Hepatitis C virus treatment failure: Clinical utility for testing resistance-associated substitutions
Ezequiel Ridruejo, Matías Javier Pereson, Diego M Flichman, Federico Alejandro Di Lello
Ezequiel Ridruejo, Hepatology Section, Department of Medicine, Centro de Educación Médica e Investigaciones Clínicas Norberto Quirno “CEMIC”, Ciudad Autónoma de Buenos Aires C1425AS, Unspecified, Argentina
Matías Javier Pereson, Federico Alejandro Di Lello, Facultad de Farmacia y Bioquímica, Instituto de Investigaciones en Bacteriología y Virología Molecular (IBaViM), Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires 1113, Argentina
Diego M Flichman, Instituto de Investigaciones Biomédicas en Retrovirus y Síndrome de Inmunodeficiencia Adquirida (INBIRS), Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires 1113, Argentina
Abstract The hepatitis C virus has a high mutation capacity that leads to the emergence of resistance-associated substitutions (RAS). However, the consequence of resistance selection during new direct-acting antiviral drug (DAA) treatment is not necessarily the therapeutic failure. In fact, DAA treatment has shown a high rate (> 95%) of sustained virological response even when high baseline RAS prevalence has been reported. In the context of RAS emergence and high rates of sustained viral response, the clinical relevance of variants harboring RAS is still controversial. Therefore, in order to summarize the data available in international guidelines, we have reviewed the clinical utility of testing RAS in the era of new pangenotypic DAA drugs.
Key Words: Hepatitis C virus; Treatment failure; Resistance; Direct-acting antiviral
INTRODUCTION
For years, the only available treatment for chronic hepatitis C virus (HCV) infection was pegylated interferon and its combination with ribavirin (PEG-IFN/RBV) therapy. However, the sustained viral response (SVR) to treatment of infected patients was limited, varying between 42% and 46% for HCV genotype 1, about 60% for HCV genotype 4, and 76% to 80% for HCV genotype 2 or 3[1-5]. The outcomes were troublesome in patients coinfected with human immunodeficiency virus /HCV, whose SVR rates were even lower[6-9]. Fortunately, treatment against HCV infection has improved significantly in the last decade, changing from a nonspecific immunomodulatory therapy with multiple and severe side effects, such as PEG-IFN/RBV, to specific viral target options such as direct-acting antiviral (DAA) drugs against NS3, NS5A, and NS5B proteins. Thus, since the development of the latest generation of DAA drugs, the SVR is achieved in 95% to 99% of treated patients[10]. Although this scenario is very encouraging, the 1% to 5% of patients who do not achieve SVR are the pitfall of DAA therapy. Therefore, the current complex challenge is to rescue patients who fail to one or more DAA schemes.
Response to treatment with PEG-IFN/RBV was associated with viral variants and single nucleotide polymorphisms[11-17]. The introduction of DAA drugs implied a higher specific and targeted pressure on the virus, which favor the selection of resistance-associated substitutions (RAS) to different antiviral agents. In this context, virological failure was associated with RAS that may be present either from the beginning (baseline RAS) of treatment or acquired during it[18].
Naturally, HCV produces approximately 1012viral particles per day[19]. In addition, the viral replication complex lacks proofreading activity, resulting in a large amount of viral variants in each infected individual. Although, in theory, all possible mutants can be produced in just 1 day, not all of them are able to remain in the population. That is because some viral genome regions have constraints and most mutations generate variants that impair viral fitness and, therefore, do not proliferate. As a result, a large mutant spectrum known as quasispecies is generated[20]. The quasispecies, that represent the lowest level of viral diversity, drives virus adaptability and constitute the greatest challenge to treatment resistance[20].
DAA drug administration inhibits wild-type HCV variants allowing the selection of reduced susceptibility variants, which present a better fitness to this environment. Although initially they do so inefficiently, over time they develop compensatory amino acid substitutions that have a higher fitness and increase the frequency of resistant variants in the quasispecies spectrum (Figure 1). Additionally, each antiviral drug has a different genetic barrier that is characterized by a threshold above which DAA resistance develops. The threshold is determined by several factors including the number of required nucleotide mutations, the level of resistance, and the viral variant fitness. Therefore, even when a viral variant with a RAS emerges, it does not mean that it is sufficient to lead to therapeutic failure. In that way, therapeutic outcome will depend on a finely poised and complex balance between the DAA genetic barrier and viral-resistant variant fitness. Consequently, a highly resistant strain with a low replication capacity will be clinically less relevant than a less resistant one that replicates more efficiently. Fortunately, more powerful DAA drugs with greater genetic barriers have been developed in the last few years[21].
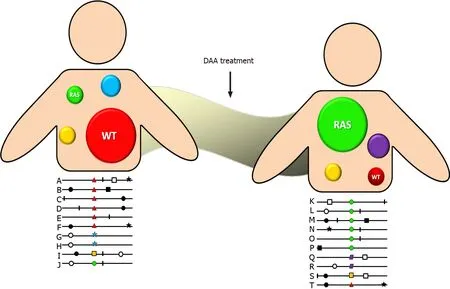
Figure 1 Quasispecies distribution.
In preclinical and in real-life studies, the reported prevalence rate of baseline RAS is around 5% to 40%, raising concern of the effect on reducing SVR[22-28]. Eventually, the adverse impact of baseline RAS could be minimized by extending treatment duration or optimizing DAA regimens. However, that is not always clinically possible, as a considerable proportion of treatment failures are caused by RAS acquired during it[29,30]. Table 1 shows the most relevant RAS reported for the currently most used DAA drugs.
RAS DETERMINATION
Unfortunately, the lack of a large market of standardized commercial assays for RAS determination has led to developing in-house RAS assays, which has created a great disparity the techniques that are used, the determined RAS, and their interpretation. Two main techniques for RAS detection have been applied. One is direct sequencing (Sanger) with sensitivity that allows detecting viral species present in between 15% and 25% within quasispecies, and the second is next generation sequencing (NGS), which allows the detection of variants present in less than 1%[31,32]. NGS is thus a more sensitive technique, but it is also much more expensive. It is therefore very likely that direct sequencing will continue to be the technique of choice because of its cost/benefit in the context of the high SVR rates of currently used DAA regimens.
Since the implementation of DAA agent, the main question that has been asked is the extent to which the RAS frequency impacts the outcome of treatment. It has been reported that the presence of a low proportion of viral variants carrying RAS within the quasispecies of an infected patient would have a lesser impact on SVR rates. In fact, some studies have reported a 15% cutoff of the viral population harboring RAS from in which a drop in the virological response rate was observed. Ikedaet al[33] (2017) reported that the SVR rates to daclatasvir (DCV)/asunaprevir (ASV) in HCVinfected patients with Y93H ratios of < 1%, 1%–25%, 26%-75%, and > 76% were 99%, 100%, 71%, and 23%, respectively[33]. Similarly, using a 15% NS5A pretreatment cutoff of ledipasvir (LDV)-specific RASs, Zeuzemet al[23] (2017) reported significant differences in SVR rates in patients treated with sofosbuvir (SOF)/LDV[23]. Overall, it has been established that SVR decreases as the proportion of RAS in the quasispecies infecting a patient increases. The second question was whether there was a differential impact of RAS depending on whether the patients were treatment naïve or previouslytreated. That question will be discussed in more detail below.
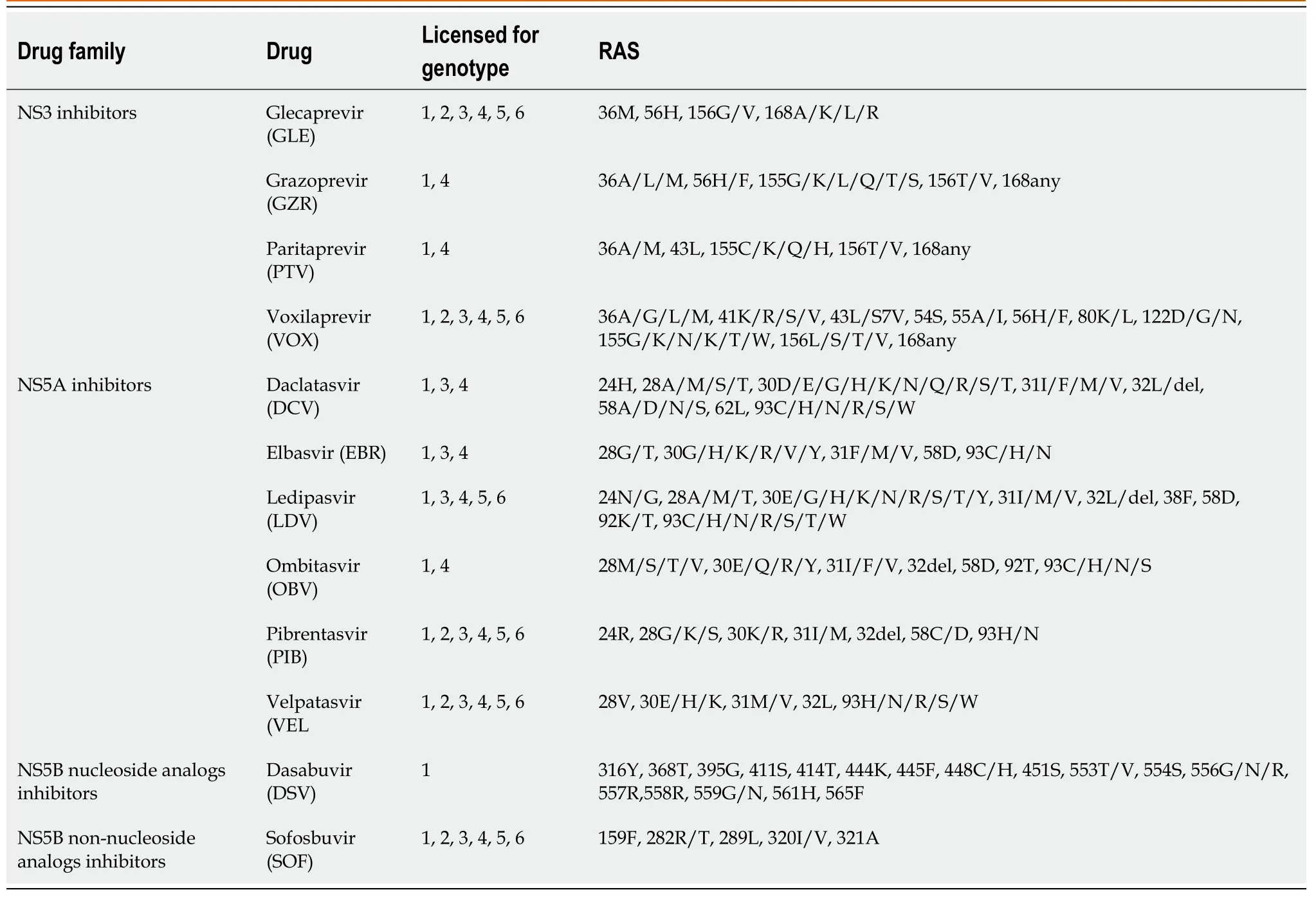
Table 1 Hepatitis C virus resistance-associated substitutions to currently used direct-acting antiviral drugs
CLINICAL UTILITY OF RAS DETECTION
The clinical impact of RAS depends particularly on both the HCV genotype/subtype and the administered DAA regimen, which varies in efficacy according to the type of RAS as well as the treatment experience and presence of cirrhosis.
Naïve patients
In naïve patients, the prevalence of RAS that significantly affect the response to treatment is estimated to be approximately 5%. In that case, the SVR rates of patients with RAS would be 91%, while for patients without RAS it would be approximately 99%[23,34,35]. In summary, RAS assessment prior to the beginning of treatment is not recommended for naïve patients. In previously treated patients, the situation is more complex and refers to subjects who have failed to respond to treatment with a DAA compound. In that case, the presence of post-failure RAS is more than 75%, and SVR rates are more affected. In fact, it has been reported that SVR rates are between 75% and 85% in patients with RAS, while for patients without RAS they continue to be remarkably high (> 95%)[23,34,35].
Identifying the HCV genotype/subtype before starting therapy in naïve patients, in the pangenotypic treatment era, remains useful and may be necessary when drug availability or lack of affordability require genotype-specific treatment or optimal treatment regimens. In that sense, HCV genotyping and subtyping should be performed by nucleotide sequence analysis of some coding regions, generally the core, NS3, or the NS5B coding regions, which accurately discriminates HCV subtypes[36,37]. Furthermore, the use of the NS3 or NS5B regions to determine the viral genotype and subtype also allows the detection of the baseline RAS[36]. On the other hand, as HCV subtypes, including 1l, 3b, 3g, 4r, 6u, 6v, among others, harbor a high frequency of baseline RAS, knowing the HCV subtype before treatment in regions or countries where these subtypes are prevalent (i.e.China, South-East Asia, and sub-Saharan Africa) is strongly recommended in order to optimize treatment[38-41]. Indeed, infrequent subtypes harboring RAS that confer resistance to NS5A inhibitors should be considered for treatment with the fixed-dose combinations SOF/velpatasvir (VEL)/ voxilaprevir (VOX) for 12 wk.
HCV-1 is the most prevalent genotype worldwide (46.2%), and one third of the HCV-1 that infects patients belongs to subtype 1a[42]. Several studies have reported that DAA-naïve individuals infected with HCV-1a are more difficult to treat than those infected with HCV-1b[23,43-45]. In fact, it has been observed that in the presence of cirrhosis, high baseline viral load, or failure of previous treatment with PEGIFN/RBV, the SVR rates of patients treated with elbasvir (EBR)/grazoprevir (GZR), or SOF/LDV were significantly lower for HCV-1a compare with HCV-1b infected individuals[23,43-45]. In EBR/GZR phase III clinical studies, the SVR rate was as low as 58% in HCV-1a treatment-naïve infected patients who harbored baseline NS5A RAS[46]. On the contrary, SVR rates were high (> 97%) in HCV-1b infected patients[46]. Nevertheless, the effect of RAS in HCV-1a infected patients can be overcome by extending treatment to 16 wk and adding RBV to patients with baseline NS5A RAS[44]. Therefore, NS5A resistance testing at baseline is recommended for HCV-1a infected patients with a viral load above 800.000 IU/mL if 12 wk treatment duration is intended.
In addition, pretreatment genotyping is recommended if cirrhotic patients will be treated with SOF/VEL, as baseline RAS reduce SVR rates in HCV-3 cirrhotic patients treated with that regimen. Moreover, a recent study analyzing 539 HCV-3 infected patients showed that patients with baseline Y93H and/or A30K RAS had an SVR rate of 72.2%, while HCV-3 infected patients without NS5A RASs achieved an SVR rate of 95.7% (P= 0.002)[47]. Accordingly, a large meta-analysis that included more than 6500 subjects with chronic HCV infection reported reduced effectiveness of GLE/PIB in HCV-3 infected patients with baseline RAS like A30K, Y93H, and P53del, and recommended, in order to improve prognosis of treatment outcome and selection of therapy, testing of RAS in such patients[48].
According to the American Association for the Study of Liver Diseases guidelines, pretreatment RAS testing is recommended in cirrhotic HCV-3 infected patients because those without a baseline Y93H RAS in NS5A are eligible for 12 wk of SOF/VEL therapy. On the other hand, cirrhotic HCV-3 infected patients with baseline Y93H RAS should be treated with SOF/VEL plus RBV or SOF/VEL/VOX for 12 wk[49]. However, since HCV-3 infections are frequent in developing countries, the benefit of pretreatment screening for RAS should be weighed. On the contrary, the European Association for the Study of the Liver (EASL) guidelines recommend the same therapeutic regime for all compensated cirrhotic patients regardless of viral genotype[50].
Retreatment for DAA failures
Even in the context of a low treatment failure rate (< 5%), the number of patients requiring retreatment is quite high because of the large number of patients with chronic HCV infection who are treated with DAA worldwide[22-24,29-30]. Currently, the main international treatment guidelines do not recommend massive testing of RAS before starting DAA treatment, although there are exceptions[49,50].
Treatment with SOF/VEL/VOX for 12 wk is one of the most promising pangenotypic regimens for rescuing patients who have failed treatment. Two phase III trials, POLARIS-1 and POLARIS-4, assessed the safety and efficacy of the SOF/VEL/VOX regimen for 12 wk in patients who failed treatment with NS3 and/or NS5A inhibitors[51]. In the POLARIS-1 study, which included 263 patients with NS5A inhibitor failure, the overall retreatment SVR rate was 96% (one breakthrough and six relapses). As expected, cirrhotic patients, who constituted 46% of the study population, had lower SVR than noncirrhotic patients (93%vs99%, respectively). It is important to highlight that neither the HCV genotype nor the RAS profile at the beginning of retreatment influenced SVR[51,52]. Unlike POLARIS-1, the POLARIS-4 study included previously treated patients without NS5A inhibitors. Cirrhotic patients were equally represented in both studies (46%). In POLARIS-4, the overall SVR rate of retreatment with SOF/ VEL/VOX for 12 wk was 98% (178/182; one relapse) compared with 90% (136/151; one breakthrough and 12 relapses) in patients retreated with SOF/VEL for 12 wk[51,52]. Regardless of patient gender, body mass index, HCV genotype, and baseline HCV-RNA levels, several real-life studies have confirmed the high SVR rates achieved with the SOF/VEL/VOX scheme in randomized clinical trials[53-56].
The other available pangenotypic option for the treatment of patients with resistant variants is GLE/PIB. However, the combination did not have a suitable genetic barrier to achieve optimal SVR rates in patients failing previous DAA treatment[57]. In the MAGELLAN-1 Part 2 study, GLE/PIB was used for the retreatment of previous DAA failures. SVR12 was achieved by 89% and 91% of HCV-1 and HCV-4 infected patients who received 12 wk and 16 wk of treatment, respectively. Previous treatment with one inhibitor class (protease or NS5A) had no impact on SVR12, whereas past treatment with both classes of inhibitors was associated with lower SVR12 rates[57]. Another study adds support of the efficacy of the 16 wk regimen for retreatment of HCV-1 infected patients with a history of sofosbuvir/NS5A inhibitor treatment failure[58]. Consequently, treatment with GLE/PIB is recommended as an alternative regimen for the retreatment of patients who failed to a prior DAA regimen including a, NS5A or NS3 inhibitor. It is not recommended for patients who have failed treatment with the combination of both inhibitors[50]. Therefore, at present, the SOF/VEL/VOX combination is the regimen of choice for the retreatment of patients who did not achieve SVR after a course of DAA treatment. RAS determination is not necessary before initiating treatment[49,50].
Currently, the most challenging scenario is represented by patients who failed combinations containing the latest generation of pangenotypic DAA agents GLE/PIB and SOF/VEL/VOX. Thus, such patients who are very difficult to cure, the combinations of SOF/VEL/VOX or SOF/GLE/PIB with RBV for 12 wk, or without RBV for 16-24 wk, are the recommended options. In a previous study, 31 patients who failed GLE/PIB were retreated with SOF/VEL/VOX achieved an SVR of 94% despite the presence of NS5A RAS in 90% of the cases[59]. On the other hand, in the ongoing MAGELLAN-3 study, 23 patients who failed GLE/PIB and received treatment with SOF/GLE/PIB combined with RBV achieved an SVR of 96%, despite the presence of RAS in the NS5A region in 91% of them[60].
Recently, failure to SOF/VEL/VOX has been reported in 40 patients[61]. RAS testing after SOF/VEL/VOX failure showed that all HCV-1a had either NS3 or NS5A RAS. On the contrary, in HCV-1b, individual NS3 RAS were rather rare (11%), and the overall frequency of NS5A RAS was moderate (33%). Finally, for HCV-3, RAS in NS5A (56%) and in NS3 plus NS5A (28%) were relatively frequent. In 22 of the cases, rescue treatment with SOF/GLE/PIB, with or without RBV, for 12-24 wk achieved an SVR rate of 79%. Unfortunately, as all types of DAA drugs have been used in most developing countries; failure is a real possibility. Therefore, surveillance of circulating viral variants is imperative. From a practical point of view, if DAA treatment fails, there are two possibilities: (1) To determine RAS and adjust the new DAA regime according to the result; and (2) to administer empirical DAA treatment following clinical practice guidelines.
The EASL currently recommends first line therapy regimens that do not require pretreatment RAS detection. The 2020 EASL Recommendations on Treatment of Hepatitis C state that in areas where the regimens are not available or not reimbursed, physicians who have access to reliable resistance tests can use the results to guide their decisions, according to[50]. Thus, the selected retreatment option depends on the availability of RAS testing, the actual access to the DAA agent indicated in the event of the failure, and the preference of the treating physician.
CONCLUSION
In the current clinical setting, there is no need for baseline detection of RAS before DAA therapy initiation in naïve patients. The use of adequate pangenotypic regimes may overcome the effect of RAS in the first treatment. After treatment failure, RAS may be determined when available. Otherwise, SOF/VEL/VOX for 12 wk is the regimen of choice, as it has shown the highest SVR rates. GLE/PIB for 16 wk is an alternative regime and it may be used in patients who have failed NS5A or NS3 inhibitors, but not a combination of both. Failure to treatment with multiple DAA regimens may be the clearest clinical scenario for RAS detection. In such cases, rescue treatment can be guided based on the results. If after many failures, RAS detection is not available, treatment should be evaluated by multidisciplinary teams. SOF/VEL/ VOX or SOF/GLE/PIB with RBV for 12 wk or without RBV for 16-24 wk are the regimens of choice as they have shown effectiveness in curing these difficult-to-treat patients.
ACKNOWLEDGEMENTS
To Silvina Heisecke, from CEMIC-CONICET, for copyediting the manuscript.
 World Journal of Hepatology2021年9期
World Journal of Hepatology2021年9期
- World Journal of Hepatology的其它文章
- Coronavirus disease 2019 and non-alcoholic fatty liver disease
- Epigenetic mechanisms of liver tumor resistance to immunotherapy
- Advances in the management of cholangiocarcinoma
- Herbal and dietary supplement induced liver injury: Highlights from the recent literature
- Challenges in the discontinuation of chronic hepatitis B antiviral agents
- Liver Kidney Crosstalk: Hepatorenal Syndrome
