Liquid chromatography-mass spectrometry method for the quantification of an anti-sclerostin monoclonal antibody in cynomolgus monkey serum
Yuxiong Gao , Zhendong Chen , Changyong Yang , Dafang Zhong ,*
a State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201210, China
b University of Chinese Academy of Sciences, Beijing,100049, China
c Preclinical Department, Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu Province, 222047, China
Keywords:Anti-sclerostin monoclonal antibody(SHR-1222)LC-MS/MS Anti-drug antibody Toxicokinetic
ABSTRACT Liquid chromatography tandem mass spectrometry (LC-MS/MS) has gradually become a promising alternative to ligand binding assay for the bioanalysis of biotherapeutic molecules, due to its rapid method development and high accuracy. In this study, we established a new LC-MS/MS method for the determination of the anti-sclerostin monoclonal antibody (SHR-1222) in cynomolgus monkey serum,and compared it to a previous electrochemiluminescence method. The antibody was quantified by detecting the surrogate peptide obtained by trypsin digestion. The surrogate peptide was carefully selected by investigating its uniqueness, stability and MS response. The quantitative range of the proposed method was 2.00-500 μg/mL, and this verified method was successfully applied to the toxicokinetic assessment of SHR-1222 in cynomolgus monkey serum.It was found that the concentrations of SHR-1222 in cynomolgus monkeys displayed an excellent agreement between the LC-MS/MS and electrochemiluminescence methods (ratios of drug exposure, 0.8-1.0). Notably, two monkeys in the 60 mg/kg dose group had abnormal profiles with a low detection value of SHR-1222 in their individual sample. Combining the high-level anti-drug antibodies (ADAs) in these samples and the consistent quantitative results of the two methods, we found that the decreased concentration of SHR-1222 was due to the accelerated clearance mediated by ADAs rather than the interference of ADAs to the detection platform. Taken together, we successfully developed an accurate, efficient and cost-effective LC-MS/MS method for the quantification of SHR-1222 in serum samples, which could serve as a powerful tool to improve the preclinical development of antibody drugs.
1. Introduction
Monoclonal antibodies (mAbs) have been mushrooming in recent years, which exhibit good therapeutic potentials against various diseases, including cancer and autoimmune diseases [1,2].Given the recent success of therapeutic mAbs, a reliable bioanalytical method is needed to allow their quantitative determination in biological matrix and to further apply in pharmacokinetic(PK) or toxicokinetic (TK) studies.
The ligand binding assays (LBAs), such as electrochemiluminescence (ECL) and enzyme-linked immunosorbent assay (ELISA), are recognized as the gold standard for targeted protein quantitation[3].However,LBAs often require more time for the assay development, and their detection accuracy may be influenced by cross-reactions [4-9]. Recently, liquid chromatography tandem mass spectrometry (LC-MS/MS) has emerged as a useful complementary technique to LBAs, because of its rapid method development and high accuracy. Generally, this technique involves sample processing, such as denaturation, reduction,alkylation and digestion of protein analyte, and ultimately LC-MS/MS quantification of surrogate peptides [10-14]. LC-MS/MS offers a wider linear dynamic range, multiplex analysis and higher selectivity compared to LBAs[15].Besides,LC-MS/MS measures the total antibody drugs by protein precipitation method[16],and also serves as a method to tolerate antidrug antibodies (ADAs). LC-MS/MS may be a basis for determining the presence or absence of ADA-induced accumulation of bioactive drugs [17,18,20]. When a decrease in drug exposure is observed, it may be difficult to distinguish whether the decrease in measured drug concentration is due to ADA-mediated determination interference or to ADAmediated accelerated clearance. It is notable that evidence exists for both mechanisms[21-23].LC-MS/MS can be adopted to explain this phenomenon. At present, there is no regulatory guidance for the LC-MS/MS detection of biotherapeutic antibodies, but several recommendations have been proposed to handle these measures[24].
Sclerostin, an endogenous protein (22.3 kDa) encoded by SOST gene in the osteocytes,has been shown to inhibit osteoblastic bone formation [25,26]. Sclerostin causes osteoporosis in the elderly population, especially postmenopausal women, which seriously affects human health [27]. Compounds that can increase the degradation or inhibit the activity of sclerostin are expected to promote osteoblastic bone formation [28]. SHR-1222 is a humanized mAb with IgG1 structure, produced by Jiangsu Hengrui Medicine (Jiangsu, China), which can be used to target sclerostin. It is now undergoing Phase I clinical trial for the treatment of osteoporosis.
In this study, a new LC-MS/MS method was developed for the detection of SHR-1222 in cynomolgus monkey serum. An appropriate surrogate peptide was selected carefully, method validation was carried out, and the TK assessment of SHR-1222 was conducted.The concentration of SHR-1222 measured using the LC-MS/MS method was compared with that measured using the Meso Scale Discovery electrochemiluminescence (MSD-ECL) method, a typical LBA established previously [29]. The advantages and disadvantages of these two methods were further compared. The findings revealed that the specificity and dynamic range of our LCMS/MS method were markedly improved compared to those of the ECL method. Additionally, the present LC-MS/MS method was relatively efficient and cost-effective,because this method does not require specific antigens antibodies.
2. Experimental
2.1. Chemicals and reagents
SHR-1222 injection (100 mg/mL, purity 99.2%) was purchased from Jiangsu Hengrui Medicine (Jiangsu, China). The blank cynomolgus monkey serum was obtained from Guangxi Guidong Primate Animal (Guangxi, China). The isotope-labeled peptide LLIYYTSNR [15N,13C] (internal standard, IS) was supplied by ChinaPeptides (China). Sequencing trypsin was obtained from Promega (Madison, WI, USA). HPLC-grade acetonitrile (ACN) and methanol were supplied by Merck KGaA (Darmstadt, Germany).HPLC-grade ammonium bicarbonate, ammonium sulfate, dithiothreitol,formic acid(FA),iodine acetamide,sodium dodecyl sulfate(SDS) and trifluoroacetic acid (TFA) were purchased from Dalian Meilun Biotechnology (Dalian, China).
2.2. Preparation of calibration standard (CS) and quality control(QC) samples
CS and QC samples were prepared by serial dilutions from stock solutions from the same source and concentration by cynomolgus monkey serum. The final concentrations of CS were prepared at 8 different points ranging from 2.00 to 500 μg/mL. The final concentrations of QC samples were 2.00 (lower limit of quantification(LLOQ)),5.00(low QC),200(middle QC),and 400(high QC)μg/mL.IS working solution(250 ng/mL)was prepared by diluting its stock solution with 20% ACN containing 0.1% FA.
2.3. Sample preparation
Sample pretreatment was carried out by protein precipitation and tryptic digestion [16]. A 2.0 μL aliquot of serum sample was diluted 10fold with phosphate-buffered saline solution containing 0.1% SDS. The diluted sample was added into 20 μL dithiothreitol(100 mM) and incubated at 60°C for 30 min. Then,alkylation was initiated by incubation with iodine acetamide (100 mM) at room temperature for 15 min in the dark. Subsequently, the proteins in the sample were precipitated by adding 180 μL ice-cold methanol,followed by centrifugation for 10 min. After removing the supernatant, 80 μL of ammonium bicarbonate buffer (50 mM, pH 8.2),20 μL of IS working solution (250 ng/mL) and 10 μL of trypsin(100 μg/mL) were added into the retained pellet. The mixture was incubated at 60°C for 90 min with violent vortexing, in order to completely dissolve the pellet. Protein digestion was quenched by adding 30 μL of 60%ACN solution containing 1% TFA.
2.4. LC-MS/MS conditions
2.4.1. Identification of candidate peptides by nano-LC orbitrap fusion
Easy-nano LC 1000 system tandem with orbitrap fusion mass spectrometer (Thermo Scientific, Waltham, MA, USA) was employed to comprehensively separate the complex peptide mixtures and identify the tryptic peptides. Digestion of the SHR-1222 solution was performed as described in Section 2.3. Peptide separation was conducted on an Easy-nano LC 1000 system equipped with a home-made C18reverse-phase column (75 μm i.d. × 18 cm length).The mobile phase contained 0.1%FA in 2%ACN and 0.1%FA in 90%ACN for A and B,respectively.A 60 min shallow gradient was applied to the nano-LC column:0-10 min, 5%-11% B; 10-45 min,11%-35%B; 45-50 min, 35%-45%B; 50-55 min,45%-80%B; and 55-60 min, 80% B. The eluted peptides were ionized under 2.1 kV and detected by the orbitrap fusion mass spectrometer. The full mass was obtained with an orbitrap analyzer at 120,000(m/z 200)resolution and m/z 350-1800 scan range. The automatic gain control and maximum ion injection time were set to 1000,000 and 50 ms,respectively.The precursor ions were selected and subjected to fragmentation through higher-energy collision dissociation.The normalized collision energy was fixed at 32%. The fragment ions were detected by ion trap. The maximum ion injection time was limited to 35 ms.The mass spectrometric data were transformed to MGF format and searched by Mascot search engine(version 2.3.01).Enzyme type was set as trypsin/P. The maximal number of missed cleavage sites was set to 2.Carbamidomethyl(C)and oxidation(M)were imposed as fixed and variable modifications,respectively.The mass tolerance levels of peptides and fragments were adjusted to±10 ppm and±0.5 Da,respectively.The peptides were then filtered with a Mascot score of ≥20.
2.4.2. Ultra-high-performance liquid chromatography (UHPLC)-MS for the detection of surrogate peptide
After searching against the Homo sapiens and Macaca fascicularis Swiss-Prot databases, a surrogate peptide that is absent in the proteome of human and cynomolgus monkey was chosen. The surrogate peptide was detected using the Agilent 1290 infinity UHPLC coupled with 6495 triple quadrupole (QqQ) MS. Chromatographic separation was performed on a rapid resolution high definition C18column(2.1 mm×50 mm,1.7 μm;Agilent,USA).The temperatures of autosampler and column were adjusted to 4°C and 40°C, respectively. The mobile phase contained 0.1% FA in water and 0.1%FA in ACN for A and B,respectively.After sample injection(3.0 μL), the mobile phase was delivered at a flow rate of 300 μL/min. The gradient program was as follows: 0-1.0 min, 16% B;1.0-3.0 min, 16%-60% B; 3.0-3.1 min, 60%-90% B; 3.1-4.0 min,90%B;and 4.1-5.0 min,16%B.The total run time was 5.0 min.The operating parameters were as follows: capillary voltage, 4000 V;drying gas temperature, 200°C; drying gas flow,14 L/min; nebulizer pressure, 30 psi; sheath gas temperature, 325°C; and sheath gas flow,11 L/min. Ionization was conducted in positive ion electrospray mode,and detection was carried out by multiple reaction monitoring with the transitions of m/z 571.8→803.4 for LLIYYTSNR and m/z 576.5→813 for LLIYYTSNR[15N,13C].The peak area ratios of analytes-to-IS versus analyte concentrations were plotted using a weighted least-squares regression method (liner,1/concentrationsquared,1/x2).
2.5. Application of LC-MS/MS to a TK study
The validated method was applied to a TK study approved by the local ethical committee and regulatory authority. The animal experiments were conducted in JOINN Laboratories (Suzhou, China).TK study was performed separately on 40 cynomolgus monkeys(n=10 in each group, with equal numbers of females and males).The monkeys were subcutaneously administered with different doses (20, 60 and 200 mg/kg) of SHR-1222, while those in control group received a placebo. The drug was administered once every two weeks for 12 weeks,7 times in total,i.e.,at day 1,15,29,43,57,71 and 85,respectively.Blood sampling was carried out at 0(before treatment), 1, 4, 24, 48, 72, 96, 168, and 336 h after the first administration, 5 min after the second administration, and 0(before the third to sixth administration)and 5 min after the third to sixth administration,and 0(before the last administration),1,4,24,48,72,96,168,336,504 and 672 h after the last administration.The samples were collected at 1, 4, and 24 h for the first three animals of each gender in each group. After collected into the anticoagulant-free tubes, the blood samples were centrifuged at 1800 g for 10 min to separate the serum fractions. All serum samples were kept at-70°C until further use.Phoenix WinNonlin(ver.6.4; Pharsight, USA)was used to analyze TK parameters through a non-compartmental model.
3. Results and discussion
3.1. Surrogate peptide candidates
The basis for an accurate and robust LC-MS/MS method is the selection of the appropriate surrogate peptide. In this study, the peptides in the tryptic mixture were identified using nano-LCorbitrap fusion and Mascot search engine as described in Section 2.4. Fig.1 shows the total ion chromatogram (TIC) of all SHR-1222 peptides separated by the nano-LC system. Non-unique peptides were excluded after blasting against Homo sapiens (for the future use in clinical trials) and Macaca fascicularis protein databases. To increase the efficiency of selecting the most suitable peptide, the peptide mixture was incubated at 60°C for 6 h after the quenching of tryptic digest and prior to the detection of the peptide mixture.The long-time and high-temperature incubation could help to reduce the interference from unstable peptides. All the results are presented in Table 1.
3.2. Optimization of LC-MS/MS conditions
The optimization of LC-MS/MS method was carried out by adjusting MS parameters,improving LC conditions,simplifying the pretreatment process,and selecting the most appropriate surrogate peptide.
After identification of candidate peptides,the quantitative work was performed on Agilent 6495 QqQ. It is worth noting that the sensitivity of QqQ MS is generally better than that of highresolution MS. Peptides tend to carry multiple charges in the MS and it is time consuming to optimize them one by one. Hence,Skyline software was introduced and combined with the Agilent MassHunter data acquisition software.Multiple scanning windows were set up to optimize the MS parameters of the candidate peptides simultaneously through a single LC-MS/MS analysis (Fig. 2).The peptide with LLIYYTSNR sequence from the light chain of the antibody was eventually selected for quantification, and the monitoring transition was m/z 571.8→803.4 and the collision energy was 19.0 V. In addition, the monitor transition for LLIYYTSNR[15N,13C] was m/z 576.5→813 and the collision energy was 18.7 V.
3.3. Method validation
The validation of LC-MS/MS method was conducted in accordance with the guidelines of China Food and Drug Administration(CFDA) [30] and European Medicines Agency (EMA) [31]. The accuracy and precision of LC-MS/MS method were evaluated by 3 different batches of analytical tests conducted with 2 analysts for at least 2 days, and the results of batch analysis were deemed acceptable. Fig. 3 displays the representative LC-MS/MS chromatograms of IS and surrogate peptide for the determination of SHR-1222. The data of method validation are summarized in Table 2. The matrix effects of hemolyzed samples did not significantly affect the accuracy and precision of the quantification. A target mAb, instead of surrogate peptide, was included prior to tryptic digestion.Notably,the accuracy and precision of QC samples were excellent, suggesting that both extraction recovery and digestion efficiency can meet the requirement of method validation. As shown in Table 3, the target mAb was stable in serum sample for more than 5.0 h at room temperature, 191 days at-70°C, and 5 freeze-thaw cycles.
3.4. LC-MS/MS profiles of SHR-1222 in TK study
The validated method was applied to the TK assessment of SHR-1222 (60 mg/kg) in cynomolgus monkeys (n=6). Fig. 4 demonstrates the mean serum concentration versus time profiles of SHR-1222. Moreover, the results of TK parameter estimation are presented in Table 4. MSD-ECL data were obtained from a previously published study [29] and are shown here only a more intuitively comparison with LC-MS/MS method. The ratio of drug exposure between the last administration (the 7th time) and the first administration was 1.21-1.45, indicating that SHR-1222 had been accumulated slightly in cynomolgus monkeys after its subcutaneous injection (60 mg/kg) for 12 weeks. The TK profiles and statistical data also revealed that the concentrations of SHR-1222 quantified by LC-MS/MS method were consistent with those determined by MSD-ECL method [29], with the ratios of Cmaxand drug exposure ranging from 0.89 to 0.95 and 0.80 to 1.00,respectively.
3.5. Outliers in detection and ADA-tolerant LC-MS/MS method
With MSD-ECL method [29], in the TK study of 60 mg/kg SHR-1222 group, it was found that the serum concentrations of SHR-1222 on the 28th and 42nd days after administration were significantly lower (38.4 μg/mL and 1.55 μg/mL, respectively) in a monkey (ID 002) than those of SHR-1222 in other monkeys(approximately 300-800 μg/mL) in the same group. Similarly, the serum concentration of SHR-1222 in a monkey(ID 004)on the 28th day after administration was also significantly lower (41.6 μg/mL)than that of SHR-1222 in other monkeys in the same group(Fig.5).To explain these outliers,the immunogenicity of the monkeys was first investigated [29]. The results showed that the levels of anti-SHR-1222 ADA in the three abnormal samples were very high,with a titer of ≥16,while the ADA titers of other samples were only about 2.It can be inferred that the profiles of SHR-1222 in these two monkeys changed significantly due to the high ADA level.

Fig.1. The total ion chromatogram (TIC) of all peptides of SHR-1222 digest separated by the nano-LC system.

Table 1 Candidate peptides for surrogate peptide selection.
However, the detection accuracy of LBA method is easily interfered by ADA level.When a decrease in drug exposure is observed by LBA method, it may be difficult to distinguish whether the reduced drug concentration is caused by ADA interference or ADAmediated accelerated clearance in vivo.Therefore,it is necessary to establish an ADA-tolerant method to shield the interference of ADA.The LC-MS/MS method of ADA tolerance can truly reflect the change of SHR-1222 concentration in vivo. The present LC-MS/MS method could overcome ADA interference by precipitation of serum proteins and releasing ADA-bound drug through enzymatic digestion, in order to ensure the measurement of total drug in serum samples. The established LC-MS/MS method was further applied to the TK assessment of SHR-1222 in cynomolgus monkeys.The results showed that the detection values of SHR-1222 in the three abnormal serum samples were also significantly lower than those of other monkeys in the same group (Fig. 5B). The serum concentrations of SHR-1222 in the monkey 002 on the 28th and 42nd days after administration were 37.4 and 5.55 μg/mL, respectively, while the serum concentration of SHR-1222 in the monkey 004 on the 28th day after administration was 60.5 μg/mL. These findings were consistent with those obtained from MSD-ECL method. Therefore, it is speculated that high ADA level can accelerate the clearance of SHR1222 in vivo,which is characterized by a decrease in drug exposure rather than ADA interfering with the detection. In addition, an interesting phenomenon observed in these two animals was that the drug concentration-time profiles recovered at later time points.It is hypothesized that the recovery of the PK profile at a later time point was possibly due to the shift in the subtype[29]or amount of anti-SHR1222 ADAs produced by the two animals after their fifth administration, the binding affinity between ADAs and SHR-1222 decreased,and ADAs no longer had a strong neutralization and clearance effect.
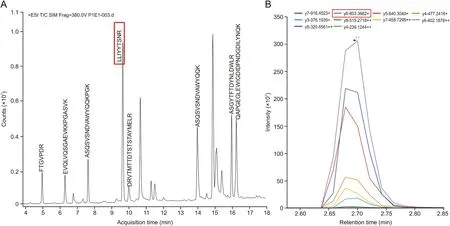
Fig.2. Optimization of MS parameters of the surrogate peptide.(A)The peptide LLIYYTSNR on the light chain of SHR-1222 showed the highest signal in full mass scan and(B)the transitions and collision energy of LLIYYTSNR were optimized using Skyline software.
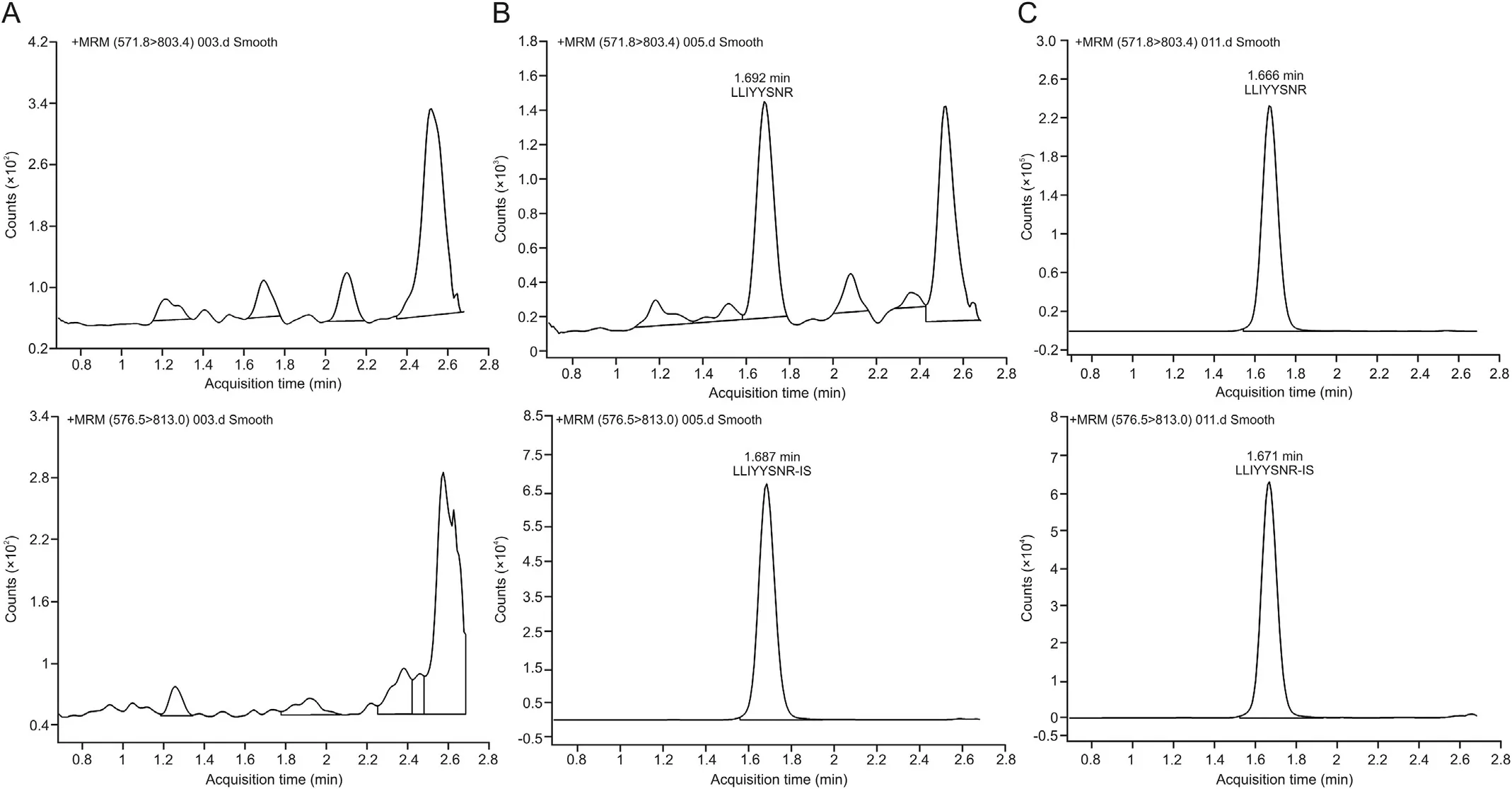
Fig. 3. Representative LC-MS chromatograms of the surrogate peptide and IS for the quantification of SHR-1222. (A) a blank serum sample, (B) an LLOQ sample, and (C): an unknown cynomolgus monkey serum sample (from a monkey of 60 mg/kg group after the first administration of SHR-1222).

Table 2 Method validation data of the LC-MS/MS method.

Table 3 The stability of SHR-1222 in cynomolgus monkey serum.
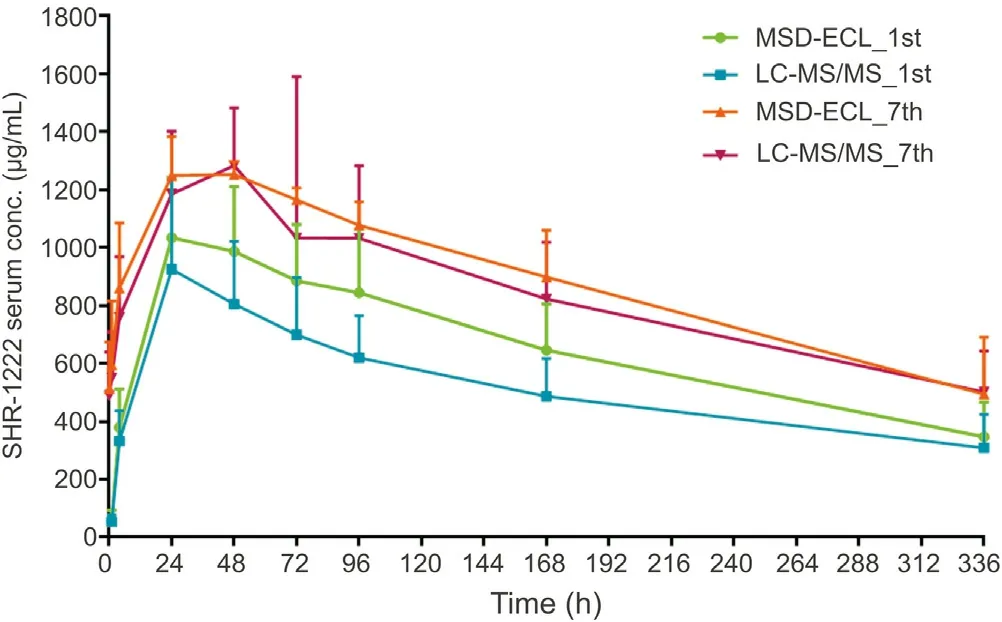
Fig. 4. The PK profiles of mean serum concentrations of SHR-1222 vs time after subcutaneous injection to cynomolgus monkeys. The monkeys were subcutaneously injected with SHR-1222 at 60 mg/kg (medium-dose group) every 2 weeks for 7 times and the profiles after the 1st and the 7th administration are shown here (MSD-ECL results are from the previous published study [29] and are shown here only for comparison with LC-MS/MS more intuitively). n =6.
As stated earlier, in the absence of pharmacodynamics data or safety biomarkers,the investigation of the influence of ADA on the overall drug level is necessary. PK assessment using ADA-tolerant methods, such as LC-MS/MS, may serve as a basis for determining the presence or absence of ADA-induced accumulation of bioactive drugs. On the other hand, sclerostin is a soluble target, and the antagonistic therapeutic mAbs against soluble targets generally do not produce long-term biological activities due to the impact of ADA. This is probably due to that they are often neutralized or cleared rapidly after binding to ADA. In this study, the similar detection results between LC-MS/MS and MSD-ECL methods were in agreement with the characteristics of this type of drugs.
3.6. Method comparison
Although both LC-MS/MS and MSD-ECL methods can be used to measure the serum concentrations of SHR-1222 in cynomolgus monkeys (the ratio of AUC0-t/AUC0-∞>0.8), the specificity and dynamic range of the developed LC-MS/MS method were remarkably improved compared to those of MSD-ECL method. LBA-based method usually involves the time-consuming step of acquiring reagents, including a particular antibody.Interestingly,the LC-MS/MS method provided a more rapid method development andenhanced assay specificity. Besides, a simpler pre-treatment process was developed for the present LC-MS/MS method compared to the MSD-ECL method,which met the specific requirements of highthroughput TK analysis.In comparison with the MSD-ECL method,the present LC-MS method was relatively efficient and costeffective. Low sensitivity, however, is the major defect of LC-MS/MS method for large molecular drug quantitation. On the one hand, this is due to the high homogeneity of plasma proteins in matrix although the multiple reaction monitoring mode can improve the detection sensitivity to some extent. On the other hand, peptides tend to carry multiple charges that lead to the reduced ionization efficiency and dispersed signal. Although lowflow LC-MS (such as nano-LC-MS) and hybrid-LC/MS methods have been developed to enhance the detection sensitivity,they are at the cost of low reproducibility and throughput.Therefore,a more technical innovation is needed and the balance between sensitivity and throughput should be achieved according to the analytical requirement.In the present work, the sensitivity of our LC-MS/MS method could meet the quantitative requirements due to the high concentration of monoclonal antibody detected in vivo.

Table 4 Mean pharmacokinetic parameters of SHR-1222 after two-weekly subcutaneous administration of 60 mg/kg to monkeys (MSD-ECL results are from the previous published study [29] and are shown here for comparison with LC-MS/MS method). n =6.
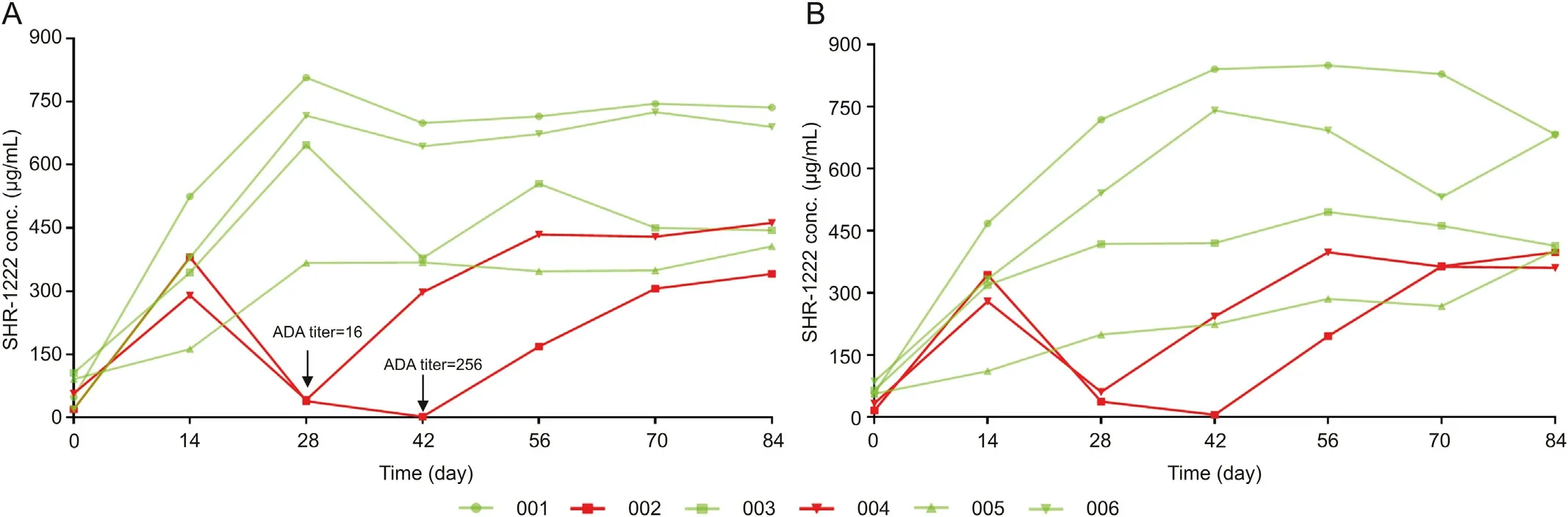
Fig.5. The profiles of individual serum concentrations of SHR-1222 vs time after subcutaneous injection to cynomolgus monkeys.(A)MSD-ECL method,and(B)LC-MS/MS method.The monkeys were subcutaneously injected with SHR-1222 at 60 mg/kg (medium-dose group) every 2 weeks for 7 times. The valley concentrations of each administration are connected, which only illustrates the effect of ADA and does not reflect the actual pharmacokinetics.
4. Conclusions
In this work, we described a new LC-MS/MS method (ADAtolerant) for the determination of SHR-1222 in cynomolgus monkeys.The method was validated in accordance with EMA and CFDA guidelines,and was successfully applied to a TK study.The suitable surrogate peptide was selected accurately and rapidly. The quantification results of LC-MS/MS method were compared with those of a previously established MSD-ECL method.It was noted that the measured concentrations of SHR-1222 displayed an excellent agreement between the above two methods. Two monkeys in the 60 mg/kg dose group had abnormal profiles with extremely low detection values of SHR-1222 in their individual sample.Combining the high-level ADAs in these samples and consistent quantitative results of the two methods, we found that the decreased concentration of SHR-1222 was due to the accelerated clearance mediated by ADAs rather than the interference of ADAs to the detection platform. In addition, LC-MS/MS method exhibited obvious advantages in its specificity and dynamic range, and was more costeffective and efficient than LBA method. Therefore, it could turn into a powerful tool for improving the developmental process of antibody drugs.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 81521005) and the National Key Research Project of the Chinese Academy of Sciences (Grant No.XDA12050306).
 Journal of Pharmaceutical Analysis2021年4期
Journal of Pharmaceutical Analysis2021年4期
- Journal of Pharmaceutical Analysis的其它文章
- A simplified LC-MS/MS method for the quantification of the cardiovascular disease biomarker trimethylamine-N-oxide and its precursors
- Reducing SARS-CoV-2 pathological protein activity with small molecules
- UHPLC-MS/MS analysis of cAMP and cGMP in rat plasma as potential biomarkers of Yin-Yang disharmony in traditional Chinese medicine
- Plasma-metabolite-based machine learning is a promising diagnostic approach for esophageal squamous cell carcinoma investigation
- Simultaneous determination of fourteen components of Gumiganghwal-tang tablet in human plasma by UPLC-ESI-MS/MS and its application to pharmacokinetic study
- Development of the general chapters of the Chinese Pharmacopoeia 2020 edition: A review
