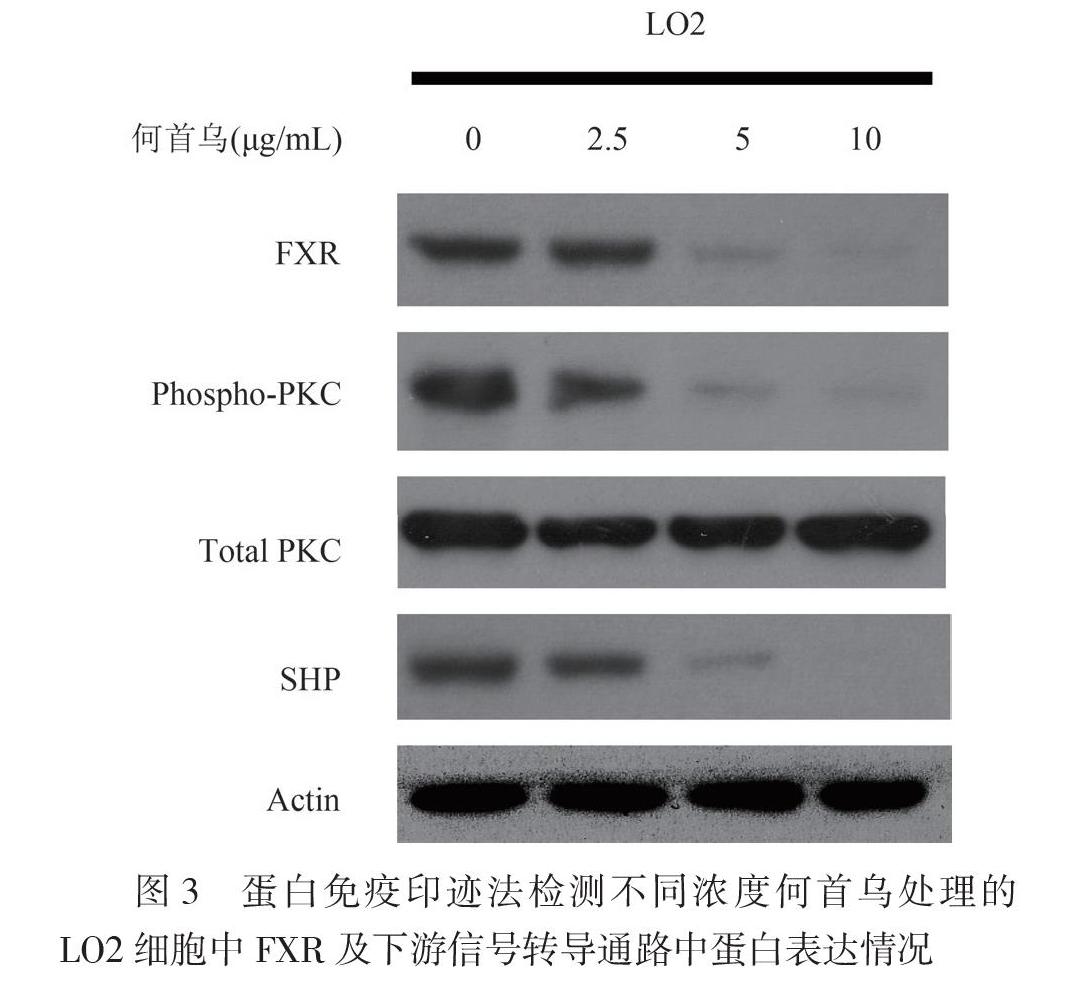
三、何首乌通过FXR通路的介导促进了LO2肝细胞的脂肪沉积
为了探索FXR通路在何首乌介导的LO2肝细胞的脂肪沉积作用,使用不同浓度何首乌提取物(2.5、5、10 μg/ml)与FFA共同处理LO2细胞24 h后,采用蛋白免疫印迹法检测FXR及其下游通路相关蛋白的表达水平。结果显示,经何首乌处理后,FXR的蛋白水平明显降低,蛋白激酶C(PKC)蛋白磷酸化水平明显降低,而PKC的总蛋白表达水平无明显改变,同时SHP的蛋白水平也明显降低(图3)。此结果提示何首乌能通过抑制FXR-PKC/SHP信号转导通路,促进LO2肝细胞内脂肪的沉积。
讨论
近年来关于何首乌肝毒性报道频出,给临床安全性和合理用药带来挑战。国家食品药品监督管理局(SFDA)不良反应监测中心报告提示何首乌及其相关制剂的不良反应报告超过2万份,主要为肝损害的不良反应。SFDA不良反应监测中心监测到的数据只反映了中药肝毒性的一部分,在中医处方、老百姓自行服用的情况下发生肝损伤更严重[11-15]。国外非常重视何首乌引起肝毒性,2012年9月美国国家医学图书馆发布的LiverTox(Clinical and Research Information on Drug-induced Liver Injury)数据库中收录约600种具有肝损伤的西药和中草药,其中何首乌作为一个专题被收录,数据库专门收录了何首乌及其制剂肝损伤的报道,其中何首乌导致肝内胆汁淤积的病例数据最多见,其治疗效果欠佳,是目前临床上亟需解决的问题之一[11-14]。
FXR是核受体超家族成员[16-18]。FXR通过调控一系列胆汁酸相关基因的表达,在胆汁酸合成、转运和代谢中发挥了重要作用。FXR主要通过2条通路激活,其中第一条即在肝细胞内激活,第二条通路在小肠上皮细胞激活[16-18]。胆汁酸本身可激活肠上皮细胞的FXR,FXR是成纤维细胞生长因子(FGF)19(在小鼠为FGF15)的上游转录因子,可启动肠上皮细胞大量表达FGF19。肠腔内FGF19释放到循环血液中,通过门脉循环到达肝脏后,FGF19结合和激活肝FGF受体4并与b-Klotho组成受体复合物后进一步激活c-jun氨基末端激酶与PKC/SHP等信号转导通路,来抑制CYP7A1等因子的合成,从而抑制胆汁酸合成,由此也构成了胆汁酸的负反馈调节通路[16-18]。
本研究团队长期致力于FGF15/19信号轴介导药物性肝损伤的临床和基础研究,发现FGF15具有促进小鼠肝细胞再生的作用,但其作用不依赖于胆汁酸。此外,本团队也通过研究揭示了酒精性肝损伤的主要原因是肠道Fgf15缺失,并证明了Fgf15缺失后肠道的通透性增加,从而加重肝损伤[19-21]。
本研究从体外细胞试验发现何首乌对LO2肝细胞脂肪沉积的促进作用,LO2细胞中何首乌诱导的脂质积累,其机制可能通过抑制FXR信號通路相关基因的表达所致。何首乌能通过抑制FXR-PKC/SHP信号转导通路,促进LO2肝细胞内脂肪的沉积。因此,本研究提示何首乌可导致肝细胞内FXR基因表达下调,FXR基因胆汁转运信号通路受到抑制,进一步介导何首乌抑制胆汁转运信号通路,促进肝细胞内脂质沉积。由此,揭示了何首乌导致药物性胆汁淤积致病分子机制,为临床上治疗胆汁淤积所导致的肝损伤提供新的理论依据。
参 考 文 献
[1] Navarro V J, Khan I, Bj?rnsson E, Seeff L B, Serrano J, Hoofnagle J H. Liver injury from herbal and dietary supplements. Hepatology,2017,65(1):363-373.
[2] Kantor E D, Rehm C D, Haas J S, Chan A T, Giovannucci E L. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA,2015,314(17):1818-1831.
[3] Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci,2015,17(1):14.
[4] Teka T, Wang L, Gao J, Mou J, Pan G, Yu H, Gao X, Han L. Polygonum multiflorum: recent updates on newly isolated compounds, potential hepatotoxic compounds and their mechanisms. J Ethnopharmacol,2021,271:113864.
[5] Liu Y, Wang W, Sun M, Ma B, Pang L, Du Y, Dong X, Yin X, Ni J. Polygonum multiflorum-induced liver injury: clinical characteristics, risk factors, material basis, action mechanism and current challenges. Front Pharmacol,2019,10:1467.
[6] Xue X, Quan Y, Gong L, Gong X, Li Y. A review of the processed Polygonum multiflorum (Thunb.) for hepatoprotection: clinical use, pharmacology and toxicology. J Ethnopharmacol,2020,261:113121.
[7] Dong Q, Li N, Li Q, Zhang C E, Feng W W, Li G Q, Li R Y, Tu C, Han X, Bai Z F, Zhang Y M, Niu M, Ma Z J, Xiao X H,
Wang J B. Screening for biomarkers of liver injury induced by Polygonum multiflorum: a targeted metabolomic study. Front Pharmacol,2015,6:217.
[8] Kumari A, Pal Pathak D, Asthana S. Bile acids mediated potential functional interaction between FXR and FATP5 in the regulation of lipid metabolism. Int J Biol Sci,2020,16(13):2308-2322.
[9] Venetsanaki V, Karabouta Z, Polyzos S A. Farnesoid X nuclear receptor agonists for the treatment of nonalcoholic steatohepatitis. Eur J Pharmacol,2019,863:172661.
[10] Shin D J, Wang L. Bile acid-activated receptors: a review on FXR and other nuclear receptors. Handb Exp Pharmacol,2019,256:51-72.
[11] Zhang Y, Wang N, Zhang M, Diao T, Tang J, Dai M, Chen S, Lin G. Metabonomics study on Polygonum multiflorum induced liver toxicity in rats by GC-MS. Int J Clin Exp Med,2015,8(7):10986-10992.
[12] Bounda G A, Feng Y U. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacognosy Res,2015,7(3):225-236.
[13] Dong H, Slain D, Cheng J, Ma W, Liang W. Eighteen cases of liver injury following ingestion of Polygonum multiflorum. Complement Ther Med,2014,22(1):70-74.
[14] Lei X, Chen J, Ren J, Li Y, Zhai J, Mu W, Zhang L, Zheng W, Tian G, Shang H. Liver damage associated with Polygonum multiflorum thunb.: a systematic review of case reports and case series. Evid Based Complement Alternat Med,2015,2015:459749.
[15] 卢俊竹,陈广成,陈慧,刘思齐,詹俊. 药物性肝损伤与慢性肝病急性加重相关性的临床研究——附301例分析. 新医学, 2020, 51(2): 138-142.
[16] Zhu Y, Liu H, Zhang M, Guo G L. Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B,2016,6(5):409-412.
[17] Miao J, Xiao Z, Kanamaluru D, Min G, Yau P M, Veenstra T D,
Ellis E, Strom S, Suino-Powell K, Xu H E, Kemper J K. Bile acid signaling pathways increase stability of Small Heterodimer Partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev,2009,23(8):986-996.
[18] Li T, Chiang J Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev,2014,66(4):948-983.
[19] Zhang M, Kong B, Huang M X, Wan R X, Armstrong L E, Schumacher J D, Rizzolo D, Chow M D, Lee Y H, Guo G L.
FXR deletion in hepatocytes does not affect the severity of alcoholic liver disease in mice. Dig Liver Dis,2018,50(10):1068-1075.
[20] Kong B, Zhang M, Huang M, Rizzolo D, Armstrong L E, Schumacher J D, Chow M D, Lee Y H, Guo G L. FXR deficiency alters bile acid pool composition and exacerbates chronic alcohol induced liver injury. Dig Liver Dis,2019,51(4):570-576.
[21] Kong B, Sun R, Huang M, Chow M D, Zhong X B, Xie W, Lee Y H, Guo G L. Fibroblast growth factor 15-dependent and bile acid-independent promotion of liver regeneration in mice. Hepatology,2018,68(5):1961-1976.
(收稿日期:2021-06-06)
(本文編辑:杨江瑜)

