亚致死浓度吡虫啉对麦二叉蚜多个世代生命表的影响
谢佳燕,吴聪,林佳,李睿
摘要:【目的】探究不同吡蟲啉亚致死浓度处理麦二叉蚜[Schizaphis graminum(Rondani)]后对其试验种群亲代及连续2个子代世代的影响,以揭示麦二叉蚜多个世代对吡虫啉亚致死浓度的响应,为田间合理用药提供理论依据。【方法】采用不同亚致死浓度(LC20和LC40)吡虫啉处理当代麦二叉蚜后,利用生命表技术分析经吡虫啉亚致死浓度胁迫后麦二叉蚜亲代(F0)及连续2个子代世代(F1和F2)存活及生命表参数的变化。【结果】不同亚致死浓度吡虫啉对麦二叉蚜的存活和发育影响不同,与对照相比,LC20浓度吡虫啉对麦二叉蚜亲代种群及子代种群的存活率和寿命均无显著影响(P>0.05,下同);LC40浓度吡虫啉显著降低亲代麦二叉蚜种群的中位生存期(P<0.05,下同),为对照的71.55%,但对2个子代种群的中位生存期无显著影响;不同世代间,LC40浓度处理的存活率和寿命随世代数的增加而增加。与对照相比,LC20浓度吡虫啉对麦二叉蚜亲代及2个子代种群的繁殖均无显著影响;LC40浓度吡虫啉可显著抑制麦二叉蚜亲代种群的繁殖,但对F1及F2世代种群的繁殖无显著影响。生命表分析结果表明,与对照相比,2个亚致死浓度吡虫啉对麦二叉蚜同一子代世代的种群生命表参数均无显著影响;同一亚致死浓度吡虫啉对2个子代世代的种群生命表参数也无显著影响。【结论】不同亚致死浓度(LC20和LC40)吡虫啉对亲代麦二叉蚜的生存率、寿命和繁殖率存在显著影响,且呈明显的剂量效应,但对2个子代种群的生命表参数均无显著影响。
关键词: 麦二叉蚜;吡虫啉;胁迫;存活率;生命表
中图分类号:S433 文献标志码: A 文章编号:2095-1191(2021)09-2375-07
Effects of sublethal concentrations of imidacloprid on the transgenerational life table characteristics of Schizaphis graminum (Rondani)
XIE Jia-yan, WU Cong, LIN Jia, LI Rui
(School of Life Science and Technology, Wuhan Polytechnic University, Wuhan 430023, China)
Abstract:【Objective】The effects of the imidacloprid on the parental generation and two successive filial progeny of the Schizaphis graminum(Rondani) were assessed. It was beneficial for revealing the response of an experimental population of S. graminum to sublethal concentrations of imidacloprid through multiple generations, which could provide a theoretical basis for the reasonable usage of insecticides in the field. 【Method】The life table and survival characteristics of S. graminum in the parental generation (F0) and two successive filial progeny (F1 and F2) were detected after the parental individuals have been exposed to different sublethal concentrations of imidacloprid(LC20 and LC40). 【Result】Different concentrations of imidacloprid exerted different influences on the survival rate and growth of S. graminum. The LC20 concentration of imidacloprid had no significant effect on the survival rate and median survival time of the parental and successive two filial generations of S. graminum populations(P>0.05, the same below). However,the LC40 concentration of imidacloprid in the F0 generation significantly reduced the survival rate of aphids(P<0.05, the same below), and median survival time of the LC40 group was only 71.55% compared with the control aphids. However, there was no significant effect on the median survival of the two offspring populations. The survival rate and longevity of the LC40 group increased with numbers of generations in three successive generations. The LC20 concentration of imidacloprid had no significant effect on the fecundity of the parental and successive two filial generations of S. graminum populations compared with control. Moreover, the LC40 concentration of imidacloprid significantly reduced the fecundity of aphids in the F0 generation,while without a significant effect on the fecundity of S. graminum populations in the F1 and F2 generations. In addition, within generations, the values of life table characteristics of S. graminum in the LC20 or LC40 group were no significantly different compared with those of the control of both two successive filial generations. Moreover,the values of life table characteristics of aphids to the same sublethal concentrations of imidacloprid(the LC20 or LC40 group) were not significantly different between their two successive filial progeny. 【Conclusion】The results suggests that the survival rates, life span, and fecundity of parental aphids are significantly different from groups(LC20 and LC40) exposed the two sublethal concentrations of imidacloprid with obvious dose effect, while this effect on life table characteristics of S. graminum in two treated groups is not significantly different in the two successive filial generations.
Key words: Schizaphis graminum(Rondani); imidacloprid; stress; survival rate; life table
Foundation item: National Natural Science Foundation of China(31201729); College Students Innovation and Entrepreneurship Training Program of Hubei(S202010496022); Teaching Research Project of Wuhan Polytechnic University(XM2020004)
0 引言
【研究意义】麦二叉蚜[Schizaphis graminum (Rondani)]是一种广泛分布的害虫,也是我国北方小麦产区的优势种,可直接危害麦类作物并能传播大麦黄矮病毒,引起麦类作物大量减产,导致严重的经济损失(曹雅忠等,2006)。目前,化学防治仍是麦蚜综合治理的主要措施,但长期不合理使用化学农药,导致麦蚜抗药性水平显著上升(彭丽年等,2000;Bass et al.,2015)。杀虫剂在田间喷洒后,随着时间的推移,可逐步降解,并逐渐递减至亚致死浓度,从而对田间害虫产生亚致死效应(王泽华等,2017;韩瑞等,2020)。有效评估杀虫剂对害虫产生的亚致死效应,可更好地理解害虫对杀虫剂的响应,对于指导田间科学合理用药具有重要的实践意义。【前人研究进展】亚致死浓度的杀虫剂不会直接杀死整个害虫种群,但可对害虫的行为、生长发育及生理生态等生物学特征产生影响(Koo et al.,2015;汝阳等,2017;Rix and Cutler,2018;辛天蓉等,2019;Ullah et al.,2020),还可通过作用于害虫的生存和繁殖参数对害虫的迁移和种群动态产生进一步影响(Ayyanath et al.,2013;Rix et al.,2016;Cao et al.,2019;Hunn et al.,2019),并影响到未接触杀虫剂的后代(Costa et al.,2014)。研究发现,LC25浓度的吡虫啉可显著降低F0代瓜蚜(Aphis gossypii)的寿命、繁殖力、内禀增长率和净增殖率,并延长其种群倍增时间,对其种群增长产生负面影响(Jam et al.,2014)。桃蚜(Myzus persicae)种群经吡虫啉亚致死剂量胁迫后,可降低其F0代成蚜的繁殖力并缩短其寿命,降低F1代种群的内禀增长率、净增殖率和周限增长率,延长其平均世代历期和种群加倍时间,压低种群趋势指数,对桃蚜具有持续控制作用(曾春祥等,2006)。采用不同亚致死浓度吡虫啉处理禾谷缢管蚜(Rhopalosiphum padi)后,可明显抑制禾谷縊管蚜种群的增长,并降低F1代的内禀增长率、周限增长率和净增殖率,延长种群倍增时间(Li et al.,2018)。另有研究发现,低浓度吡虫啉还可刺激害虫的繁殖能力,导致田间害虫再猖獗(James and Price,2002;Cordeiro et al.,2013;Guedes and Cutler,2014;Cao et al.,2019),且这种影响还能延迟到继代才出现,从而导致害虫种群暴发滞后(Cutler et al.,2009;Cutler,2013;Rix et al.,2016)。亚致死浓度(LC15及LC5)的吡虫啉虽然显著降低了当代棉蚜(A. gossypii)的寿命和繁殖力,但却显著提高其F1代的寿命、繁殖力、内禀增长率和净增殖率(Ullah et al.,2019)。【本研究切入点】目前,有关杀虫剂亚致死效应的研究主要集中在当代和子一代种群,其对害虫更长世代种群参数影响的研究报道较少。【拟解决的关键问题】采用生命表的方法分析亚致死浓度吡虫啉对麦二叉蚜亲代及连续2个子代种群生长和繁殖的影响,探讨吡虫啉对麦二叉蚜的亚致死效应,为后期在田间科学、合理使用吡虫啉防治麦二叉蚜提供理论依据。
1 材料与方法
1. 1 试验材料
麦二叉蚜种群于2011年采集自拉萨市麦田,于实验室内未接触任何药剂人工饲养至2020年。采用盆栽普通小麦苗饲养于人工气候培养箱内,饲养条件为温度(24±1)℃、光照周期14L∶10D、湿度60%~70%(下同)。供试95.3%吡虫啉原药为江苏常隆化工有限公司产品。
1. 2 吡虫啉亚致死浓度确定
采用浸渍法(张晓宁等,2014)进行室内生物测定。选择约100头麦二叉蚜成蚜接于新鲜麦苗上,置于人工气候培养箱内饲养,24 h后弃成蚜留若蚜继续饲养,以第4 d的麦二叉蚜作为试虫。将杀虫剂用丙酮配制5 mg/mL的母液,再用含0.1% Trition-100的蒸馏水稀释成不同浓度梯度。将麦二叉蚜连同小麦叶片放于细纱小笼中浸于预先稀释的吡虫啉溶液中,10 s后取出,滤纸吸去多余药液,放入置有滤纸的培养皿中,于人工气候培养箱中饲养。药剂处理24 h后统计麦二叉蚜的存活和死亡个体数,以毛笔轻触虫体,试虫不动记为死亡,计算死亡率和校正死亡率。以不含药剂的处理为对照,每处理30头麦二叉蚜,每个浓度3次重复。
1. 3 生命表组建
选择约100头麦二叉蚜成蚜接于新鲜麦苗上,置于人工气候培养箱内饲养,24 h后弃成蚜留若蚜继续饲养,于第4 d按照生物测定方法用亚致死浓度的吡虫啉处理该试虫(F0世代),以不含药剂的处理为对照。处理24 h后,随机选取存活个体接于新鲜麦苗上并于人工气候培养箱内单头单株饲养。每日8:00记录麦二叉蚜存活、死亡及产蚜数,并将每日新产子代蚜虫计数后挑离麦苗,至成蚜死亡。子一代(F1世代)及子二代(F2世代)试虫均为其前一世代试虫所产第1批若蚜进行单头单株饲养,并按照相同的方法进行观察和记录。每处理3个重复,每个重复15~20头蚜虫。
1. 4 统计分析
试验数据采用SPSS 14.0进行毒力回归方程及统计分析。对不同吡虫啉亚致死浓度处理组和不同世代进行单因素方差分析(One-way ANOVA),采用Student-Newman-Keuls (SNK)多重比较检验法进行多重比较。采用Weibull分布的生存函数进行存活曲线拟合和计算(梁霞等,2018),生存函数公式为Sp(t)= exp[-(t/b)c],式中,t表示存活时间,b为尺度参数(Scale parameter),c为形状参数(Shape parameter)。采用Wilcoxon (Gehan)进行生存时间的比较。生命表构建参照徐汝梅和成新跃(2005)的方法,分别计算麦二叉蚜各处理组种群的净增殖率(R0)、内禀增长率(rm)、周限增长率(λ)和种群倍增时间等参数。
2 结果与分析
2. 1 吡虫啉对麦二叉蚜的亚致死浓度
根据室内生物测定结果,得到吡虫啉对麦二叉蚜的亚致死浓度(表1)。其毒力回归方程的Perarson拟合度检验为x 2=1.26,P=0.74。分别采用3.94和9.46 mg/L的吡虫啉浓度作为处理的亚致死浓度。
2. 2 亚致死浓度吡虫啉对麦二叉蚜不同世代存活曲线参数的影响
将麦二叉蚜用不同亚致死浓度吡虫啉处理,测定其亲代及连续2个子代世代麦蚜种群存活曲线的变化,结果(表2)表明,同一世代中,不同處理间存活曲线的c值无显著差异(P>0.05,下同),且不同世代中麦二叉蚜种群的c值均大于1,为I型存活曲线,表明在不同亚致死浓度吡虫啉胁迫下,麦蚜个体的死亡主要发生在生长发育后期。
同一世代中,F0世代LC20处理的b值与CK无显著差异,而LC40处理的b值显著低于CK(P<0.05,下同);F1及F2世代中,LC20和LC40处理的b值均与CK无显著差异。不同世代中,LC20处理各世代间b值无显著差异(F=3.92,P=0.08);LC40处理随世代数增加b值呈递增趋势,且差异显著(F=12.77,P=0.007),表明LC40处理F0世代麦二叉蚜的存活率显著低于F1和F2世代。
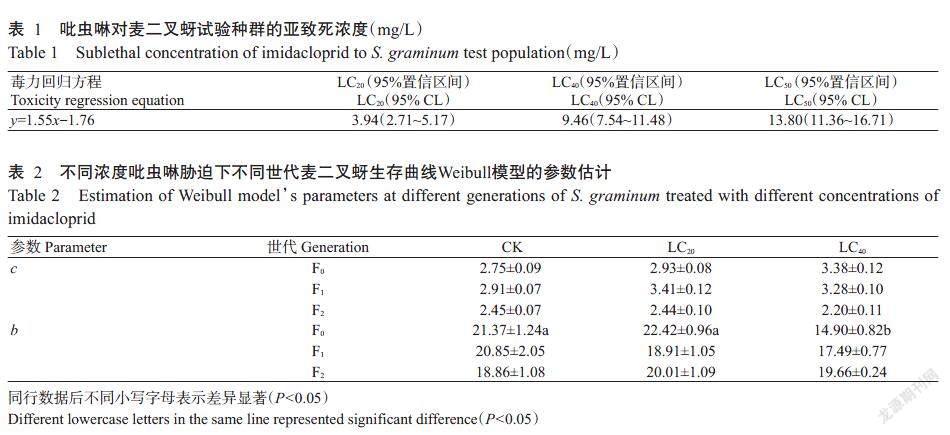
2. 3 亚致死浓度吡虫啉对麦二叉蚜生存的影响
由图1可知,同一世代中,不同亚致死浓度吡虫啉对麦二叉蚜F0世代种群的中位生存期产生显著影响,LC20处理试虫的中位生存期较CK增加7.32%,而LC40处理试虫的中位生存期显著缩短为CK的71.55%;F1和F2世代中,LC20处理和LC40处理试虫的中位生存期与CK无显著差异。不同世代间,LC20处理试虫F0世代的中位生存期显著高于F1及F2世代[Wilcoxon (Gehan)统计量为6.87,P=0.030];LC40处理试虫的中位生存期随世代数递增而增加,其F0世代中位生存期分别是F1及F2世代的87.50%[Wilcoxon (Gehan)统计量为1.77,P=0.180]和84.10%[Wilcoxon (Gehan)统计量为3.93,P=0.047]。
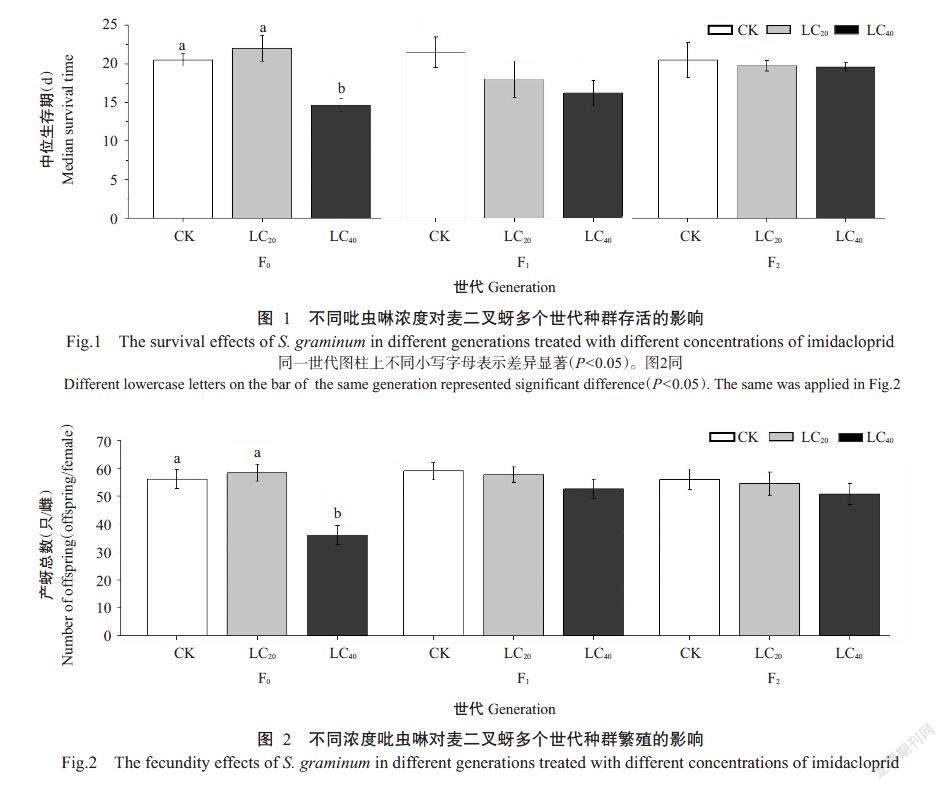
2. 4 亚致死浓度吡虫啉对麦二叉蚜繁殖的影响
采用不同亚致死浓度吡虫啉处理麦二叉蚜后检测对其亲代和子代繁殖的影响,结果(图2)表明,同一世代中,亚致死浓度吡虫啉显著影响F0世代麦二叉蚜的繁殖(F=13.70,P=0.00),LC40处理平均单雌产蚜总数显著低于CK和LC20处理,产蚜总数仅为CK的64.19%;F1和F2世代中,LC20处理和LC40处理试虫的单雌产蚜总数与CK无显著差异。不同世代间,LC20处理试虫的单雌产蚜总数在连续3个世代间无显著差异(F=0.36,P=0.70);而LC40处理试虫的每雌产蚜总数随世代数延长而显著增加(F=6.38,P=0.002),其F0世代单雌产蚜总数分别是F1和F2世代的68.51%和70.95%。
2. 5 亚致死浓度吡虫啉对麦二叉蚜不同世代生命表参数的影响
亲代麦二叉蚜暴露于不同亚致死浓度吡虫啉后,测定吡虫啉对其子代生命表参数的影响,结果(表3)发现,同一世代中,麦二叉蚜种群的LC20和LC40处理的生命表参数与对照组无显著差异;对于相同亚致死浓度处理组,所有生命表参数在2个世代间也无显著差异。
3 讨论
化学防治仍是目前我国田间作物害虫防治的重要措施。田间喷洒的杀虫剂会随着时间的延续逐渐递减至亚致死浓度,从而对昆虫产生亚致死效应(Rix et al.,2016;Cao et al.,2019)。众多研究表明,杀虫剂亚致死浓度可影响昆虫的发育、存活和生长水平(Rix and Cutler,2018;Ullah et al.,2020)。大豆蚜(A. glycines)种群经LC30吡虫啉胁迫后其存活率急剧下降(张傲楠等,2020)。LC30浓度吡虫啉能显著延长萝卜蚜(Lipaphis erysimi)的发育历期(李昭等,2018)。亚致死浓度杀虫剂对害虫生长和存活的影响可能因杀虫剂种类及使用杀虫剂浓度不同而有所差异(惠婧婧等,2009)。本研究发现,不同亚致死浓度吡虫啉会显著影响亲代麦二叉蚜的存活和生长,且不同浓度间存在差异,并存在明显的剂量效应;但对子代连续2个世代种群的存活均无显著影响。
亚致死浓度杀虫剂处理昆虫还可影响其生存及繁殖能力,昆虫通过生命表参数的不同响应,从而对其种群数量波动产生影响(Ayyanath et al.,2013;Rix et al.,2016;Cao et al.,2019;Hunn et al.,2019)。有研究发现,当昆虫暴露于杀虫剂的亚致死浓度后,可降低昆虫的生存及繁殖,影响种群的生命表参数,从而抑制种群数量的增长(Miao et al.,2014;张傲楠等,2020)。LC30浓度吡虫啉处理大豆蚜后,其繁殖率降低,净生殖率、内禀增长率及周限增长率明显降低,种群倍增时间延长,种群数量的增长受到抑制(张傲楠等,2020)。以不同亚致死浓度吡虫啉处理豌豆蚜(Acyrthosi phonpisum)可导致豌豆蚜寿命和繁殖力均显著低于对照组,且对当代和下一代豌豆蚜均表现为抑制作用(惠婧婧等,2009)。麦长管蚜(Sitobion avenae)取食以LC10浓度吡虫啉处理的小麦后,其生长和繁殖力与对照组无明显差异,但经LC50浓度处理后,其生长和繁殖力显著降低(Miao et al.,2014)。本研究发现,经LC40浓度吡虫啉处理后,可导致亲代麦二叉蚜的单雌产蚜量显著降低;LC20浓度吡虫啉对亲代麦蚜的单雌产蚜量无显著影响。但2个亚致死浓度吡虫啉对2个子代麦二叉蚜的种群生命表参数均无显著影响。采用环氧虫啶处理棉蚜后,也发现其亚致死浓度能显著缩短当代蚜虫的产卵期,但对F1代种群的生命表参数未产生明显影响(Cui et al.,2018)。因此,亚致死浓度杀虫剂对昆虫生长、发育和生命表参数的影响与杀虫剂剂量、昆虫响应世代数和昆虫种类密切相关。
4 结论
不同亚致死浓度(LC20和LC40)吡虫啉对亲代麦二叉蚜的生存率、寿命和繁殖率存在显著影响,且呈明显的剂量效应,但对2个子代种群的生命表参数均无显著影响。实践过程中,通过评估害虫多个世代种群对不同杀虫剂亚致死浓度胁迫的响应,对于指导田间合理用药具有重要的实践意义。
参考文献:
曹雅忠,尹姣,李克斌,张克诚,李贤庆. 2006. 小麦蚜虫不断猖獗原因及控制对策的探讨[J]. 植物保护,32(5):72-75. [Cao Y Z,Yin J,Li K B,Zhang K C,Li X Q. 2006. Discussion on causes of continuous aphid of wheat aphids and control counter measures[J]. Plant Protection,32(5):72-75.] doi:10.3969/ j.issn.0529-1542.2006.05.022.
韩瑞,杨帆,吴梅,肖春. 2020. 丹皮酚對马铃薯块茎蛾成虫的亚致死效应及其对生殖行为的影响[J]. 江西农业学报,32(12):55-59. [Han R,Yang F,Wu M,Xiao C. 2020. Sublethal effect of paeonol on adult potato tuber moth and its effect on reproductive behavior[J]. Acta Agriculturae Jiangxi,32(12):55-59.] doi:10.19386/j.cnki.jxnyxb.2020. 12.11.
惠婧婧,刘长仲,孟银凤,陈洁. 2009. 吡虫啉对豌豆蚜的亚致死效应[J]. 植物保护,35(5):86-88. [Hui J J,Liu C Z,Meng Y F,Chen J. 2009. Sublethal effects of imidacloprid to Acyrthosiphon pisum[J]. Plant Protection,35(5):86-88.] doi:10.3969/j.issn.0529-1542.2009.05.019.
李昭,郭圆,刘勇,王小平,朱智慧. 2018. 亚致死剂量杀虫剂对两种蚜虫翅型、发育和生殖的影响[J]. 应用昆虫学报,55(5):896-903. [Li Z,Guo Y,Liu Y,Wang X P,Zhu Z H. 2018. The effects of sublethal levels of insecticide on the wing dimorphism,development and reproduction of two aphid species[J]. Chinese Journal of Applied Entomology,55(5):896-903.] doi:10.7679/j.issn.2095-1353.2018.109.
梁霞,赵贝,李媛,胡祖庆,赵惠燕. 2018. 紫外长期胁迫对高世代麦长管蚜生命表参数的影响[J]. 生态学报,38(17):6228-6234. [Liang X,Zhao B,Li Y,Hu Z Q,Zhao H Y. 2018. Effects of ultraviolet continuous processing on life table parameters of high generations of Sitobion avenae(Fabricius)[J]. Acta Ecologica Sinica,38(17):6228-6234.] doi:10.5846/stxb201709221699.
彭丽年,张小平,叶建生,左燕. 2000. 四川省麦长管蚜(Macrosiphum avenae F.)的抗药性研究[J]. 农药学学报,2(3):13-18. [Peng L N,Zhang X P,Ye J S,Zuo Y. 2000. Study of resistance to insecticides in Macrosiphum avenae F. in Sichuan Province[J]. Chinese Journal of Pesticide Science,2(3):13-18.] doi:10.3321/j.issn:1008-7303.2000.03.003.
汝阳,陈耀年,尚素琴,张新虎. 2017. 阿维菌素亚致死剂量对二斑叶螨解毒酶系的影响[J]. 甘肃农业大学学报,52(1):87-91. [Ru Y,Chen Y N,Shang S Q,Zhang X H. 2017. Effect of sublethal dose of avermectin on the activi-ties of detoxifying enzymes in Tetranychusurticae[J]. Journal of Gansu Agricultural University,52(1):87-91.] doi:10.13432/j.cnki.jgsau.2017.01.015.
王泽华,范佳敏,陈金翠,宫亚军,魏书军. 2017. 氟啶虫胺腈亚致死浓度对桃蚜生长和繁殖的影响[J]. 中国农业科学,50(3):496-503. [Wang Z H,Fan J M,Chen J C,Gong Y J,Wei S J. 2017. Sublethal effects of sulfoxaflor on the growth and reproduction of the green peach aphid Myzus persicae[J]. Scientia Agricultura Sinica,50(3):496-503.] doi:10.3864/j.issn.0578-1752.2017.03.008.
辛天蓉,練涛,李雪儿,王静,邹志文,夏斌. 2019. 亚致死浓度除虫脲对朱砂叶螨生长和繁殖的影响[J]. 应用昆虫学报,56(4):736-743. [Xin T R,Lian T,Li X E,Wang J,Zou Z W,Xia B. 2019. Sublethal effects of diflubenzuron on the growth and reproduction of an experimental population of Tetranychus cinnabarinus(Boisduval)(Acari:Tetranychidae)[J]. Chinese Journal of Applied Entomology,56(4):736-743.] doi:10.7679/j.issn.2095-1353. 2019.085.
徐汝梅,成新跃. 2005. 昆虫种群生态学[M]. 北京:科学出版社. [Xu R M,Cheng X Y. 2005. Insect population ecology[M]. Beijing:Science Press.]
曾春祥,王进军,曾智平,曹高. 2006. 吡虫啉亚致死剂量对桃蚜实验种群的胁迫效应[J]. 中国农学通报,22(12):335-338. [Zeng C X,Wang J J,Zeng Z P,Cao G. 2006. Impact of sublethal doses of imidacloprid on experimental population of Myzus persicae[J]. Chinese Agricultural Science Bulletin,22(12):335-338.] doi:10.3969/j.issn. 1000-6850.2006.12.078.
张傲楠,韩岚岚,赵奎军,张雯林,肖建飞,陈娟,高丽瞳. 2020. 大豆蚜对不同浓度吡虫啉药剂胁迫的适应性[J]. 应用昆虫学报,57(3):676-681. [Zhang A N,Han L L,Zhao K J,Zhang W L,Xiao J F,Chen J,Gao L T. 2020. Effects of semilethal and sublethal doses of imidacloprid on Aphis glycines(Hemiptera:Aphididae)[J]. Chinese Journal of Applied Entomology,57(3):676-681.] doi:10. 7679/j.issn.2095-1353.2020.068.
张晓宁,陈巨莲,程登发,曾建国,程辟,孙京瑞. 2014. 博落回提取物对3种麦蚜的生物活性[J]. 植物保护,40(3):187-190. [Zhang X N,Chen J L,Cheng D F,Zeng J G,Cheng P,Sun J R. 2014. Biological activity of Macleaya cordata extract against three species of wheat aphids[J]. Plant Protection,40(3):187-190.] doi:10.3969/j.issn.0529- 1542.2014.03.036.
Ayyanath M,Cutler G C,Scott-dupree C D,Sibley P K. 2013. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid[J]. PLoS One,8(9):e74532. doi:10.1371/journal.pone.0074532.
Bass C,Denholm I,Williamson M S,Nauen R. 2015. The global status of insect resistance to neonicotinoid insecticides[J]. Pesticide Biochemistry and Physiology,121:78-87. doi:10.1016/j.pestbp.2015.04.004.
Cao Y,Yang H,Li J,Wang C,Li C,Gao Y L. 2019. Sublethal effects of imidacloprid on the population development of western flower thrips Frankliniella occidentalis(Thysanoptera:Thripidae)[J]. Insects,10(1):3. doi:10. 3390/insects10010003.
Cordeiro E M G,de Moura I L ,Fadini M A,Guedes R N. 2013. Beyond selectivity:Are behavioral avoidance and hormesis likely causes of pyrethroid-induced outbreaks of the southern red mite Oligonychus ilicis?[J]. Chemosphere,93(6):1111-1116. doi:10.1016/j.chemosphere. 2013.06.030.
Costa M A,Moscardini V F,da Costa Gontijo P,Carvalho G A,de Oliveira R L,de Oliveira H N. 2014. Sublethal and transgenerational effects of insecticides in developing Trichogramma galloi(Hymenoptera:Trichogrammatidae)[J]. Ecotoxicology,23:1399-1408. doi:10.1007/s10646-014-1282-y.
Cui L,Yuan H Z,Wang Q Y,Wang Q Q,Rui C H. 2018. Sublethal effects of the novel cis-nitromethylene neonicotinoid cycloxaprid on the cotton aphid Aphis gossypii Glo-ver(Hemiptera:Aphididae)[J]. Scientific Reports,8:8915. doi:10.1038/s41598-018-27035-7.
Cutler G C. 2013. Insects,insecticides and hormesis:Evidence and considerations for study[J]. Dose Response,11(2):154-177. doi:10.2203/dose-response.12-008.
Cutler G C,Ramanaidu K,Astatkie T,Isman M B. 2009. Green peach aphid,Myzus persicae (Hemiptera:Aphididae),reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin[J]. Pest Management Science,65(2):205-209. doi:10.1002/ps.1669.
Guedes R N,Cutler G C. 2014. Insecticide-induced hormesis and arthropod pest management[J]. Pest Management Science,70(5):690-697. doi:10.1002/ps.3669. Epub 2013 Dec 2.
Hunn J G,Macaulay S J,Matthaei C D. 2019. Food shortage amplifies negative sublethal impacts of low-level exposure to the neonicotinoid insecticide imidacloprid on stream mayfly nymphs[J]. Water,11(10):2142. doi:10.3390/w11102142.
Jam N A,Kocheyli F,Mossadegh M S,Rasekh A,Saber M. 2014. Lethal and sublethal effects of imidacloprid and pirimicarb on the melon aphid,Aphis gossypii Glover (Hemiptera:Aphididae) under laboratory conditions[J]. Journal of Crop Production,3(1):89-98.
James D G,Price T S. 2002. Fecundity in two spotted spider mite (Acari:Tetranychidae) is increased by direct and systemic exposure to imidacloprid[J]. Journal of Economic Entomology,95(4):729-732. doi:10.1603/0022-0493-95.4.729.
Koo H,Lee S,Yun S,Kim H K,Kim G. 2015. Feeding response of the cotton aphid,Aphis gossypii,to sublethal rates of flonicamid and imidacloprid[J]. Entomologia Experimentalis Applicata,154(2):110-119. doi:10.1111/eea. 12260.
Li W Q,Lu Z B,Li L L,Yu Y,Dong S,Men X Y,Ye B H. 2018. Sublethal effects of imidacloprid on the performance of the bird cherry-oat aphid Rhopalosiphum padi[J]. PLoS One,13(9):e0204097. doi:10.1371/journal.pone.0204097.
Miao J,Du Z B,Wu Y Q,Gong Z J,Jiang Y L,Duan Y,Li T,Lei C L. 2014. Sub-lethal effects of four neonicotinoid seed treatments on the demography and feeding beha-viour of the wheat aphid Sitobion avenae[J]. Pest Manage-ment Science,70(1):55-59. doi:10.1002/ps.3523.
Rix R R,Ayyanath M M,Cutler G C. 2016. Sublethal concentrations of imidacloprid increase reproduction,alter expression of detoxification genes,and prime Myzus persicae for subsequent stress[J]. Journal of Pest Science,89(2):581-589. doi:10.1007/s10340-015-0716-5.
Rix R R,Cutler G C. 2018. Does multigenerational exposure to hormetic concentrations of imidacloprid precondition aphids for increased insecticide tolerance?[J]. Pest Management Science,74(2):314-322. doi:10.1002/ps.4731.
Ullah F,Gul H,Desneux N,Gao X W. 2019. Imidacloprid-induced hormesis effects on demographic traits of the melon aphid,Aphis gossypii[J]. Entomologia Generalis,39(3-4):325-337. doi:10.1127/entomologia/2019/0892.
Ullah F,Gul H,Tariq K,Desneux N,Gao X W,Song D L. 2020. Thiamethoxam induces transgenerational hormesis effects and alteration of genes expression in Aphis gossypii[J]. Pesticide Biochemistry and Physiology,165:104557. doi:10.1016/j.pestbp.2020.104557.
(責任编辑 麻小燕)

