Potential role of an athlete-focused echocardiogram in sports eligibility
Stefano Palermi,Alessandro Serio,Marco Vecchiato,Felice Sirico,Francesco Gambardella,Fabrizio Ricci,Franco Iodice,Juri Radmilovic,Vincenzo Russo,Antonello D'Andrea
Stefano Palermi,Alessandro Serio,Felice Sirico,Francesco Gambardella,Public Health Department,University of Naples Federico II,Naples 80131,Italy
Marco Vecchiato,Sport and Exercise Medicine Division,Department of Medicine,University Hospital of Padova,Padova 35128,Italy
Fabrizio Ricci,Department of Neuroscience,Imaging and Clinical Sciences,“G.d’Annunzio”University of Chieti-Pescara,Chieti 66100,Italy
Fabrizio Ricci,Department of Clinical Sciences,Faculty of Medicine,Lund University,Clinical Research Center,Malmö 21428,Sweden
Fabrizio Ricci,Casa di Cura Villa Serena,Pescara 65013,Italy
Franco Iodice,Vincenzo Russo,Antonello D'Andrea,Unit of Cardiology,Department of Translational Medical Sciences,University of Campania “Luigi Vanvitelli”,Monaldi Hospital,Naples 80131,Italy
Juri Radmilovic,Antonello D'Andrea,Unit of Cardiology and Intensive Coronary Care,“Umberto I” Hospital,Nocera Inferiore 84014,Italy
Abstract Sudden cardiac death (SCD) of an athlete is a rare but tragic event and sport activity might play a trigger role in athletes with underlying structural or electrical heart diseases.Preparticipation screenings (PPs) have been conceived for the potential to prevent SCD in young athletes by early identification of cardiac diseases.The European Society of Cardiology protocol for PPs includes history collection,physical examination and baseline electrocardiogram,while further examinations are reserved to individuals with abnormalities at first-line evaluation.Nevertheless,transthoracic echocardiography has been hypothesized to have a primary role in the PPs.This review aims to describe how to approach an athlete-focused echocardiogram,highlighting what is crucial to focus on for the different diseases (cardiomyopathies,valvulopathies,congenital heart disease,myocarditis and pericarditis) and when is needed to pay attention to overlap diagnostic zone (“grey zone”) with the athlete's heart.Once properly tested,focused echocardiography by sports medicine physicians may become standard practice in larger screening practices,potentially available during first-line evaluation.
Key Words:Echocardiogram;Athletes;Sport eligibility;Pre-participation screening;Sudden cardiac death;Sport cardiology
INTRODUCTION
The advantages of sport practicing in improving cardiovascular (CV) health are evident,but a concomitant raise in CV events has been seen during exercise[1].Physical activity therefore is a double-edge sword,with both benefits and risks mostly in predisposed subjects,even if not all people are conscious about it[2].
Sudden cardiac death (SCD) of an athlete is as uncommon as shocking event[3].Sport practice might be a trigger event of cardiac arrest in athletes with underlying heart diseases,with syncope as a frequent first manifestation[4].Indeed,these diseases are often clinically silent and unsuspecting[5].Athletes have an increased risk of SCD compared with their non-active counterpart,related to pathologies theoretically identifiable through pre-participation screening (PPS)[6].Therefore,several PPSs have been developed with the aim to uncover possible hidden and fatal CV condition in athletes.There is a significative debate about the efficacy and feasibility of different kind of PPSs proposed by national and international society worldwide.While the American Heart Association has never recommended a history collection and a physical examination,opposing the routine use of electrocardiogram (ECG)[7],the European Society of Cardiology (ESC) protocol focuses on history collection,physical examination,and ECG[8] and it has been adopted by different sport committees and international federations[9].Controversies arise regarding the sensitivity and specificity of individual tests as well as the availability of expertise and cost effectiveness[10-13].
Nevertheless,some cardiac structural diseases can be difficult to detect on physical examination and ECG,but they may definitely be recognized with further cardiac investigations.About 10% of SCD cases have been related to cardiac diseases showing structural but no electrical manifestations[14].While in Italy an exercise stress test is mandatory before engaging in competitive sports,transthoracic echocardiography has also been hypothesized to have a role in PPS:it might be a useful,convenient and noninvasive tool to increase diagnostic power of screening evaluation[15].Even if echocardiography is frequently adopted as a first-line screening tool for athletes[16],also by major sporting bodies such a Federation Internationale de Football Association(FIFA) or Union of European Football Association (UEFA),and mainly as a valuable second line investigation to the diagnose malignant cardiac conditions[17,18],it has never been recommended as a first-line screening tool in athletes[19,20],nor included in recent ESC 2020 guidelines on sport cardiology[3].Diagnostic necessity,time constrains and cost-effectiveness are probably unfavorable reasons for its use[21],even if there are only few data[22] regarding the cost-effectiveness of including an echocardiography in the PPS.
However,the use of echocardiography is highly increasing among non-cardiologist physicians and it could represent a powerful tool in the sport eligibility process by sport medicine physicians.
Therefore,the aim of the present paper is to review athlete-related echocardiographic features in order to develop a first-line screening athlete-focused echocardiogram.How should an echocardiography of the athlete be performed? What should a sport physician know about echocardiography? What would an athlete-focused echocardiogram be like?
EPIDEMIOLOGY AND ETIOLOGY OF SCD
SCD could literally be defined as “natural death due to cardiac causes,heralded by abrupt loss of consciousness within one hour after the onset of symptoms”[23].Current epidemiology of the incidence of SCD in professional athletes varies from almost 1/1000000 to 1/5000 subjects per year[24,25].Indeed,the definition of the precise frequency of SCD is made difficult by heterogeneous study methodologies.In literature,the incidence of SCD in young athletes is fluctuating[14,26-28],with a prevalence of CV predisposing conditions of about 0.2%-0.7%[29].
Several causes of SCD in athletes are related to the age of onset.In patients from birth to adolescence,the primary cause of SCD is a congenital abnormality[30].The great majority of young victims (age ≤ 35 years) present a concealed structural heart disease[31].Coronary artery disease is the most frequent cause of SCD in aged athletes(35 years or older)[32].Main causes of SCD are summarized in Table 1.

Table 1 Common cardiovascular diseases associated with sudden cardiac deaths in athletes (adapted from[15,21])
ECHOCARDIOGRAPHY IN ATHLETE’S HEART
Even if a minimum dataset is recommended for all echocardiograms[33] (Table 2),previous knowledge of some demographics data,patient’s personal history and physical examination,and ECG findings will help to focus the examination and the interpretation of findings:sex,age,body mass index,ethnicity,ECG changes,symptoms,training volume,type of sport and level,and family history of unexplained cardiac death<40 years[34].A focused echocardiogram with real-time interpretation can considerably cut the cost and time spent performing the study,as previously demonstrated[35].Indeed,Niederseeret al[21] proposed to include a double screening echocardiography in the athlete:in adolescence to rule out structural heart disease,and over the age of 30 to evaluate pathological cardiac remodeling to exercise,cardiomyopathies and wall motion anomalies.Often coronary artery disease is missed by classing screening method,so in a master athlete (>35 years) echocardiography detection of atherosclerotic plaque[36],could represent a valid example of a focused exam.

Table 2 Minimum dataset for transthoracic echocardiography (adapted from[33])

PLAX:Parasternal long axis view;PSAX:Parasternal short axis view;A4C:Apical 4 chambers view;A5C:Apical 5 chambers view;A2C:Apical 2 chambers view;LV:Left ventricle;BSA:Body surface area;EF:Ejection fraction;RVOT:Right ventricle outflow tract;RV:Right ventricle;FAC:Fractional area charge;LAVI:Left atrium ventricle index;LA:Left atrium;IVS:Inter ventricular septum;PW:Power doppler wave;CW:Continuous doppler wave;RA:Right atrium;STJ:Sinotubular junction;OCA:Origin of coronary arteries;PDA:Patent ductus arteriosus;COA:Aortic coarctation;ASD:Atria septum defect;VSD:Ventricular septal defect;RVD:Right ventricle diameter;TAPSE:Tricuspid annular plane excursion.
Moreover,prolonged physical activity causes structural,functional,and electrical heart modifications that represent the physiological responses (“adaptation”) of the heart during physical effort:this series of remodeling is named as “athlete's heart”[37]and it can be appreciated both in ECG[38] and in echocardiograms.These adaptations,involving all the heart chambers[39] and strictly dependent upon on the duration,type and intensity of training,are often benign and physiological but,sometimes,may predispose to pathological conditions[40,41].The challenges posed by athlete’s heart require detailed assessment in order to distinguish between physiological adaptation and life-threatening cardiomyopathies[16,42-44],that often coexist in the so called“grey zones”.
Also,novel advanced echocardiographic techniques play an emerging role in the echocardiographic investigation of athlete[41]:Exercise stress echocardiography in the differentiation between athlete’s heart and dilated cardiomyopathy,coronary artery disease and pulmonary hypertension;speckle tracking [with left ventricular (LV)global longitudinal strain] in the differentiation between athlete’s heart and dilated cardiomyopathy and hypertrophic cardiomyopathy,other than the characterization of wall motion abnormalities and right ventricle description;3D echocardiography in estimation of cardiac mass.
Therefore,echocardiography has a major role in sports cardiology,and it can help physicians not only to investigate structural diseases invisible both at anamnesis and ECG,but also to rule out these physio-pathological modifications.
ATHLETE-FOCUSED ECHOCARDIOGRAM
The concept of point-of-care ultrasound is increasingly emerging in literature[45],in different body systems[46,47],including heart.Even if it is crucial to consider the ultrasound scanner and the degree of competence and knowledge of the sonographer[48],a focused protocol for point-of-care echocardiography could be developed for physicians to help screen out major CV diseases afflicting athletes in granting sport eligibility.Such protocols may gain much more attention by the fact that nowadays,portable and handheld ultrasound devices are spreading and have proven to be equally reliable and accurate in evaluating cardiac features[49,50].
Several of athlete-focused echocardiography protocols have been proposed in literature[19,51,52] by different authors[53-55],and some are reported in Table 3.Some of them are direct to rule out specific diseases,while others are designed for global viewing but only last a few minutes.Moreover,several sportive federations,such as FIFA,have their own precompetition medical assessment that includes echocardiographic examination.

Table 3 Athlete-focused echo protocols
We want to propose our version of first-line athlete-focused echocardiography,as seen in Table 4 and Figures 1 and 2.Some measurements are intentionally missed:visually estimated left ventricular ejection fraction and wall motion has been found to be closely related to formal quantitative methods[56,57];valvular heart diseases can be firstly screened by color doppler[58].We also consider a visual assessment of cardiac chambers’ dimensions.All these first-line measurements,if pathological,will be eventually followed by other measurements (“second-line”),as seen in Table 5.

Figure 1 Parasternal long-axis,short axis and suprasternal windows showing left and right chambers and aortic valve and arch.

Figure 2 Apical 4-chamber,5-chamber and subcostal windows showing left and right chambers,inferior cava vein and pericardium.

Table 4 Proposed echocardiographic protocol for athletes
Below we will describe main echo findings and grey zone in the more frequent CV conditions in athletes (Table 5),with cut off based on latest recommendations[42].

Table 5 Main echo findings of cardiovascular pathologies in athletes

PLAX:Parasternal long axis view;PSAX:Parasternal short axis view;A4C:Apical 4 chambers view;HCM:Hypertrophic cardiomyopathy;DCM:Dilated cardiomyopathy;LVNC:Left ventricle non compaction;ARVC:Arrhythmogenic right ventricular cardiomyopathy;BAV:Bicuspid aortic valve;AOCA:Anomalous origin of coronary arteries;ASD:Atrial septum defects;VSD:Ventricular septal defects;PDA:Patent ductus arteriosus;CoA:Aortic coarctation;M:Male;F:Female;LV:Left ventricle;BSA:Body surface area;EF:Ejection fraction;LVOT:Left ventricle outflow tract;NC/C:Noncompact/compact;RVOT:Right ventricle outflow tract;RV:Right ventricle;FAC:Fractional area charge;PAPS:Pulmonary artery systolic pressure;LAVI:Left atrium ventricle index;RA:Right atrium;ToF:Tetralogy of fallot;TgA:Transposition of great arteries;DCRV:Double-chambered right ventricle.
CARDIOMYOPATHIES
The diagnosis of these cardiomyopathies and their differentiation with athlete’s heart still represent one of the biggest challenges of sport cardiology
HYPERTROPHIC CARDIOMYOPATHY
Hypertrophic cardiomyopathy (HCM) is one of the most common etiologies of SCD in athletes and represents one of the most frequent reason for denied sport eligibility according to both American and European guidelines[59-61].
Echocardiography is crucial for the diagnosis and monitoring of HCM.In the absence of another reason of hypertrophy,it can be diagnosed with a maximal enddiastolic wall thickness of ≥ 15 mm in any zone of the left ventricle;less marked hypertrophy (13-14 mm) can be diagnostic when present in relatives of a patient with HCM or along with a positive genetic test.For children,a threshold of z>2.5 may be useful to identify early HCM in asymptomatic subject with no family history,while for children with a positive family history or a positive genetic test,a threshold of z>2 could be sufficient for early diagnosis.
Measurements of LV wall thickness should be executed at end diastole,in parasternal long axis (PLAX) or in parasternal short axis (PSAX) views[15].
Grey zone:The diagnosis of HCM in competitive athletes may be difficult when LV wall thickness ranges from 13 to 16 mm[62-64].The overlap between athlete’s heart and HCM is challenging in Afro-American individuals[65].Indeed,the extreme value of LV hypertrophy in athletes is found in male (15 mm in white and 16 mm in Afro-American athletes[66]),while lower values are found in female (11 mm in whites and 13 mm in Afro-Americans[67]).The type and the intensity of sport practiced are also important aspects to consider.
Differential diagnosis features between HCM and athlete’s heart in the gray-zone is seen in Table 6.The most practical morphologic criterion is the assessment of LV cavity size and geometry,enlarged (end-diastolic diameter>54 mm) and with an eccentric pattern only in athlete’s heart[62,68].On the other side,an asymmetric and heterogeneous pattern of LV hypertrophy represents features of HCM[62].Furthermore,athletes usually show a homogeneous distribution of wall thickness and a normal diastolic function[69],besides a reduction of wall thickness after a detraining period.

Table 6 Differential diagnosis between hypertrophic cardiomyopathy and athlete’s heart,in the grey-zone (adapted from[42])
What to assess? LV wall thickness.If pathological,what to assess? LV end diastolic diameter + LV mass + LV outflow tract (LVOT) obstruction + LV wall thickness distribution + LV diastolic function (E/A).
DILATED CARDIOMYOPATHY
Dilated cardiomyopathy (DCM) is a not-so rare cause of SCD in athletes[70,71].It is defined as (left) ventricular systolic dysfunction and dilatation not explained by other loading conditions.Systolic dysfunction is measured by abnormal LV ejection fraction(EF) (through biplane Simpson’s rule).LV dilatation is defined by LV end-diastolic diameters>2 SD from normal,indexed by body surface area (BSA),age and gender.LV shape can provide additional information (“sphericity index”)[72].
Grey zones:Left ventricular cavity increasing in athletes should be interpreted in the context of sport played and it is usually associated with increased wall thickness[73,74].A significant dilation is often seen in endurance athletes.Almost 40% of athletes showed an increase of LV end diastolic diameter (>55 mm),and almost 10%even bigger cavity (>60 mm,with 99thpercentile corresponding to 65 mm).LV dilation in athletes is frequently presented with EF>55%.Moreover,indices of diastolic function are within normal values[75].In case of mildly reduced ejection fraction,it may be useful to perform a stress test echocardiography,along with the measure of stroke volume.
Differential diagnosis features between DCM and athlete’s heart in the grey zone is showed in Table 7.

Table 7 Differential diagnosis between dilated cardiomyopathy and athlete’s heart (adapted from[42])
What to assess? LV end diastolic diameter.If pathological,what to assess? EF.
LEFT VENTRICLE NON COMPACTION
Left ventricle non-compaction (LVNC) is a cardiomyopathy identified by increased myocardial trabeculations and inter-trabecular recesses[76,77],ranging clinically from asymptomatic to SCD outcomes.Diagnosis is based on three proposed echocardiographic criteria,showing high non-compacted to compacted ratio within the LV myocardium[78,79].
Grey zones:About 1 in 5 athletes has LV hypertrabeculation,with about 10%complying LVNC diagnostic criteria[80,81].This may be due to cardiac adaptation to load training.ESC guidelines on cardiovascular imaging[42] proposed the threshold for defining an LV trabeculations on echocardiography:a non-compacted to compacted (NC/C) layer ratio>2.0 in systole[82].
In athletes achieving criteria for LVNC,an history of cardiac symptoms or family SCD,are suggestive of pathology.Additional echocardiography features of LVNC include low EF not improving with exercise,altered diastolic function and a thickness of compacted layer in systole<8 mm.
Differential diagnosis features between LVNC and athlete’s heart in the gray-zone is showed in Table 8.

Table 8 Differential diagnosis between left ventricle non compaction and athlete’s heart,in the grey zone (adapted from[42])
What to assess? LV trabeculation.If pathological,what to assess? FE (Simpson) +thickness of compact layer in systole + LV diastolic function (E/A).
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a cardiomyopathy characterized by progressive waste of myocytes that are substituted by fibrofatty tissue.It typically (but not only) involves the right ventricle (RV).
Diagnostic imaging criteria have been defined in 2010[83]:Disproportionate enlargement of the right ventricle outflow tract (RVOT) and RV motion abnormalities are essential characteristics of this disease[84].
Grey zones:Long-term physical activity could promote the expression of ARVC:exercise remodeling includes RV (basal) enlargement and mild reduction of global RV peak systolic longitudinal strain[15].ESC guidelines on cardiovascular imaging[42]propose that only major dimensional criteria,indexed by BSA,should be used to define RV enlargement in athletes:specifically,RVOT>19 mm/m2in PLAX,and/or>21 mm/m2in PSAX.Other useful measures include the ratio of RV inflow dimension(in apical 4 chambers view (A4C))/LV end-diastolic dimension (PLAX)>0.9[85],the RV fractional area change (FAC)<33%[86],and a subclinical RV dysfunction with reduced RV global longitudinal strain[87].About 50% of ARVC subjects could have regional akinesia,dyskinesia,or aneurysmal deformation.
Differential diagnosis features between ARVC and athlete’s heart is seen in Table 9.
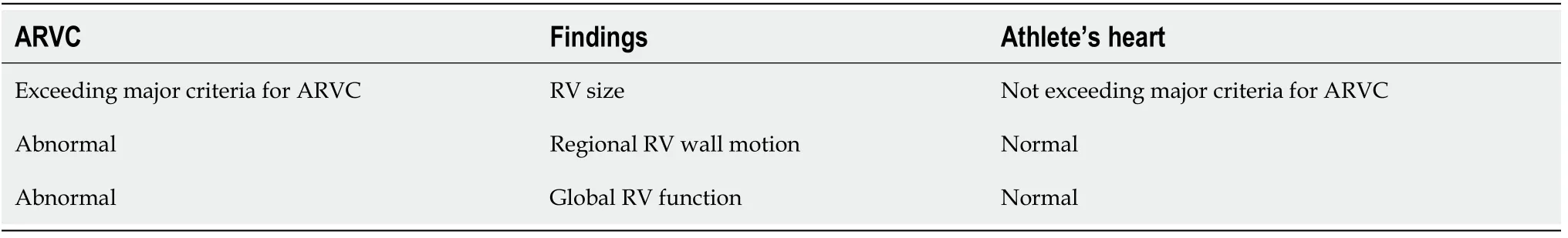
Table 9 Differential diagnosis between arrhythmogenic right ventricular cardiomyopathy and athlete’s heart,in the grey-zone (adapted from[42])
What to assess? RVOT PSAX + RVOT PLAX.If pathological,what to assess? RV inflow apical/LV end diastolic PLAX + RV regional wall abnormalities + RV FAC.
AORTIC DISEASES
Aortic pathologies,such as aortic dissection or aortic aneurism,are important etiologies of SCD in athletes[88,89] and could be related to Marfan disease.Practically,aortic root diameter measurements should be performed from the PLAX view using the leading edge-to-leading edge convention (except for the measurement of the annulus,in which it is used inner edge to inner edge convention) and preferring 2D measurements[90,91].Otherwise,in children and in subjects ≤ 25 years,aortic root dimensions should be carried out using the inner-wall-to-inner-wall technique during systole[92].Measurements should embrace the aortic valve annulus,the sinuses of Valsalva,the sinotubular junction and the proximal ascending aorta.
Grey zone:Remodeling of the aortic root in athletes is strictly related to the overload training[93-95].Large increases in aortic size are rare in athletes and often related to an underlying potentially fatal aortic disease[96].The American College of Cardiology and the American Heart Association have proposed the use of z-scores to identify subjects with significant aortic root dilation[97].If dilated aortic root dimensions are found,it is recommended to exclude the presence of associated conditions such as Marfan Syndrome of bicuspid aortic valve (BAV)[98,99].In athletes with dilated aortic root,a periodical diagnostic assessment to monitor dimension is recommended.
What to assess? aortic root dimension + ascending aortic diameter.If pathological,what to assess? BAV + aortic valve regurgitation.
VALVULOPATHIES
Mitral valve prolapse
Mitral valve prolapse (MVP) is a common valve abnormality in population that,in some case,has been associated to SCD especially in young individuals[100].Echocardiography,ideally in PLAX view,is essential for MVP diagnosis[40]:It is literally defined by “abnormal systolic bulging of one or both leaflets toward the left atrium(LA) with displacement of coaptation point into the LA (>2 mm beyond a line connecting the annular hinge point)”.Leaflets are usually elongated and thickened:“classic” MVP is characterized by a leaflet thickness ≥ 5 mm,while “non-classic” MVP is defined by leaflet thickness<5 mm.Furthermore,mitral annulus is usually enlarged.
Grey zone:MVP is one of the most frequent cause of mitral valve (MV)regurgitation in athletes[101].If rare,cases of malignant arrhythmic prolapse have been reported and therefore this is a must be ruled-out condition[102-104].Athletes usually has mild mitral regurgitation (MR),even if severe MR must be excluded(Table 10).Also pulmonary artery pressures (PAPS) and reverse flow in the pulmonary veins assessment should be performed.LV and LA dimensions must be evaluated to exclude cardiac chamber dilatation associated.
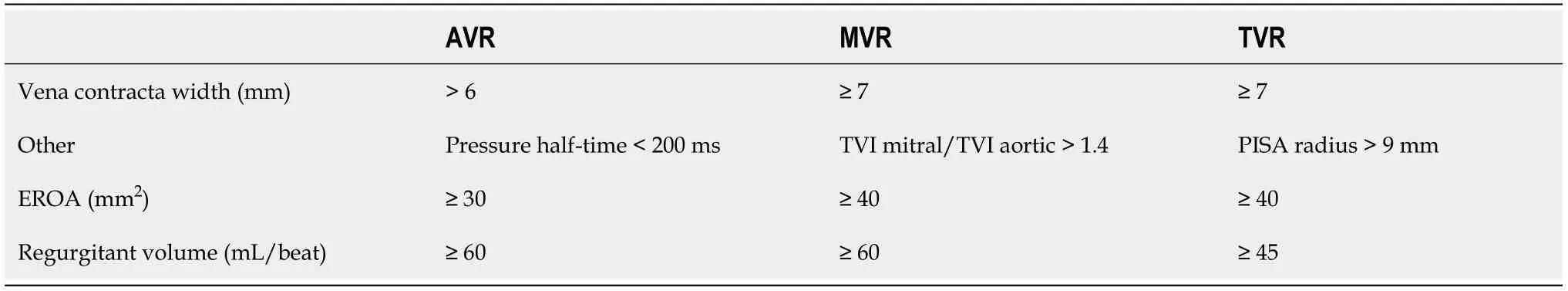
Table 10 Echocardiographic criteria for the definition of severe valve regurgitation (adapted from[165])
What to assess? MV prolapse.If pathological,what to assess? MR (vena contracta,CW jet area,PISA,MR flow,CW doppler,E wave) + PAPS + pulmonary vein flow +LV mass + LV function + LA dimension.
Other valvulopathies
Valvular heart disease is frequently related to degenerative processes and usually agerelated[14],but may also involve younger individuals.Moreover,specific recommendations exist for athletes with valvulopathies[105].Congenital defects are common cause of valvular heart disease in young individuals.Although cardiac magnetic resonance offers a better quality of imaging[106],these are conditions to be considered when performing an echocardiogram in athletes.Right-sided valvular pathologies and regurgitant lesions are better tolerated[107].
Valvular Stenosis
Aortic valve stenosis (AVS) counts for 5% of congenital heart defects,and is frequently caused by BAV[108].Even if mostly asymptomatic,these subjects have a small but significant risk of sudden death[109].Echocardiography is the gold standard diagnostic tool[110] (Table 11).

Table 11 Echocardiographic parameters indicative of the degree of severity of different valve stenosis (adapted from[111,165])
Mitral valve stenosis (MVS) in young individuals can be of rheumatic origin.It results in elevated LA pressure and eventually pulmonary hypertension.An exerciserelated cardiac output increase can lead to acute pulmonary edema[111].The degree of MVS can be categorized through echocardiography (Table 8).
Tricuspid valve stenosis (TVS) can be caused by rheumatic fever and is frequently associated with MVS,rather than alone[111].
Pulmonary valve stenosis accounts for about 10% of the congenital heart syndrome[112].It is commonly present in tetralogy of Fallot,Nooan syndrome and maternal rubella syndrome[113].Most of these patients are asymptomatic,but rarely SCD.Evaluation of pulmonary stenosis is best evaluated with echocardiography[114,115].
Valvular regurgitation
Valvular regurgitation is a common and often harmless condition in athletes.In particular,mitral and tricuspid regurgitation are widely diffused in young subjects[116].
The more common causes of aortic valve regurgitation (AVR) include BAV,rheumatic fever,endocarditis,Marfan syndrome and aortic dissection.AVR causes LV dilatation with subsequent LV pressure and volume loading.LV dilatation should be evaluated by echocardiography.Often LV cavity dimension can be raised in healthy athletes[111].Quantification of AVR is seen in Table 9.
There are many causes of mitral valve regurgitation (MVR),including MVP,endocarditis,rheumatic heart disease,coronary artery disease,and dilated cardiomyopathy[117].In athletes,mitral regurgitation is mostly related to MVP.Quantification of MVR is showed in Table 9.
Tricuspid valve regurgitation (TVR) is often the result of RV dilatation.Primary TVR leads to RV volume overload and increased venous pressure.The severity of TVR can be determined by echocardiography (Table 10)[111].
CONGENITAL HEART DISEASES
A once-in-a-lifetime echocardiography allows to identify congenital heart disease in order to plan an individualized follow-up and exercise recommendation,other than screening of the athlete’s family.
ANOMALOUS ORIGIN OF CORONARY ARTERIES
Anomalous origin of coronary arteries (AOCA) is a rare but recognized cause of sports related SCD[108],often missed to ECG.Identification of AOCA is not easy because these are often asymptomatic individuals and do not necessarily exhibit distinctive features on ECG stress testing.Even if echocardiography is not the gold standard for its diagnosis,this examination is the first line test recommended when there is such a suspicion also because it is the only noninvasive tool to visualize the ostia and first tracts of coronary arteries[118-120].Each coronary artery (CA) usually originates from its respective sinus above the aortic valve leaflets,with the right CA (RCA) arising from the right sinus of Valsalva and the left main CA (LCA) arising from the left sinus of Valsalva[121].Not all AOCA have the same prognostic impact:the most at-risk anomalies include an anomalous origin from a wrong aortic sinus [aortic anomalous origin of coronary arteries (AAOCA)[15]].
About the AAOCA,the ostium and proximal course of the left main and right coronary arteries can be visualized by echocardiography respectively in over 98% and 80% of subjects[122],especially from a PSAX view.The left main CA origin is approximately 4 o'clock and the RCA origin at 11 o'clock.Additional color flow mapping can help in the identification process[123].Occasionally,clockwise rotation of the transducer in the PSAX view frequently allows the identification of the left main CA as it bifurcates into the left anterior descending branch and the left circumflex branch.
In infants and small children with good subcostal windows,the LCA origin can be seen from a coronal plane;the RCA origin is frequently best seen from a PLAX view.
What to assess? Origin of coronary arteries (PSAX).if pathological,what to assess?Additional color flow mapping of the inter-arterial space.
BICUSPID AORTIC VALVE
BAV is one of the most common congenital cardiac abnormalities[124].Subjects with BAV may consequently have larger dimensions of the aortic sinus,ascending aorta,and aortic arch[15,89],beside possible progression to subsequent valvulopathies.The most common fusion pattern involves the right and left cusps[125].Moreover,BAV may be associated with other diseases such as coarctation of the aorta,interrupted aortic arch,patent ductus arteriosus,coronary anomaly,as well as Williams or Turner syndrome[126,127].The leaflets number and fusion,and the presence of a ‘raphe’ can be easily evaluated in the PSAX view,while systolic doming is better seen in PLAX view.Doppler echocardiography permits assessment of valve dysfunction[110,128]
What to assess? Aortic valve morphology (PSAX).If pathological,what to assess?Aortic stenosis + aortic regurgitation + dimension of aortic root (PLAX) + other congenital defects (coarctation of the aorta,interrupted aortic arch,patent ductus arteriosus,coronary anomaly or hypoplastic left heart,as well as Williams or Turner syndrome).
ATRIAL SEPTAL DEFECTS
Atrial septal defect (ASD),the failure to close the communication between RA and LA,afflicts 1 in 4 children[129].There are five subtypes of atrial septal defects:patent foramen ovalis,ostium secundum defect,ostium primum defect,sinus venosus defect,and coronary sinus defect[89,130].The atrial septum can be assessed from subcostal,apical and PSAX[131,132] views,even if subcostal is the preferred one.The fourchamber view allows hemodynamic assessment of the ASD (RA and RV dilatation) RV pressure estimation (TVR jet velocity).Associated lesions include anomalous pulmonary venous connection,pulmonary valve stenosis,and mitral valve prolapse.This condition is frequent in Ebstein anomaly[89].
What to assess? ASD (subcostal).If pathological,what to assess? ASD (A4C/PSAX)+ RA dimension + RV dimension + PAPS.
VENTRICULAR SEPTAL DEFECTS
Ventricular septal defects (VSD) are the most common congenital cardiac abnormality in young individuals and the second one in adults,following only BAV[133].Four locations of the defect within the interventricular septum are possible.Multiple windows can be used to assess the ventricular septum[131],such as PSAX and A4C views.VSD is also a common component of complex anomalies,such as Tetralogy of Fallot (ToF) and transposition of the great arteries (TGA)[89].Key findings to provide are location,number,and size of defects,severity of LV volume overload,and estimated PAPS.Double chambered right ventricle (DCRV) and sinus of Valsalva aneurysm must be ruled out[89].
What to assess? VSD (A4C).If pathological,what to assess? LV mass + PAPS +aortic regurgitation + other congenital defects (aneurysm of Valsalva sinus,ToF,TGA,DCRV).
PATENT DUCTUS ARTERIOSUS
Patent ductus arteriosus (PDA) is the lasting communication between the proximal left pulmonary artery (PA) and the descending aorta,just distal to the left subclavian artery[89].Echocardiography is choice diagnostic tool131].PDA is best seen from the PSAX and suprasternal windows,where the duct can be visualized along the left border of the pulmonary artery:there is bidirectional flow across the duct,confirmed on pulsed wave doppler (PW);moreover,LA/Aortic root ratio ≥ 1.4 is suggestive of shunt[134].A complete echocardiographic assessment should be performed to exclude pulmonary atresia or coarctation of the aorta.Furthermore,it must be assessed the degree of LV volume overload,PAPS,PA size,and right heart change.
What to assess? PDA (PSAX).If pathological,what to assess? PWPDA (PSAX) +LA/Aortic root ratio ≥ 1.4 + LV mass + PAPS + PA size + RV dimension + RA dimension.
AORTIC COARCTATION
Aortic coarctation (CoA) is considered a part of a more general arterial disease[89]:It occurs as a stenosis or as a hypoplastic aortic (arch) segment.Associated lesions include BAV ascending aortic aneurysm,aortic stenosis,mitral stenosis or complex congenital heart defects.Echocardiography provides information regarding site,structure,and extent of CoA,LV function and LVH,and aortic vessel diameters[89].
What to assess? Aortic coarctation (PSAX).If pathological,what to assess? Aortic stenosis + mitral stenosis + FE + LV mass + other congenital defects (BAV,ascending aortic aneurysm).
OTHER CONGENITAL DISEASES
Improvements in modern medicine has led to an increase in the number of adults with congenital heart disease engaging in sports activities[89,135].Therefore,an echocardiography assessment of others various congenital heart diseases is crucial for sport eligibility.
ToF is a congenital cardiac malformation made by VSD,RVOT obstruction,override of the ventricular septum by the aortic root,and RV hypertrophy[136].The initial presentation of ToF varies depending on the severity lung blood flow obstruction.Echocardiography has become the standard modality in its diagnosis[137].The ventricular septal defect,the aortic override,and RV hypertrophy can be seen in PLAX view,while the pulmonary outflow tract obstruction in PSAX view[138].
Transposition of the great vessels is a group of congenital heart defects involving an abnormal spatial arrangement of any of the great vessels[139].Congenital heart diseases involving only the primary arteries (pulmonary artery and aorta) belong to a sub-group called TGA:the aorta aligns with the RV and the pulmonary artery aligns with the LV[140].It is the fourth most common type of major cardiac defect[141] and the second most common cyanotic lesion after ToF[140];if not treated,it is the leading cause of SCD in neonates and infants[142].Important echocardiographic assessments in case of TGA are position of the great arteries in PLAX or subcostal views,presence of ASD and VSD,presence of LVOT obstruction,eventual anomalies of coronary arteries[140].
Ebstein's anomaly (EA) is a malformation of the tricuspid valve (TV) with myopathy of the RV[143].Initial presentation of EA in adulthood is common,and natural history demonstrates decreased survival with biventricular failure[144].Echocardiography is the imaging standard in EA and provides a platform for TV leaflet,determination of right-heart size and function and dynamic evaluation of intracardiac shunts and defects,through color doppler[143,145].
Complete atrioventricular canal,also referred to as complete atrioventricular septal defect,is characterized by an ostium primum atrial septal defect,a common atrioventricular valve and a variable deficiency of the ventricular septum inflow[146].It is a rare congenital heart condition.Echocardiography is the key tool for its diagnosis and anatomic classification[147].
OTHER PATHOLOGIES
Even if these are acute conditions that usually manifest with other symptoms,these pathologies are often cause of sudden cardiac death in athletes and therefore need to be screened.
MYOCARDITIS
Myocarditis accounts for almost 15% of SCDs in younger populations,especially in athletes[148].The clinical presentation varies from chest pain,exertional dyspnea and fatigue,to cardiogenic shock[148].Some patients heal completely while other may evolve in DCM[149].An higher incidence of sport related SCD in case of myocarditis is documented[148],for the higher rate of life-threatening ventricular arrhythmias[150].In acute form,echocardiography may show global LV dysfunction[151],wall motion abnormalities and pericardial effusions[15,152,153],an diffuse myocardial edema(increased wall thickness)[154-157].In healed form,echocardiographic LV systolic dysfunction is found rarely because there is often small myocardial fibrosis,with undetectable scar at echocardiography[158]:Its evaluation still represent one of the bigger challenges of sport cardiology.
What to assess? EF;LV wall motion abnormalities;pericardial effusion;increased LV wall thickness.
PERICARDITIS
In athletes presenting with chest pain,the diagnosis of pericarditis should always be taken in consideration[159],particularly in younger ones[160].Pericarditis and myocarditis may coexist in 20%-30% of patients[135].Specific sport recommendations exist for patients with this disease[135].Echocardiography is the diagnostic test of choice.The presence of pericardial fluid is recorded as an echo-free space between the posterior pericardium and left ventricular epicardium (in case of small effusion) or anterior right ventricular epicardium (in case of larger effusion)[159].It can be assessed mostly in subcostal view.
What to assess? Pericardial effusion.
KAWASAKI DISEASE
Kawasaki disease (KD) is an acute vasculitis affecting young children,resulting in coronary artery abnormalities in case of delayed diagnosis[161,162].
Echocardiography is the imaging modality of choice for its detection:LV function and wall motion,of valvar regurgitation (particularly the mitral and aortic valves),and pericardial effusion should be assessed[163,164].
What to assess? Coronary artery abnormalities (PSAX/PLAX);EF;LV wall motion abnormalities;mitral regurgitation;aortic regurgitation;pericardial effusion.
CONCLUSION
Echocardiography is a valuable tool helping in detecting several CV conditions afflicting athletes.As physicians become more experienced with sonography,focused echocardiography by sports medicine physicians may become standard practice in larger screening practices.This technique could help to detect some hidden CV condition and to distinguish between physiological and pathological adaptation to physical activity,assisting in sport eligibility process.Even if we admit that a good manual skill,a training period and echocardiography experience should be required to perform it,our athlete-focused echocardiography could be an effective time-saving first line evaluation tool in sport eligibility process,eventually followed by a carefully exam.As such,a focused cardiac ultrasound examination may optimize cost-effectiveness:early detection of asymptomatic structural heart conditions could have important prognostic implications,with a relative less expensive cost than a complete echocardiogram.Nevertheless,further clinical trials should investigate its efficacy,accuracy,and applicability.
 World Journal of Cardiology2021年8期
World Journal of Cardiology2021年8期
- World Journal of Cardiology的其它文章
- ISCHEMIA trial:How to apply the results to clinical practice
- Shortened dual antiplatelet therapy in contemporary percutaneous coronary intervention era
- Multimodality imaging in the diagnosis and management of prosthetic valve endocarditis:A contemporary narrative review
- In-depth review of cardiopulmonary support in COVID-19 patients with heart failure
- Surgical strategies for severely atherosclerotic (porcelain) aorta during coronary artery bypass grafting
- Angiotensin receptor blocker neprilysin inhibitors
