Nogo-A aggravates oxidative damage in oligodendrocytes
Yang-Yang Wang, Na Han, Dao-Jun Hong, Jun Zhang,
Abstract Nogo-A is considered one of the most important inhibitors of myelin-associated axonal regeneration in the central nervous system. It is mainly expressed by oligodendrocytes. Although previous studies have found regulatory roles for Nogo-A in neurite outgrowth inhibition,neuronal homeostasis, precursor migration, plasticity, and neurodegeneration, its functions in the process of oxidative injury are largely uncharacterized. In this study, oligodendrocytes were extracted from the cerebral cortex of newborn Sprague-Dawley rats. We used hydrogen peroxide (H2O2) to induce an in vitro oligodendrocyte oxidative damage model and found that endogenously expressed Nogo-A is significantly upregulated in oligodendrocytes. After recombinant virus Ad-ZsGreen-rat Nogo-A infection of oligodendrocytes, Nogo-A expression was increased, and the infected oligodendrocytes were more susceptible to acute oxidative insults and exhibited a markedly elevated rate of cell death. Furthermore, knockdown of Nogo-A expression in oligodendrocytes by Ad-ZsGreen-shRNA-Nogo-A almost completely protected against oxidative stress induced by exogenous H2O2. Intervention with a Nogo-66 antibody, a LINGO1 blocker, or Y27632, an inhibitor in the Nogo-66-NgR/p75/LINGO-1-RhoA-ROCK pathway, did not affect the death of oligodendrocytes. Ad-ZsGreen-shRNA-Nogo-A also increased the levels of phosphorylated extracellular signal-regulated kinase 1/2 and inhibited BCL2 expression in oligodendrocytes. In conclusion, Nogo-A aggravated reactive oxygen species damage in oligodendrocytes, and phosphorylated extracellular signal-regulated kinase 1/2 and BCL2 might be involved in this process. This study was approved by the Ethics Committee of Peking University People’s Hospital, China (approval No. 2018PHC081) on December 18, 2018.
Key Words: BCL2; H2O2; LINGO1; NgR; Nogo-A; oligodendrocytes; phosphorylated extracellular signal-regulated kinase 1/2; reactive oxygen species
Graphical AbstractThe mechanism of Nogo-A involving oxidative injury of oligodendrocytes

Introduction
In recent decades, Nogo has been investigated as one of several neurite growth inhibitors (He and Koprivica, 2004;Schwab, 2010). The Nogo gene encodes three protein products, Nogo-A, Nogo-B, and Nogo-C, which share a 188-amino acid sequence comprising a 66-amino acid extracellular domain (Nogo-66) and a conserved C-terminal domain. The longest isoform, Nogo-A is the principal family member found in the central nervous system, and is expressed largely in oligodendrocyte plasma membranes, but also in neurons. Physiologically, Nogo-A is a critical factor for oligodendrocyte maturation and myelin formation (Pernet et al., 2008; Huang et al., 2012), cortical development and neuronal maturation (Mingorance-Le Meur et al., 2007), and synaptic transmission and memory formation (Karlén et al.,2009; Zemmar et al., 2014). In addition, Nogo-A is implicated in several degenerative diseases, including amyotrophic lateral sclerosis (ALS) (Jokic et al., 2006; Yang et al., 2009),Alzheimer’s disease (Park and Strittmatter, 2007; Zhou et al.,2011), Parkinson’s disease (Simunovic et al., 2009; Schawkat et al., 2015), multiple sclerosis (Jurewicz et al., 2007; Lee and Petratos, 2013; Kim et al., 2018), and psychiatric diseases(Budel et al., 2008; Willi et al., 2010).
ALS has a close relationship with a mutation in the gene encoding superoxide dismutase 1 (SOD1), which protects cells from superoxide radical damage. Nogo-A expression is upregulated in the skeletal muscle of the Cu/Zn-SOD1 transgenic mutant mouse (Bros-Facer et al., 2014), and Nogo-A plays a role in the pathophysiology of ALS (Dupuis et al., 2002; Schwab, 2010). Bros-Facer et al. (2014) determined that treatment with an anti-Nogo-A antibody significantly improved neuromuscular function in the SOD1G93A mouse model of ALS, at least during the early stages of the disease.However, a randomized, double-blind, placebo-controlled,phase 2 trial demonstrated that ozanezumab, a humanized monoclonal antibody against Nogo-A, did not show efficacyversusplacebo in patients with ALS (Meininger et al., 2017).Although Nogo-A does not seem to be an effective therapeutic target in ALS, the relationship between ALS and Nogo-A is close and complicated. In addition, it is not only motor neurons but also glial cells that are involved in the pathology of ALS. Oligodendrocytes contribute to motor neuron death in ALS via a SOD1-dependent mechanism (Ferraiuolo et al., 2016). Hence, oligodendrocytes are an important area of ALS research. The relationship between Nogo-A and oligodendrocytes, however, has not been researched.
The fundamental pathophysiology of ALS is oxidative injury;therefore, we have studied oligodendrocytic Nogo-A in the presence of oxidative stress. First, anin vitromodel of oligodendrocyte oxidative injury was established using hydrogen peroxide (H2O2). Then the level of Nogo-A was calculated in the oxidative-injured oligodendrocytes. After oligodendrocytes were infected with recombinant viruses,Ad-ZsGreen-rat Nogo-A or Ad-ZsGreen-shRNA-Nogo-A,the antioxidative abilities of Nogo-A were estimated to demonstrate its function in oligodendrocytes oxidative injury.The mechanism of action of Nogo-A in oligodendrocyte oxidative injury was further assessed.
Materials and Methods
Oligodendrocyte culture
This study was approved by the Ethics Committee of Peking University People’s Hospital, China (approval No. 2018PHC081)on December 18, 2018. Twenty pregnant Sprague-Dawley rats (Charles River, Cambridge, MA, USA) were bred and maintained under specific pathogen-free conditions in the Animal Center of Peking University People’s Hospital.
Oligodendrocytes were prepared from the brains of newborn(≤ 24 hours) Sprague-Dawley rats as described previously(Chen et al., 2007). Briefly, the cerebral cortex was aseptically removed and then digested with 0.25% trypsin (Sigma, Los Angeles, CA, USA) and 0.04% ethylenediaminetetraacetic acid (Jiangsu Keygen Biotech, Nanjing, Jiangsu Province,China) and dissociated. After centrifugation, Dulbecco’s modified Eagle’s medium/F-12 (Jiangsu Keygen Biotech)with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA)was added to the precipitate to 20 mL. The cells were then seeded at 1 × 106/cm2in a T75 poly-D-lysine-coated flask incubated in a humidified atmosphere with 5% CO2at 37°C.The medium, which contained Dulbecco’s modified Eagle’s medium (Jiangsu Keygen Biotech), 4 mM L-glutamine (Jiangsu Keygen Biotech), 1 mM sodium pyruvate (Sigma), and 20%fetal bovine serum, was replaced every 3 days. After 9 days in culture, the cells were shaken on a shaker at 37°C for 2 hours at 200 r/min and purified after removal of microglia.Samples were then incubated for 3 days in oligodendrocyte progenitor cell medium [10 ng/mL platelet-derived growth factor-AA (Invitrogen) and 10 ng/mL basic fibroblast growth factor (Invitrogen) was added to Basal chemically defined medium containing Dulbecco’s modified Eagle’s medium, 4 mM L-glutamine, 1 mM sodium pyruvate, 0.1% bovine serum albumin (Sigma), 50 mg/mL apotransferrin (Sigma), 5 mg/mL insulin (Sigma), 30 nM sodium selenite (Sigma), 10 nM D-biotin (Sigma) and 10 nM hydrocortisone (Sigma)], and then oligodendrocyte differentiation medium [basal chemically defined medium containing 15 nM triiodothyronine (Sigma),10 ng/mL ciliary neurotrophic factor (Sigma) and 5 µg/mL N-acetylcysteine (Jiangsu Keygen Biotech)] was added and cells cultured for 5 days to obtain mature oligodendrocytes.
Immunofluorescence staining
Oligodendrocytes were fixed with 4% paraformaldehyde for 30 minutes, washed three times with phosphate-buffered saline (PBS) for 3 minutes each, permeabilized with 0.5%Triton X-100 (Jiangsu Keygen Biotech) in PBS for 20 minutes,and washed in PBS as before. They were then incubated with 3% H2O2for 10 minutes, washed again in PBS for 3 ×2 minutes, and blocked with 5% bovine serum albumin for 20 minutes. The following antibodies were used: polyclonal rabbit anti-galactosylceramidase (for detecting myelin; 1:150;Cat# 13251R; Bioss, Beijing, China), monoclonal mouse anti-myelin basic protein (for detecting mature myelin; 1:500; Cat#932908; R&D, Minneapolis, MN, USA), and polyclonal rabbit anti-Nogo-A (1:100; Cat# ab62024; Abcam, London, UK). Cells were incubated for 2 hours at room temperature with primary antibodies, washed with PBS for 3 × 2 minutes, and incubated with peroxidase-conjugated AffiniPure goat anti-rabbit IgG(1:100; Cat# KGAA35; Jiangsu Keygen Biotech) and peroxidase-conjugated AffiniPure goat anti-mouse IgG (1:100; Cat#KGAA37; Jiangsu Keygen Biotech) for 1 hour at 37°C. After washes, 50 µg/mL 4′,6-diamidino-2-phenylindole was added for nuclear staining. Slides were observed with a fluorescence microscope (BX43; Olympus, Tokyo, Japan).
MTT assay
Cell viability was assessed by measuring the ability of cells to metabolize 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), as described previously(Siman et al., 2001). Different concentrations of H2O2(10, 25,50, 75, 100 µM) were added to cell cultures for 12 hours and then the cells were maintained in growth medium containing 5 mg/mL MTT for 4 hours at 37°C. After removal of the medium, dimethyl sulfoxide was added to each well. After complete dissolution of the precipitate for 10 minutes at room temperature, the absorbance at 490 nm was measured using a microplate spectrophotometer (MD Spectramac M3,San Francisco, CA, USA). Apart from assessing the effects of H2O2concentrations, the MTT assay was also used to calculate the survival rates of oligodendrocytes with or without up-/down-regulated Nogo-A (achieved by infection with the recombinant viruses, Ad-ZsGreen-rat Nogo-A and Ad-ZsGreenshRNA-Nogo-A). In addition, cell survival rates were calculated when Ad-ZsGreen-Nogo-A or Ad-shRNA-Nogo-A was added to the oligodendrocyte cultures 2 hours before, simultaneously with, or 30 minutes after H2O2treatment. Furthermore,oligodendrocytes were treated with Nogo-66 antagonist(1:1000; Cat# 475221-20-6; R&D), a Leucine-rich repeat and immunoglobulin-like domain-containing protein 1 (LINGO1)blocker, a rabbit polyclonal anti-Nogo-A antibody (1:500; Cat#ab23631; Abcam), and a Rho-associated protein kinase (ROCK)inhibitor, Y27632 (5 µM; Cat# ab120129; Abcam) followed by incubation with 75 µM H2O2for 12 hours. MTT assays were also used to test cell survival rates.
Cell death assay
Oligodendrocytes with or without up-/down-regulated Nogo-A were incubated in culture medium containing 2 µg/mL propidium iodide (PI) (Cat# KGA215; Jiangsu Keygen Biotech) and 2 µg/mL Hoechst 33342 (Cat# KGA215; Jiangsu Keygen Biotech) at 37°C for 15 minutes. The percentage of cell death was calculated by PI (+)/Hoechst (+) observed with a fluorescence microscope. Cell death rates were also calculated when Ad-ZsGreen-Nogo-A or Ad-shRNA-Nogo-A was added to the oligodendrocyte cultures 2 hours before, simultaneously with, or 30 minutes after H2O2treatment.
Real-time PCR
Total RNA was extracted from oligodendrocytes using Trizol reagent (Invitrogen). Complementary DNA synthesis was performed using the RNA polymerase chain reaction (PCR) kit from Takara (Tokyo, Japan) according to the manufacturer’s instructions. Glyceraldehyde 3-phosphate dehydrogenase was chosen as the endogenous control. The reaction was performed at 37°C for 15 minutes and 85°C for 5 seconds. To ensure that the PCR products fell within the linear range, cycle dependence was examined. Quantitative real-time PCR was performed using a Veriti 96-well Thermal cycler (ABI, Carlsbad,CA, USA) and the StepOnePlus Real-Time PCR system (ABI).The primers used for real-time PCR of Nogo-A were: 5′-CCG CCT TCA AGT ACC AGT TCG T-3′ (forward) and 5′-CTC TCC AGC ACC TCC AGT TCC T-3′ (reverse). The primers used for realtime PCR of glyceraldehyde 3-phosphate dehydrogenase were: 5′-CGG CAA GTT CAA CGG CAC AGT G-3′ (forward) and 5′-CGC TCC TGG AAG ATG GTG ATG G-3′ (reverse).
Western blot assays
Total protein was extracted from oligodendrocytes using a Total Protein Extraction Kit (Jiangsu Keygen Biotech) according to the manufacturer’s instructions. Protein concentrations were determined using a bicinchoninic acid protein assay kit. The samples were separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis and transferred to nitrocellulose membranes and then blocked for 1 hour at room temperature in Tris-buffered saline containing 5% nonfat milk. The membranes were next incubated overnight at 4°C with the relevant primary antibodies. The primary antibodies used were: polyclonal rabbit anti-Nogo-A (1:1000; Cat#ab62024; Abcam), polyclonal rabbit anti-extracellular signalregulated kinase 1/2 (ERK1/2) (1:1000; Cat# ab17942; Abcam),polyclonal rabbit anti-phosphorylated ERK1/2 (p-ERK1/2)(1:1000; Cat# sc-23759-R; Santa Cruz Biotechnology, Santa Cruz, CA, USA), monoclonal rabbit antiBCL2 (1:1000; Cat#ab32124; Abcam), and polyclonal rabbit glyceraldehyde 3-phosphate dehydrogenase (Cat# KGYT5052-6; Jiangsu Keygen Biotech). For detection, horseradish peroxidaseconjugated goat anti-rabbit IgG (1:100; Cat# KGAA35; Jiangsu Keygen Biotech) was added for 2 hours at room temperature and a Chemiluminescence Western Blotting Kit (Cat#KGP1201; Jiangsu Keygen Biotech) was used. Densitometry quanti-fication was performed with the Syngene G:BOX chemiXR5 system (Syngene, London, UK) and analyzed using Gel-Pro32 software (Media Cybernetics, Rockville, MD, USA).
Recombinant virus preparation and transfection
The recombinant viruses Ad-ZsGreen-rat Nogo-A and Ad-ZsGreen-shRNA-Nogo-A were purchased from Jiangsu Keygen Biotech. The virus vectors for Ad-ZsGreen-rat Nogo-A and Ad-ZsGreen-shRNA-Nogo-A were pDC316-mCMV-ZsGreen and pDC316-ZsGreen-shRNA, respectively. The recombinant virus Ad-ZsGreen-rat Nogo-A was used to increase the expression of Nogo-A, whereas Ad-ZsGreen-shRNA-Nogo-A was used to decrease its expression. Lipofectamine 3000 (Invitrogen) was used in six-well tissue culture plates (Corning Incorporated)for plasmid transfection. In one tube, 2.5 µg plasmid was mixed with 250 µL Opti-MEM (Invitrogen) while a second tube contained 5 µL Lipofectamine 3000 and 250 µL Opti-MEM. Each tube was incubated at room temperature for 5 minutes before the two solutions were combined and allowed to incubate for a further 20 minutes at room temperature for complex formation.The entire mixture was then added to a well containing 500µL culture medium. Five hours after transfection, plasmid-Lipofectamine 3000 was removed. The over- and underexpression of Nogo-A were verified by western blot assays.
Measurement of ROS using dihydroethidium
The cell-penetrating fluorescent probe, dihydroethidium(DHE) (Cat# KGAF019; Jiangsu Keygen Biotech), was used to assess the real-time formation of superoxide anionsin oligodendrocytes as described previously (Fan et al.,2008). The cells were then dehydrated and examined for red fluorescence. Harvested cells were incubated with 10 µM DHE for 20 minutes at 37°C according to the manufacturer’s instructions. Measurement of red fluorescence was performed on a flow cytometer at an excitation wavelength of 488 nm and emission wavelength of 530 nm. Flow cytometry was conducted in duplicate in at least three independent experiments.
Statistical analysis
Statistical analysis was performed using SPSS 23.0 statistical software (IBM, Chicago, IL, USA). Data are expressed as the mean ± standard deviation (SD). Statistical significance was evaluated by one-way analysis of variance or the independentsamplest-test, and probability values (P) of < 5% were considered significant.
Results
Identification of cultured oligodendrocytes
Purified oligodendrocytes were identified using light microscopy and galactosylceramidase staining (Figure 1). The proportion of oligodendrocytes was about 90%.
The most suitable H2O2intervention conditions for rat oligodendrocytes
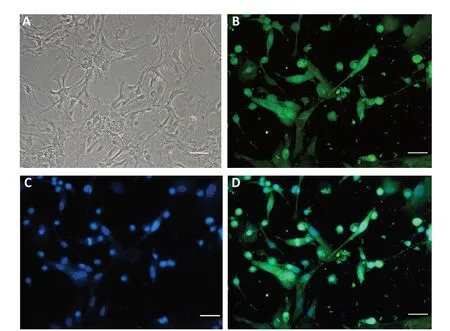
Figure 1 |Immunofluorescence staining of oligodendrocytes.(A) Morphology of oligodendrocytes observed by light microscopy. (B-D)Immunofluorescence staining of oligodendrocytes. Galactosylceramidase immunofluorescence (green, B) marked oligodendrocytes and 4′,6-diamidino-2-phenylindole counterstaining marked nuclei (blue, C). (D) Merged image of B and C. Oligodendrocytes are indicated by galactosylceramidase and 4′,6-diamidino-2-phenylindole colocalization. Scale bars: 50 µm.
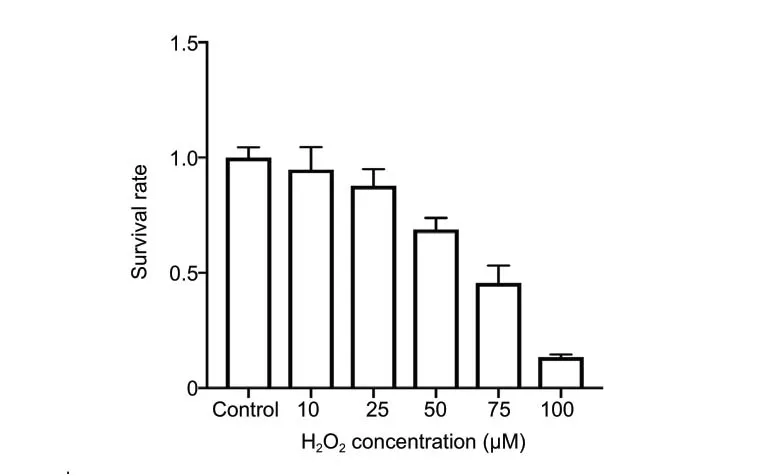
Figure 2 |Survival rates of oligodendrocytes exposed to different H2O2 concentrations for 12 hours calculated by MTT assays.A dose of 75 µM was the sublethal dose for rat oligodendrocytes. Data are expressed as the mean ± SD and were analyzed by one-way analysis of variance. H2O2: Hydrogen peroxide; MTT: 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Rat oligodendrocytes showed a H2O2concentration-dependent decrease in cell viability, with 75 µM H2O2for 12 hours leading to 50-60% cell death compared with control (Figure 2).Thus, treatment with 75 µM H2O2for 12 hours was used in subsequent experiments.
H2O2increases Nogo-A expression in oligodendrocytes
Nogo-A is predominantly expressed in oligodendrocytes,and H2O2increased Nogo-A expression in these cells in a concentration-dependent manner (Figure 3). After exposure to 75 µM H2O2for 12 hours, oligodendrocytic Nogo-A mRNA (P< 0.001) and protein (P< 0.001) levels were sharply increased compared with controls (Figure 4).
The transfection efficiency of Nogo-A shRNA in HEK293 cells
Forty-eight hours after infection, the infection efficiencies of Ad-ZsGreen-rat Nogo-A and Ad-ZsGreen-shRNA-Nogo-A in HEK293 cells were determined by fluorescence microscopy. As shown inFigure 5, there was a high percentage of fluorescent cells in both the Ad-ZsGreen-rat Nogo-A and Ad-ZsGreenshRNA-Nogo-A groups. However, it was essential to validate the extent of the over-/under-expression of the Nogo-A protein. This is usually achieved through quantitative western blot analysis of transfected cells and comparison of the levels of the targeted protein to those in untransfected or controltransfected cells. Analysis of the transfection efficiency of Nogo-A revealed that the protein levels of Nogo-A in oligodendrocytes with Ad-ZsGreen-Nogo-A and Ad-ZsGreenshRNA-Nogo-A were significantly altered compared with control groups (P< 0.001;Figure 6).

Figure 3 |Nogo-A immunopositivity in oligodendrocytes with different H2O2 concentrations.MBP stain (green) marks oligodendrocytes, red immunofluorescence marks Nogo-A, and DAPI counterstaining (blue) marks nuclei. Nogo-A-positive oligodendrocytes were identified as MBP, Nogo-A, and DAPI triple-labeled cells. The Nogo-A level was clearly increased by H2O2 in a concentrationdependent manner. Scale bars: 50 µm. DAPI: 4′,6-Diamidino-2-phenylindole;H2O2: hydrogen peroxide; MBP: myelin basic protein.
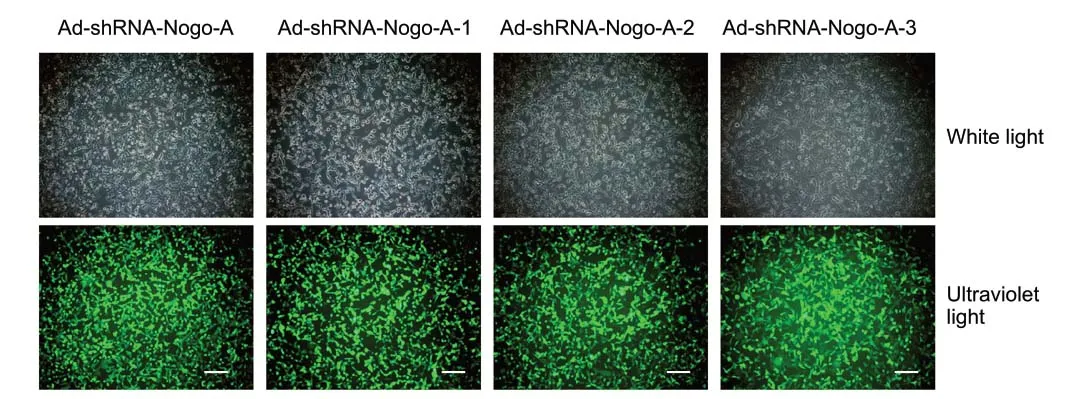
Figure 5 |ZsGreen (green fluorescent protein) expression in HEK293 cells infected with the recombinant adenoviruses Ad-ZsGreen-Nogo-A andAd-shRNA-Nogo-A visualized under fluorescence microscopy.There was a high percentage of fluorescent cells in all infected groups. Scale bars: 50 µm.
Nogo-A aggravates oxidative injury in oligodendrocytes
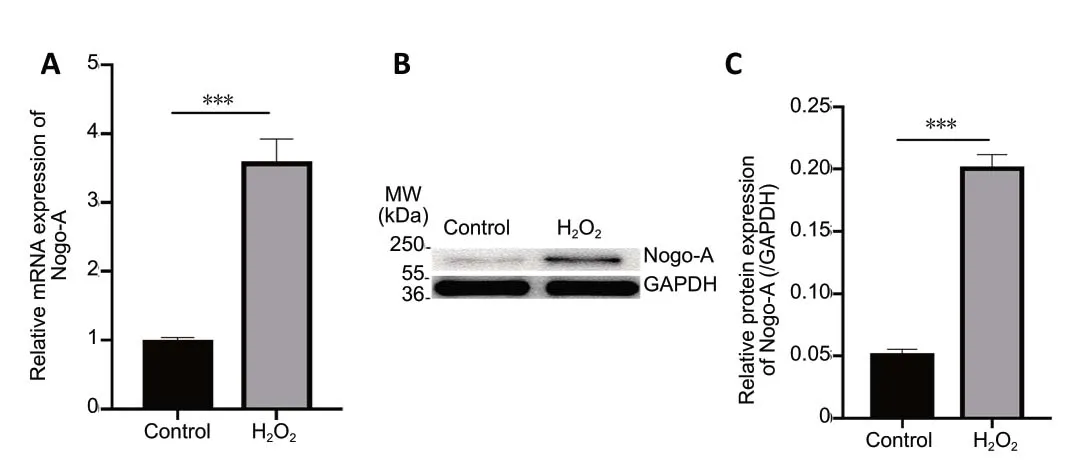
Figure 4 |Effect of H2O2 on Nogo-A levels in oligodendrocytes identified using polymerase chain reaction and western blot assays.(A) Total RNA was extracted from oligodendrocytes exposed to 75 µM H2O2 for 12 hours and used for quantitative real-time polymerase chain reaction.GAPDH was selected as an internal control. Data are expressed as the optical density ratio relative to GAPDH. (B) Proteins from oligodendrocytes exposed to 75 µM H2O2 for 12 hours were analyzed by western blotting with Nogo-A.GAPDH was selected as an internal control. (C) The Nogo-A/GAPDH ratio was quantified from the densitometric scans. Data are expressed as the mean ± SD(n = 3). ***P < 0.001 (independent-samples t-test). GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; H2O2: hydrogen peroxide; MW: molecular weight.

Figure 6 |Transfection efficiencies of Ad-ZsGreen-Nogo-A and Ad-shRNA-Nogo-A verified by western blot assays.(A) Cell lysates treated with phosphate-buffered saline (control), Ad-ZsGreen,or Ad-Nogo-A for 2 hours, and quantitative analysis of Nogo-A are shown. (B)Cell lysates treated with phosphate-buffered saline (control), Ad-shRNA, AdshRNA-Nogo-A-1, Ad-shRNA-Nogo-A-2, or Ad-shRNA-Nogo-A-3 for 2 hours,and quantitative analysis of Nogo-A are shown. GAPDH was selected as an internal control. Quantification of Nogo-A expression was performed using the Nogo-A/GAPDH ratio. Data are expressed as the mean ± SD (n = 5). ***P< 0.001 (one-way analysis of variance). GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; MW: molecular weight.
To investigate the potential role of Nogo-A in oxidative damage in oligodendrocytes, Ad-ZsGreen-Nogo-A was transfected into cells. Overexpression of Nogo-A spontaneously induced cell death and significantly increased susceptibility to H2O2(P<0.001), as revealed by PI staining in the control group (Figure 7A-C). Moreover, the MTT assay showed a similar result for oligodendrocytes transfected with Ad-ZsGreen-Nogo-A (P< 0.001;Figure 7DandE). Co-treatment of H2O2with Ad-ZsGreen-Nogo-A infection or Ad-ZsGreen-Nogo-A infection 30 minutes after H2O2treatment did not increase oxidative injury(Figure 7CandE). These results suggest that oligodendrocytic Nogo-A is involved in oxidative damage.
Suppression of Nogo-A decreases the susceptibility of oligodendrocytes to H2O2
We next knocked down the expression of Nogo-A in oligodendrocytes to further validate the role of Nogo-A in oxidative damage in oligodendrocytes. PI/Hoechst staining(Figure 8A-C) and MTT assays (Figure 8DandE) showed that silencing of Nogo-A in oligodendrocytes clearly protected oligodendrocytes from H2O2compared with control (P<0.01). In addition, we tested the time dependency of the protective effects of Ad-shRNA-Nogo-A. Pretreatment with AdshRNA-Nogo-A exerted a protective effect, but H2O2and AdshRNA-Nogo-A co-treatment or Ad-shRNA-Nogo-A infection 30 minutes after H2O2treatment had no protective effect(Figure 8CandE). These results indirectly demonstrated the negative role of Nogo-A in the oxidative damage process in oligodendrocytes.

Figure 7 |Pretreatment with Ad-ZsGreen-Nogo-A aggravates H2O2-induced oxidative injury in oligodendrocytes.(A) Oligodendrocyte apoptosis was analyzed by PI/Hoechst staining. In the control group, the cells exhibited normal nuclear morphometry, with few PIpositive cells (red fluorescence). After treatment with 75 µM H2O2 for 12 hours,the cells showed clear apoptosis, karyopyknosis, and nuclear fragmentation and there was an increase in the number of dead cells. After pretreatment with Ad-ZsGreen-Nogo-A, the quantity of abnormal cells and nuclei was increased. Scale bars: 50 mm. (B, C) The cell death rate was calculated as PI(+)/Hoechst (+). (D, E) The cell death rate was also examined by MTT assays.Data are expressed as the mean ± SD (n = 5). **P < 0.01, ***P < 0.001 (oneway analysis of variance). H2O2: Hydrogen peroxide; PI: propidium iodide; MTT:3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide.
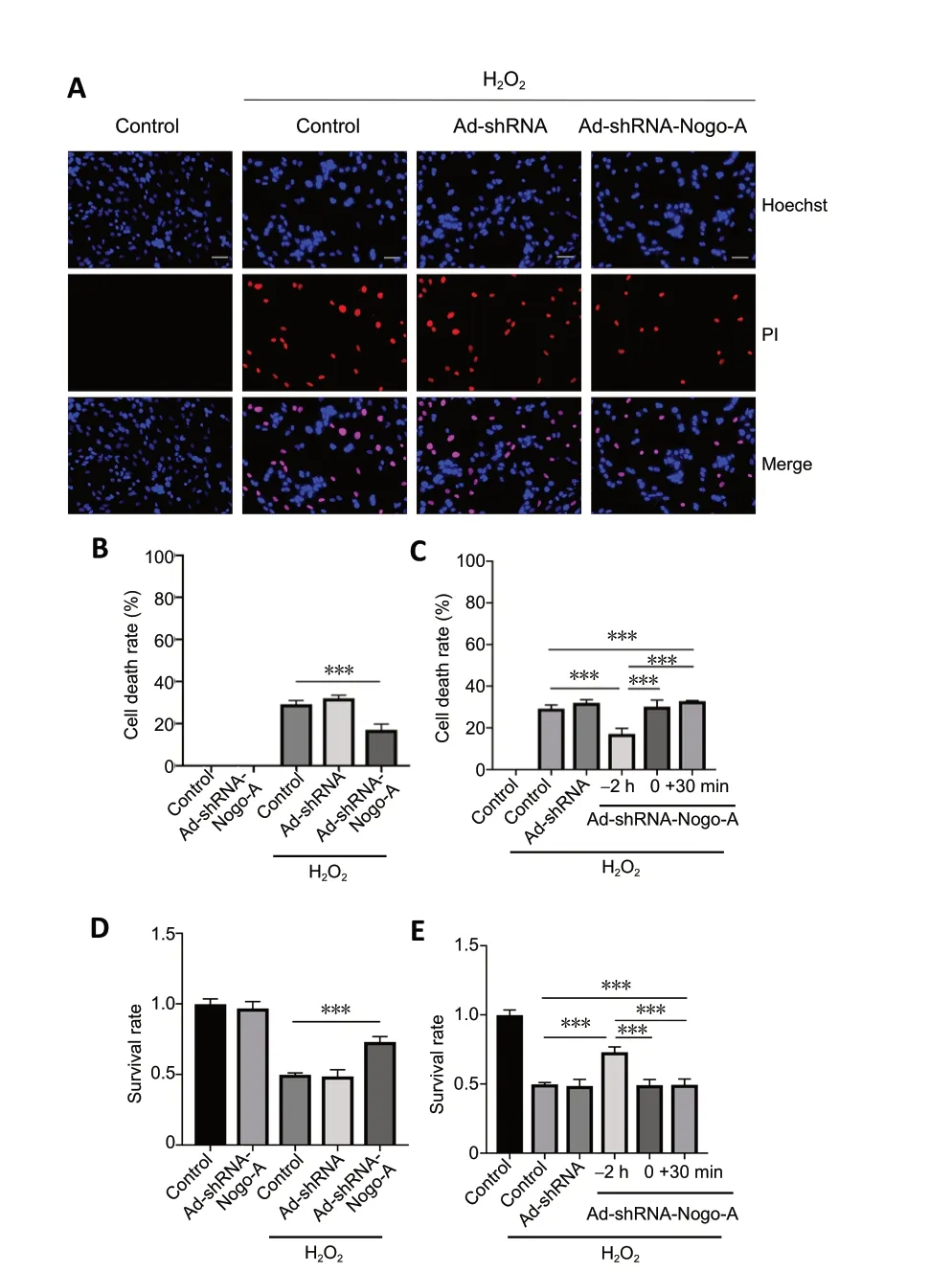
Figure 8 |Ad-shRNA-Nogo-A pretreatment protects rat oligodendrocytes against H2O2-induced cell death.(A) Oligodendrocyte apoptosis was analyzed by PI/Hoechst staining. In the control group, cells exhibited normal nuclear morphometry, with few PI-positive cells (red fluorescence). After treatment with 75 µM H2O2 for 12 hours, the cells showed clear apoptosis, karyopyknosis, and nuclear fragmentation and there was an increase in the number of dead cells.Scale bars: 50 mm. (B, C) The cell death rate was calculated by the PI (+)/Hoechst (+) ratio. (D, E) The cell death rate was also examined by MTT assays. Data are expressed as the mean ± SD (n = 5). ***P < 0.001 (one-way analysis of variance). H2O2: Hydrogen peroxide; PI: propidium iodide; MTT:3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Nogo-A increases intracellular ROS generation induced by H2O2
Once H2O2enters the cell, it triggers abundant reactive oxygen species (ROS) generation and initiates a series of signaling pathways (Schwab, 2010). We next determined whether Nogo-A could increase cellular ROS and aggravate oxidative death. ROS were thus assayed using the fluorescent probe,DHE (Figure 9A). Flow cytometry revealed that Ad-ZsGreen-Nogo-A pretreatment increased the level of H2O2-induced ROS (P< 0.001;Figure 9B). The ROS pattern (Figure 9C)corresponded with the time-dependent damage effect of Nogo-A in oligodendrocytes (Figure 7). Therefore, this finding strongly indicated that the ability of oligodendrocytes to resist H2O2is weakened through increased production of ROS by Ad-ZsGreen-Nogo-A.
The Nogo-66-NgR/LINGO1 and RhoA-ROCK signaling pathways are not involved in the oxidative injury induced by H2O2
Many of the known regulatory roles of Nogo-A rely on the Nogo-66-Nogo-66 receptor (NgR)/p75/LINGO1-RhoA-ROCK pathway (Schwab, 2010). We thus assessed whether these components participated in the oxidative injury induced by H2O2. Cell death assays found no significant differences compared with controls with several different signaling blockers, including a Nogo-66 antibody, a LINGO1 blocker, and the ROCK inhibitor Y27632 (Figure 10).
ERK and BCL2 might participate in Ad-shRNA-Nogo-Amediated oligodendrocyte survival against oxidative injury
ERK1/2 is a major mediator of oxidative stress (Subramaniam and Unsicker, 2010). Western blot data showed that Nogo-A knockdown increased the levels of p-ERK1/2, but that these levels decreased after H2O2stimulation (Figure 11A). The reduction in the levels of p-ERK1/2 induced by H2O2was substantially increased by Nogo-A knockdown (P< 0.01;Figure 11AandC). These data demonstrated that p-ERK1/2 might participate in oxidative stress.2 hours before, simultaneously with, or 30 minutes after H2O2treatment.Data are expressed as the mean ± SD (n= 3). ***P< 0.001 (one-way analysis of variance). DHE: Dihydroethidium; H2O2: hydrogen peroxide; ROS: reactive oxygen species.

Figure 9 |Ad-ZsGreen-Nogo-A increases intracellular ROS induced by H2O2.(A) Intracellular ROS was measured by flow cytometry. (B, C) Summary data of DHE-positive cells. Ad-Nogo-A was added to the oligodendrocyte cultures
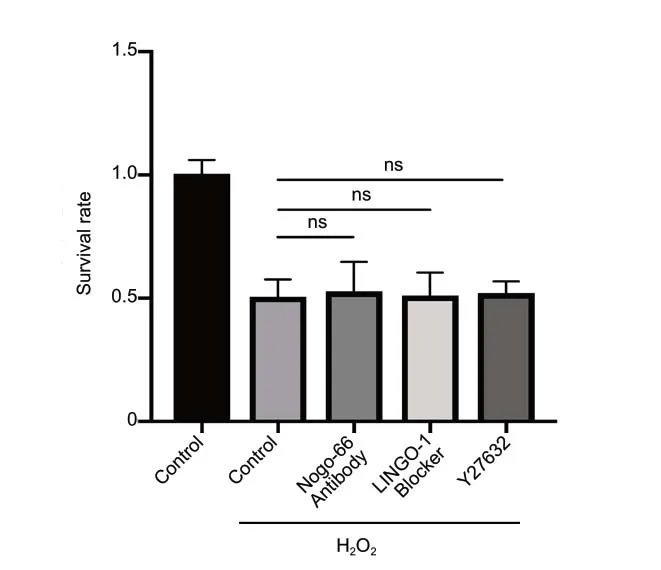
Figure 10 |Nogo-66-NgR/LINGO1 and RhoA-ROCK signaling pathways are not involved in the oxidative injury induced by H2O2.Cell death rates were examined by MTT assays. Data are expressed as the mean ± SD (n = 4), and analyzed by one-way analysis of variance. H2O2:Hydrogen peroxide; LINGO1: leucine-rich repeat and immunoglobinlike domain-containing protein 1; MTT: 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide; NgR: Nogo-66 receptor; ns: not significant;ROCK: Rho-associated protein kinase.
Krishna et al. (2011) demonstrated a relationship between the prosurvival protein BCL2 and ROS. As expected, western blotting showed that Ad-shRNA-Nogo-A prevented the decrease in BCL2 levels induced by H2O2(P< 0.01;Figure 11BandD). These findings support our results that Nogo knockdown antagonizes oligodendrocyte oxidative damage by regulating ROS and its downstream pathways.
Discussion
Nogo-A is highly expressed in oligodendrocytes and plays a negative role in traumatic injury by inhibiting neurite growth(Ueda et al., 2002), as suggested by its name “Nogo”. However,its pathophysiological role in oligodendrocytes exposed to oxidative stress has been unclear. Here, we establish a negative role of Nogo-A in the oxidative injury induced in oligodendrocytes by H2O2and identify elevated ROS levels in these Ad-Nogo-A-transfected oligodendrocytes. Endogenous Nogo-A protein was significantly overexpressedin vitroupon H2O2exposure for 12 hours, and its overexpression increased susceptibility to acute oxidative insults and markedly elevated the rate of oligodendrocyte death. However, knockdown of Nogo-A robustly protected the oligodendrocytes.
H2O2treatment reduces ERK1/2 activation in oligodendrocytes,while Nogo-A knockdown increases it. The degree of reduction in the levels of p-ERK1/2 induced by H2O2was, however,increased by Nogo-A knockdown. ERK1/2 activation was initially considered to promote neuronal survival (Grewal et al., 1999). Nonetheless, ERK1/2 activation was later discovered to also operate with a variety of neuronal death signals(Subramaniam and Unsicker, 2010). These results might result
from the bidirectional functions of p-ERK1/2, indicating that ERK1/2 is involved in the process of oxidative stress. However,its specific roles need to be explored in further experiments.As with p-ERK1/2, BCL2 was increased by Nogo-A knockdown and decreased by H2O2treatment. However, Nogo-A knockdown depressed the decrease of BCL2 induced by H2O2.It has been demonstrated that upregulation of BCL2 is an important cell survival marker, correlating with responsiveness to drug therapyin vivoandin vitro(Sartorius and Krammer,2002). Therefore, the relative increase in the level of BCL2 in oligodendrocytes after Nogo-A knockdown was reasonable for its resistance to the oxidative damage induced by H2O2.
In our study, the Nogo-66-NgR/LINGO1 and RhoA-ROCK signaling pathways were not involved in the oxidative injury of oligodendrocytes, as is seen in neurons (Mi et al., 2012).Explanation of these results should take into account that RhoA/ROCK activates downstream effectors, which regulate cytoskeletal reorganization, such as growth cone collapse and neurite outgrowth inhibition (Lehmann et al., 1999). H2O2induces cell death or apoptosis, not cytoskeleton collapse, so it might be anti-apoptotic or pro-survival factors rather than the Nogo-66 pathway that participates in oxidative stress.Thus, it is possible that the Nogo-66-NgR/LINGO1 and RhoAROCK signaling pathways do not participate in the process of oxidative injury.
Despite the deleterious role of Nogo-A in oligodendrocytes,Mi et al. (2012) found that neuronal Nogo-A might play a cell-autonomous role in improving neuronal survival against oxidative insult and scavenging of ROS. In terms of a difference in function of Nogo-A between oligodendrocytes and neurons, Zemmar et al. (2018) found that neuronal Nogo-A affects proximal dendrites, whereas oligodendrocytic Nogo-A affects distal regions. In addition, Meves et al. (2018)found that it is oligodendrocytic, and not neuronal, Nogo that restricts corticospinal axon sprouting after central nervous system injury. These recent studies illustrate the distinct roles of Nogo-A in oligodendrocytes and neurons.
Therefore, Nogo-A in different locations might be involved in different reactions to oxidative insult and our findings pave the way for a new understanding of the involvement of Nogo-A in oligodendrocyte oxidative injury. Because we found that Nogo-A is a deleterious factor in the presence of oxidative stress, we assume that Nogo-A is a negative force for oligodendrocytes in ALS, which has been confirmed to involve oxidative insult. ALS exhibits degeneration and impaired regeneration of gray matter oligodendrocytes (Kang et al.,2013). However, the role played by Nogo-A in the process of oligodendrocyte impairment in ALS is unclear. More studies should thus be performed to examine this topic. In this study,we have not explained the mechanisms by which Nogo-A damages oligodendrocytes, or the exact roles of p-ERK in Nogo-A aggravation of oxidative injuries. Furthermore, the interaction between oligodendrocytes and neurons with regard to oxidative stress is unresolved. Thus, we plan to coculture oligodendrocytes and neuronsin vitroto determine the influence of oligodendrocytes on neurons in the presence of oxidative stress.
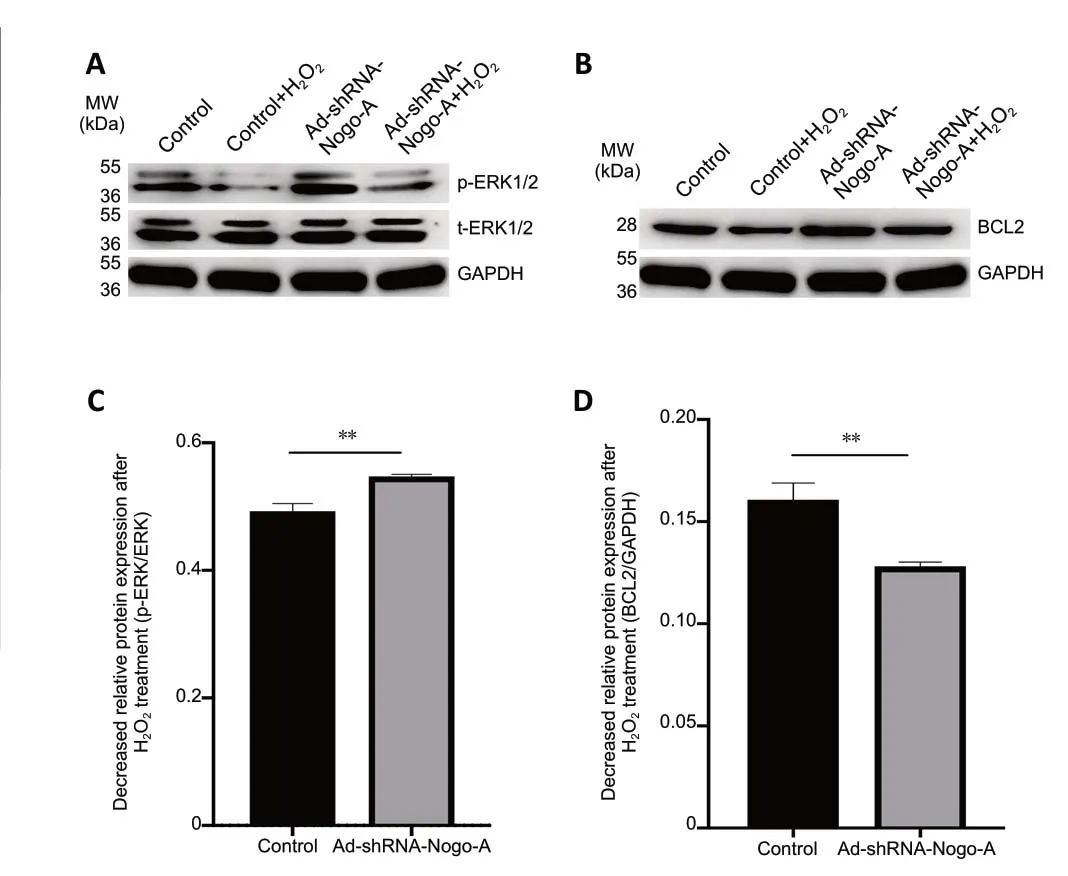
Figure 11 |Ad-shRNA-Nogo-A increases the decrease in expression of p-ERK and suppresses the decrease in BCL2 induced by H2O2.(A) Cell lysates collected from oligodendrocyte cultures treated with 75 µM H2O2 for 12 hours with or without pre-infection with Ad-shRNA-Nogo-A plasmid were subjected to western blot analysis using antibodies against p-ERK1/2 or t-ERK1/2. GAPDH was selected as an internal control. Quantification of densitometric scans was performed using the p-ERK/t-ERK ratio. (B) Lysates from oligodendrocytes treated with H2O2 for 12 hours with or without AdshRNA-Nogo-A plasmid pre-infection were subjected to western blotting with an anti-BCL2 antibody. GAPDH was selected as an internal control.Quantification of densitometric scans was performed using the BCL2/GAPDH ratio. Data are expressed as the mean ± SD (n = 3). **P < 0.01 (independentsamples t-test). ERK1/2: Extracellular signal-regulated kinase 1/2; GAPDH:glyceraldehyde 3-phosphate dehydrogenase; H2O2: hydrogen peroxide; MW:molecular weight; p-ERK1/2: phosphorylated ERK1/2; t-ERK1/2: total ERK1/2.
Acknowledgments:We thank Dr. Ling Liu (Department of Neurology, Peking University People’s Hospital, China) for her instruction in cell culture. We are also grateful to Dr. Wei-Dong Yu (Department of Medical Laboratory, Peking University People’s Hospital, China) for his tremendous advice during the process of this study.
Author contributions:Study design: JZ; experimental implementation: YYW,NH; data analysis: YYW, DJH; manuscript writing: YYW. All authors approved the final version of the manuscript.
Conflicts of interest:There were no conflicts of interest in this experiment.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81870996 (to JZ). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:This study was approved by the Ethics Committee of Peking University People’s Hospital, China (approval No.2018PHC081) on December 18, 2018.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Ilias Kazanis, University of Cambridge, UK; Rodrigo Franco, University of Nebraska-Lincoln, USA.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Information for Authors-Neural Regeneration Research
- Microglia-associated neuroinflammation is a potential therapeutic target for ischemic stroke
- Argon reduces microglial activation and inflammatory cytokine expression in retinal ischemia/reperfusion injury
- Laminin-coated multifilament entubulation, combined with Schwann cells and glial cell line-derived neurotrophic factor, promotes unidirectional axonal regeneration in a rat model of thoracic spinal cord hemisection
- Insights into stem cell therapy for diabetic retinopathy:a bibliometric and visual analysis
- Deletion of Krüppel-like factor-4 promotes axonal regeneration in mammals