Role of exosomal long non-coding RNAs in colorectal cancer
Ru Sun,Xiao-Yun He,Cheng Mei,Chun-Lin Ou
Ru Sun,Department of Blood Transfusion,Affiliated Hospital of North Sichuan Medical College,Nanchong 637000,Sichuan Province,China
Xiao-Yun He,Chun-Lin Ou,Department of Pathology,Xiangya Hospital,Central South University,Changsha 410008,Hunan Province,China
Cheng Mei,Department of Blood Transfusion,Xiangya Hospital,Central South University,Changsha 410008,Hunan Province,China
Abstract Exosomes are a class of small extracellular vesicles,30-150 nm in diameter,that transfer biological information(e.g.,DNA,RNA,and protein)via cell-to-cell communication.Exosomes play critical roles in the occurrence and development of human cancers,including colorectal cancer(CRC).Recent studies have shown that long non-coding RNAs(lncRNAs)can be encapsulated in exosomes,which transfer lncRNAs from secretory cells into recipient cells.This process affects the progression of CRC,since exosomal lncRNAs display special and extensive functions in CRC tumorigenesis,including malignant proliferation,metastasis,chemoresistance,and inflammatory response.Moreover,due to their specificity and sensitivity,exosomal lncRNAs are released into body fluids(e.g.,urine,sputum,and plasma),which have the potential to be biomarkers of CRC tumorigenesis within screening efforts and medical and epidemiologic research.In this review,we aim to clarify the function and mechanism of exosomal lncRNAs in CRC tumorigenesis and provide a strategy for early diagnosis and medical treatment of this malignancy.
Key Words:Exosomes;Long non-coding RNAs;Colorectal cancer;Chemoresistance;Inflammatory response;Therapy
INTRODUCTION
Colorectal cancer(CRC)is one of the most common gastrointestinal cancers and is currently the fourth most common malignant cancer worldwide in terms of incidence.CRC has an extremely high morbidity and mortality,with almost 1.8 million newly diagnosed cases and nearly 900000 deaths worldwide each year[1-3].With the rapid progress of cancer therapeutics,the survival time of patients with early-stage CRC has been significantly prolonged.However,the 5-year survival rate for patients with advanced CRC remains low[4-6].Therefore,exploring the molecular mechanisms of CRC tumorigenesis and identifying early and intermediate stage molecular markers are key processes in the screening and treatment of CRC.
In 1983,Hardinget al[7]and Pan and Johnstone[8]were the first to identify and define exosomes,which were considered metabolic waste of cells.With the popularization of electron microscopy,researchers discovered that exosomes,which are 40-160 nm in diameter,are produced by almost all cell types and are released freely in all body fluids(e.g.,urine,saliva,sputum,and plasma).Exosomes can transfer RNA,DNA,proteins,lipids,and metabolitesviacell-to-cell communication,transferring these molecules from secretory cells to recipient cells and thereby inducing tumors and a range of other human diseases.In recent years,exosomes have become a potentially effective method for disease diagnosis and treatment because of their extensive presence in the body and convenience of acquisition,which may provide a promising prospect in the development of precision medicine[9-11].
With the completion of the Human Genome Project and the arrival of the postgenome era,noncoding RNAs(ncRNAs)have become a hot “star” in the era of life science[12-15].Long ncRNAs(lncRNAs)are a subset of ncRNAs,which have a transcribing length of 200-100000 nt,lack a complete functional open reading frame,and rarely encode a functional short peptide[16-19].Recent studies have shown that lncRNAs play an important role in human pathophysiological processes,including tissue differentiation,immunity,and reproduction,and thereby induce a range of human diseases,including diabetes mellitus(DM),Parkinson’s disease,and tumors[20-23].
An increasing number of studies have shown that lncRNAs can be encapsulated into exosomes,which transfer the lncRNAs from secretory cells into recipient cells,thereby affecting the progression of CRC[24-27].These exosomal lncRNAs can enter receptor cells through humoral circulation and participate in multiple phenotypes of tumor progression,including malignant proliferation,metastasis,chemoresistance,and inflammatory response.In addition,due to their specificity and sensitivity,exosomal-derived lncRNAs released into the tumor microenvironment have the potential to become biomarkers of CRC.Therefore,this review aims to provide a new direction for finding new CRC biomarkers and therapeutic targets by pooling and summarizing related studies of the role of exosomal lncRNAs in CRC tumorigenesis.
BIOLOGICAL CHARACTERISTICS AND MECHANISMS OF EXOSOMAL LNCRNAS
According to their size,extracellular vesicles(EVs)can be divided into small EVs(sEVs;with a diameter less than 200 nm)and large EVs(lEVs;with a diameter more than 200 nm).Exosomes are a class of sEVs,with a diameter of approximately 40-160 nm,derived from almost all types of human cells.After being released by secretory cells,exosomes are widely distributed to all types of human body fluids,including saliva,breast milk,cerebrospinal fluid,ascites,urine,and semen[28-30].Exosomes enter target cells through humoral circulation and cell receptors in three ways:Direct fusion,endocytosis,and receptor ligand binding.Exosomes carry proteins,nucleic acids,lipids,and other important signal carriers,forming a cell-cell information transmission system that participates in cell communication,cell migration,angiogenesis,and tumor cell growth[31-33].
The biological function of lncRNAs depends on their subcellular localizations.When lncRNAs are mainly localized in the nucleus,they play a role in regulating epigenetic modification,which regulates the expression of downstream target genes through histone modification by binding the enhancer region of the gene.Cytoplasmic lncRNAs,in contrast,not only sponge microRNAs(miRNAs)to form competing endogenous RNAs(ceRNAs)to regulate the expression of miRNA target genes,but can also block the phosphorylation level of targeted proteins,thereby affecting the protein expression of molecules and downstream signaling pathways[34-36].Therefore,when lncRNAs are encapsulated into exosomes,they transfer from secretory cells into recipient cells to exert their biological function,thereby affecting the progression of tumors.After entering the recipient cells,lncRNAs regulate the target gene expression of miRNAs through sponge miRNAs and affect the phosphorylation level or transcription expression of proteins by binding to proteins after entry into the target cells.Exosomal lncRNAs further regulate core molecules and signaling pathways,leading to tumor progression(Figure 1).In CRC,numerous studies have shown that exosomal lncRNAs,after entry into the target cells,not only regulate the target gene expression of miRNAs through sponge miRNAs[37-43](Table 1),but also affect the phosphorylation level or transcription expression of proteins by binding to proteins[44-49](Table 2).Therefore,a systematic understanding of the role of exosomal lncRNAs in cancer metastasis may provide improved diagnostic and prognostic biomarkers and therapeutic targets for malignant tumors.

Table 1 Relationship between exosomal long non-coding RNAs and microRNAs in colorectal cancer

Table 2 Relationship between exosomal long non-coding RNAs and their binding molecules in colorectal cancer
EXOSOMAL LNCRNAS AND CRC
Hanahan and Weinberg[50]published a landmark review entitled “Hallmarks of cancer:the next generation” in the journalCell,which summarized and clarified the ten defining characteristics of tumors,including self-sufficiency in growth signals,sustained angiogenesis,insensitivity to anti-growth signals,resisting cell death,limitless replicative potential,tissue invasion and metastasis,avoiding immune destruction,tumor promotion through inflammation,genome instability and mutation,and deregulation of cellular energetics.Recent studies have shown that exosomal lncRNA levels are closely related to the occurrence and development of tumors and that exosomal lncRNAs are involved in malignant proliferation,invasion and metastasis,chemoresistance,and inflammatory response(Figure 2).

Figure 1 The process of exosomal long non-coding RNAs secretion,transportation,and ingestion.A:Exosomal long non-coding RNAs(lncRNAs)are secreted by colorectal cancer(CRC)cells,carcinoma-associated fibroblasts,and chemoresistant CRC cells;B:Exosomal lncRNAs are released into circulatory system;C:Exosomal lncRNAs are transferred from the secretory cell into recipient cell to exert their biological function,thereby affecting the progression of tumors.CRC:Colorectal cancer;CAF:Carcinoma-associated fibroblast.

Figure 2 Summary chart of exosomal long non-coding RNAs in colorectal cancer.Exosomal long non-coding RNAs are closely related to the occurrence and development of tumors,involved in malignant proliferation,invasion and metastasis,chemoresistance,and the inflammatory response.Orange arrows:High expression in colorectal cancer;green arrows:Low expression in colorectal cancer.LncRNA:Long non-coding RNA;CRC:Colorectal cancer;HuR:Human antigen R.
Exosomal lncRNAs and malignant proliferation of CRC cells
Malignant proliferation of tumor cells is the most common malignant phenotype in the development of tumors.Tumor cells usually lack contact inhibition and show enhanced proliferation.Thus,tumors are classified as a type of progressive hyperplastic disease[51,52].Although starting from the direction of cutting off the malignant proliferation of tumors is a good choice for cancer treatment,the current basic research and clinical results are still unsatisfactory.Identifying key effective and broad-spectrum targets for inhibiting malignant tumor proliferation remains a difficult problem for scientists[53-55].
In recent years,exosomal lncRNAs have attracted wide attention as targets for the malignant proliferation of CRC.Exosomal lncRNA-UCA1 can be transmitted into CRC cells,thereby increasing the expression of myosin VI(MYO6)by sponging with miR-143,thus promoting CRC cell proliferation[38].Moreover,Zhaoet al[42]demonstrated that circulating lncRNA-LINC02418 can be transmitted into CRC cellsviaexosomes,and lncRNA-LINC02418 can function as a ceRNA to regulate the expression ofmaternal embryonic leucine zipper kinase(MELK)by competing for miR-1273g-3p binding.Liet al[47]revealed that exosomal lncRNA-ADAMTS9-AS1 can promote the malignant proliferation of CRC cells by increasing the expression of β-catenin and activating the Wnt signaling pathway.Interestingly,exosomal lncRNA-APC1 was also found to be able to inhibit CRC cell proliferation by activating the MAPK pathway through directly binding to Ras-related protein rab-5b(Rab5b)mRNA and hence reducing its stability[45].These findings show that exosomal lncRNAs play a key regulatory role in CRC cells;hence,lncRNAs may provide a new target for CRC therapy.
Exosomal lncRNAs and CRC metastasis
The term tumor metastasis usually refers to the process by which malignant tumor cells detach from the primary tumor site and are transferred through the circulatory system to secondary tissues or organs,where they colonize and form secondary tumors.Tumor metastasis is a complex process that involves cytoskeletal reconstruction,decreased cell adhesion,extracellular matrix degradation,and so on,thereby inducing angiogenesis and the epithelial-mesenchymal transition(EMT)of tumors[56].Tumor metastasis is a major problem with regard to cancer therapy because it affects the prognosis and survivorship of tumor patients and represents the leading cause of tumor-related deaths[57].Therefore,it is of great significance to study the mechanisms of tumor metastasis for the sake of improved treatment and prevention of tumors.
Exosomal lncRNAs are a class of molecules that,in recent years,have been shown to play an important role in the CRC metastasis.A study[40]demonstrated that exosomal lncRNA-MALAT1 derived from highly metastatic CRC cells can enhance the metastatic abilities of primary CRC cells.The underlying mechanism is that exosomal lncRNA-MALAT absorbs miR-26a/26b,hence increasing the expression of fucosyltransferase 4(FUT4)and activating the PI3K/AKT/mTOR pathway.Meanwhile,exosomal lncRNA 91H was also found to be able to directly interact with heterogeneous ribosomal protein K(HNRNPK)in CRC cells,thereby positively regulating the expression of HNRNPK to enhance CRC metastasis[48].Furthermore,exosomal lncRNAs are closely related to EMT in CRC cells.Zhouet al[39]found that cancerassociated fibroblast-derived exosomal LINC00659 can promote the progression of EMT in CRC cellsviathe miR-342-3p/ANXA2 axis.These studies indicate that exosomal lncRNAs might be potential biomarkers and therapeutic targets for the prediction,screening,and treatment of CRC.
Exosomal lncRNAs and CRC chemoresistance
The chemoresistance of tumors is caused by interaction between internal and external factors.Internal factors include protective autophagy,EMT,oxidative stress,and metabolic reprogramming.External factors include hypoxia and the tumor microenvironment.Corresponding molecules and the signaling pathways of cancer cells are changed through both internal and external factors,reducing the drug sensitivity of cancer cells and ultimately inducing the chemoresistance of cancer cells[58-60].The mechanism of chemoresistance has not been fully elucidated.Thus,chemoresistance is still one of the key reasons for the failure of tumor treatment.
Recent studies have demonstrated the important role of exosomal lncRNAs in CRC chemoresistance.The chemotherapy drugs for CRC contain cetuximab,mitomycin,and oxaliplatin.Yanget al[61]reported that cetuximab-resistant CRC cells secrete the exosomal lncRNA-UCA1,which can transmit cetuximab resistance to sensitive cells,and that the expression of exosomal lncRNA-UCA1 is closely related to the clinical outcome of cetuximab therapy in CRC patients.Interestingly,Chenet al[41]revealed that lncRNA-HOTTIP is highly expressed in mitomycin-resistant CRC cells and can be encapsulated into exosomes,transferring lncRNAs from mitomycin-resistant cells to sensitive cells;after entering the sensitive cells,lncRNA-HOTTIP can upregulate the expression of karyopherin subunit alpha 3(KPNA3)by binding to miR-214,thereby promoting drug resistance in sensitive cells.Moreover,Denget al[46]found that carcinoma-associated fibroblasts(CAFs)can secrete exosomal lncRNA-CCAL to promote oxaliplatin resistance in CRC cells.Functional studies revealed that lncRNACCAL can interact directly with the mRNA stabilizing protein HuR(human antigen R)to increase the expression of β-catenin,thereby inducing chemoresistance in CRC.Targeting exosomal lncRNAs might thus be a promising strategy for overcoming drug resistance in CRC.
Exosomal lncRNAs and inflammatory response in CRC
The connection of inflammation with tumorigenesis and tumor promotion,progression,and metastasis has been a major concern in recent years.Studies have shown that inflammation can accelerate the inflammation-tumor transformational reaction chain,thereby increasing the risk of the occurrence of tumors.Therefore,this type of tumor is referred to as an inflammation-associated tumor.Inflammation is an important biological risk factor for malignancy,and is considered the seventh defining characteristic of tumors[62-64].Emerging evidence suggests that exosomal lncRNAs participate in inflammatory tumor response,which is closely related to the tumor microenvironment,comprised of immune cells,inflammatory factors,chemokines,etc.[65,66].
The association between inflammatory bowel disease(IBD)and CRC has long been recognized[64].Colitis-associated cancer(CAC)is a CRC subtype that is associated with IBD[67].By constructing an AOM/DSS-induced CAC mouse model,Renet al[37]found that CAF-derived exosomal H19 can act as a ceRNA to increase the expression of β-cateninviasponging with miR-141,thereby contributing to the stemness and development of CRC.
Furthermore,proinflammatory conditions can be reflected in the tumor microenvironment,which contains a series of immune cells,such as dendritic cells,natural killer cells,macrophages,and T helper(Th)cells[68,69].Recent studies have shown that exosomal lncRNAs play a crucial role in the differentiation of CRC immune cells.For example,Sunet al[49]demonstrated that serum exosomal lncRNA-CRNDE-h can be transmitted into CD4+ T cells to increase the Th17 cell proportion and promote Th17 cell differentiation by inhibiting the E3 ubiquitin ligase Itch-mediated ubiquitination and degradation of RAR-related orphan receptor γt(RORγt)in CRC.Moreover,Lianget al[44]found that CRC cells-derived exosomal lncRNA-RPPH1 can be transmitted into tumor-associated macrophages to mediate macrophage M2 polarization,which is associated with CRC inflammatory response.Taken together,exosomal lncRNAs may serve as a promising target for tumor immune therapy.
EXOSOMAL LNCRNAS AS NOVEL CRC BIOMARKERS AND TARGETS
Exosomal miRNAs have a higher specificity compared to proteins and are easier to extract and detect.Thus,testing exosomal miRNAs by quantitative reverse transcription-polymerase chain reaction andin situhybridization assays is more specific and sensitive than detecting proteins by an antigen-antibody reaction[70,71].Exosomal lncRNAs can be detected in body fluids,and the content of lncRNAs can provide significant information about physiological and/or pathological changes in tumor patients[72,73].Therefore,exosomal lncRNAs have been widely studied as CRC markers in recent years[37,38,40,42,44,45,47,48,74-78](Table 3).
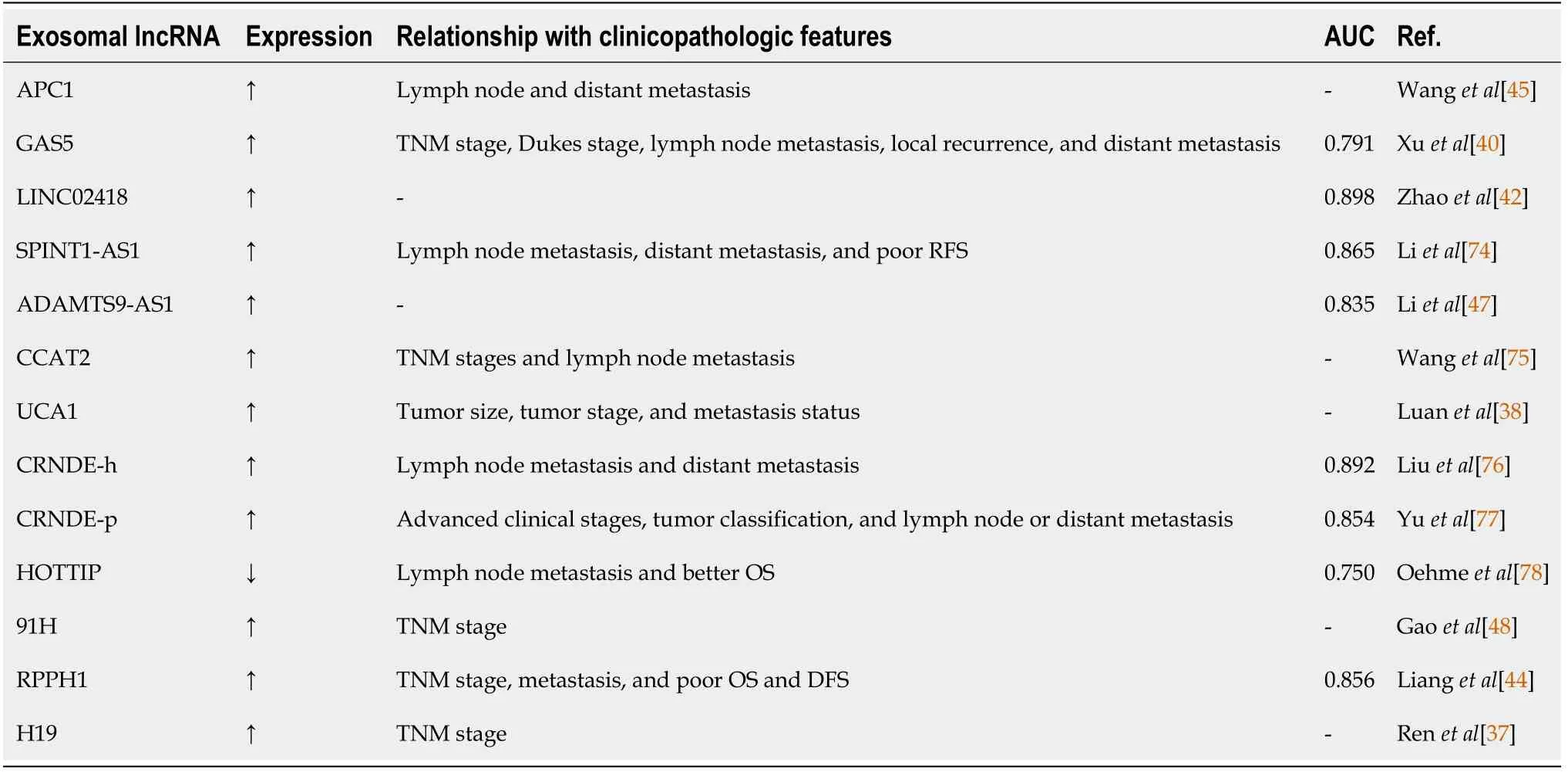
Table 3 Exosomal long non-coding RNAs as novel biomarkers and therapeutic targets for colorectal cancer
Many clinical studies have indicated a close association between exosomal lncRNAs and various clinical symptoms.Liet al[74]found that elevated expression of exosomal lncRNA-SPINT1-AS1 was associated with regional lymph node metastasis,distant metastasis,and short recurrence-free survival of CRC patients.Liuet al[43]showed that elevated expression of exosomal lncRNA-GAS5 was correlated with TNM stage,Dukes stage,local recurrence rate,and distant metastasis in CRC.In addition,elevated expression of CCAT2 was associated with local invasion and lymph node metastasis in CRC[79].
Exosomal lncRNAs serve as novel potential diagnostic and prognostic biomarkers of CRC.Using exosomal lncRNA-CRNDE-h as a diagnostic biomarker[76],receiver operating characteristic(ROC)curve analysis showed that lncRNA-CRNDE-h expression was a good candidate for distinguishing CRC patients from healthy control participants providing serum samples(sensitivity 70.3%,specificity 94.4%).The area under the ROC curve was 0.892[95% confidence interval(CI):0.860-0.918,P< 0.05].Moreover,exosomal miRNAs act as prognostic biomarkers,and Oehmeet al[78]found that low expression of exosomal lncRNA-HOTTIP was positively correlated with a poor overall survival(OS)in CRC patients(P= 0.0009),and further found that low expression of lncRNA-HOTTIP was an independent prognostic marker for OS(hazard ratio:4.5,95%CI:1.69-11.98,P= 0.0027)within a multivariate analysis.With the widespread use and development of new technologies,the detection of exosomal lncRNAs may provide a novel strategy for the screening,early diagnosis,and therapy of CRC.
FUTURE PERSPECTIVES
To summarize,an increasing number of studies have shown that exosomal lncRNAs are closely associated with the progression of CRC.Exosomal lncRNAs have been recognized as ‘new stars’ in the research field of tumors,and,as a class of novel regulatory molecules,they participate in the progression of CRC.Therefore,we can silence or activate exosomal lncRNAs in CRC patientsviaan exogenous means.For example,we can wrap the silenced or active lentiviral vector of lncRNAs into exosomesin vitro,then perform a targeted injection into the corresponding organs of humans through fluid circulation.This can be developed as a therapy for CRC.With the development of high-throughput sequencing and related technologies in recent decades[80],an increasing number of exosomal lncRNAs have been discovered and identified in human diseases.Because the extraction and detection of exosomal lncRNAs present a higher specificity and sensitivity compared to exosomal proteins,lncRNAs have great potential as biological tools for the diagnosis or treatment of tumors[81].However,there is a long road from scientific research on exosomal lncRNAs to clinical applications.Recently,research on exosomal lncRNAs has faced a series of challenges and limitations:(1)Although high-throughput sequencing has shown that many exosomal lncRNAs are abnormally expressed in tumor body fluids(e.g.,urine,sputum,and plasma),the specific mechanisms and functions of exosomal lncRNAs have not been fully understood;(2)The technology and methods for isolation and purification of exosomes,including ultra-high-speed centrifugation,filtration,precipitation,and immunoconcentration,are not mature[82,83].Existing purification methods can hardly distinguish exosomes from non-vesicle components,which may affect the subsequent functional experiments of exosomal lncRNAsin vivoandin vitro;(3)Although exosomal lncRNAs have been shown to play a role in tumor treatment in many animal models,there is still a lack of clinical trials to confirm the accuracy and safety of these findings;and(4)There are still some difficulties in developing exosome-based drug delivery systems and functionally introducing them to specific cells.
Although our existing understanding of exosomal lncRNAs is just the tip of the iceberg,novel approaches and techniques will ultimately shed light on these processes.It is likely that in the near future,detection kits and therapeutic drugs targeting exosomal lncRNAs will be used in clinical research for CRC screening,diagnosis,and treatment.
CONCLUSION
Recent studies have shown that exosomal lncRNAs play critical roles in the occurrence and development of CRC.Exosomal lncRNAs display special and extensive functions in CRC tumorigenesis,including malignant proliferation,metastasis,chemoresistance,and inflammatory response.Moreover,due to their specificity and sensitivity,exosomal lncRNAs are released into body fluids,which have the potential to be biomarkers of CRC tumorigenesis within screening efforts and medical and epidemiologic research.In this review,we aim to clarify the function and mechanism of exosomal lncRNAs in CRC tumorigenesis and provide a strategy for early diagnosis and treatment.
 World Journal of Gastrointestinal Oncology2021年8期
World Journal of Gastrointestinal Oncology2021年8期
- World Journal of Gastrointestinal Oncology的其它文章
- Diagnostic value of four serum exosome microRNAs panel for the detection of colorectal cancer
- Investigation of the factors influencing surgical treatment of duodenal gastrointestinal stromal tumors
- Development of a prognostic prediction model based on microRNA-1269a in esophageal cancer
- Diffuse reduction of spleen density is a novel prognostic marker for intrahepatic cholangiocarcinoma after curative resection
- Neutrophil-to-lymphocyte ratio and carbohydrate antigen 19-9 as prognostic markers for advanced pancreatic cancer patients receiving first-line chemotherapy
- STAT3-mediated activation of mitochondrial pathway contributes to antitumor effect of dihydrotanshinone I in esophageal squamous cell carcinoma cells
