Melatonin relieves heat-induced spermatocyte apoptosis in mouse testes by inhibition of ATF6 and PERK signaling pathways
De-Zhe Qin ,Hui Cai ,Chen He ,Dong-Hui Yang ,Jing Sun ,Wen-Lai He ,Ba-Lun Li,Jin-Lian Hua,Sha Peng,*
1 College of Veterinary Medicine, Shaanxi Centre of Stem Cells Engineering & Technology, Northwest A & F University, Yangling, Shaanxi 712100, China
ABSTRACT Normal spermatogenic processes require the scrotal temperature to be lower than that of the body as excessive heat affects spermatogenesis in the testes,reduces sperm quality and quantity,and even causes infertility. Endoplasmic reticulum stress(ERS) is a crucial factor in many pathologies.Although several studies have linked ERS to heat stress,researchers have not yet determined which ERS signaling pathways contribute to heat-induced testicular damage.Melatonin activates antioxidant enzymes,scavenges free radicals,and protects the testes from inflammation;however,few studies have reported on the influence of melatonin on heatinduced testicular damage.Using a murine model of testicular hyperthermia,we observed that heat stress causes both ERS and apoptosis in the testes,especially in the spermatocytes.These observations were confirmed using the mouse spermatocyte cell line GC2,where the Atf6 and Perk signaling pathways were activated during heat stress.Knockout of the above genes effectively reduced spermatocyte damage caused by heat stress.Pretreatment with melatonin alleviated heat-induced apoptosis by inhibiting the Atf6 and Perk signaling pathways.This mitigation was dependent on the melatonin receptors.In vivo experiments verified that melatonin treatment relieved heat-induced testicular damage.In conclusion,our results demonstrated that ATF6 and PERK are important mediators for heat-induced apoptosis,which can be prevented by melatonin treatment.Thus,our study highlights melatonin as a potential therapeutic agent in mammals for subfertility/infertility induced by testicular hyperthermia.
Keywords: Heat stress; ATF6; PERK; Melatonin;Spermatocyte
INTRODUCTION
Infertility affects 10%–15% of all reproductive-aged couples and is of major clinical,social,and economic concern (Lotti & Maggi,2018).Male infertility mainly manifests by changes in sperm density,quantity,quality,or volume (Kumar & Singh,2015).Scrotal temperature is currently recognized as an important factor of male infertility by the World Health Organization,with a strong link found between increased testicular temperature and decreased fertility (Jung & Schuppe,2007).Various factors,such as occupation,living habit,and clinical disease,can raise the temperature of the testes.Prolonged occupational heat exposure,such as for chefs and welders,can increase scrotal temperature,resulting in a decrease in semen quality (Thonneau et al.,1997).Longterm use of laptop computers and extended periods of sitting(e.g.,professional drivers) can also increase scrotal temperature,leading to decreased fertility (Garolla et al.,2013).
Spermatogenesis requires the scrotal temperature to be lower than that of the body (Danno et al.,2000).Studies have shown that men with higher scrotal temperature show subfertility/infertility,and sperm quality worsens as the temperature increases (Mieusset et al.,1987).Spermatocytes and mature sperm cells are sensitive to changes in temperature.The cells most vulnerable to heat damage in humans and rats are zygotene and pachytene spermatocytes and early round spermatozoa (Cooke & Saunders,2002).Heat stress damages spermatogenic cells through apoptosis,autophagy,DNA damage,and excessive production of reactive oxygen species.Increased testicular temperature also interferes with the function and morphology of Sertoli cells and disrupts the tight connection between cells and blood-testis barrier (Zhang et al.,2020).
The endoplasmic reticulum (ER) regulates cell function by controlling calcium ion homeostasis,lipid synthesis,and protein folding.When protein synthesis and transportation are impaired or Ca2+uptake and release are dysfunctional,many unfolded or misfolded proteins accumulate in the ER cavity,causing ER stress (ERS) (Santofimia-Castaño et al.,2016).To resist the effects of ERS,cells initiate a protective unfolded protein response (UPR) to restore ER protein folding homeostasis (Hetz & Papa,2018).ERS can be induced by three independent signaling pathways,including activated transcription factor 6 (ATF6),inositol-requiring enzyme (IRE1),and protein kinase R-like endoplasmic reticulum kinase(PERK) (Davenport et al.,2008).BiP/GRP78 is an important molecular chaperone in the ER lumen and plays an important role in the synthesis of related proteins in the cascade signaling pathway (Ibrahim et al.,2019).
The relationship between heat stress and ERS varies according to the intensity of heat stimulation and the type of cells involved.Heat stress usually accompanies other stress responses,as seen in bovine granulosa cells whereNrf2-mediated oxidative stress and ERS are triggered after heat stimulation (Alemu et al.,2018).Heat stress also has different effects on ERS signaling pathways.PERK reduces cell damage and maintains cell homeostasis by activating eIF2α phosphorylation (Khoutorsky et al.,2016).Furthermore,heat stress can cause the down-regulation of IRE1α,which effectively activates autophagy and then the UPR negative feedback system by regulating the IRE1α-XBP-1 axis (Homma & Fujii,2016).
Melatonin (N-acetyl-5-methoxytryptamine) is a neuroendocrine hormone with strong antioxidant activities secreted by the pineal gland (Tan et al.,2007) and synthesized in the testes (Frungieri et al.,2017).This hormone exhibits a distinct circadian synthesis profile,with high levels at night and low levels during the day,synchronized with the environmental light/dark cycle.Melatonin activates antioxidant enzymes,scavenges free radicals (Amaral & Cipolla-Neto,2018),and protects the testes from inflammation (Frungieri et al.,2017).It has both fat-soluble and water-soluble characteristics and can directly pass through cell membranes (Claustrat & Leston,2015).Melatonin can also regulate the homeostasis of apoptosis and autophagy.Phosphorylated BECLIN1 can bind to BCL2,increase free BAX in cells,and cause mitochondrialdependent apoptosis (Pan et al.,2018).Melatonin improves spermatogonial stem cell apoptosis caused by reactive oxygen species and P53 by promoting the expression of MnSOD and SIRT1 (Li et al.,2018).Melatonin can alleviate reproductive damage caused by heat stress by reducing apoptosis and oxidative stress and strengthening the tight junctions between Sertoli cells (Zhang et al.,2020).The function of melatonin depends on melatonin receptors 1 and 2(MT1 and MT2),which are G protein-coupled membrane receptors that can heterodimerize with the G protein receptor.Interactions between MT1 and G protein receptors can influence cellular functions (Samanta,2020).
In the current study,we explored the relationship between ERS and heat-induced testicular damage and investigated the role of ATF6/PERK in spermatocyte apoptosis induced by heat stress.We also tested whether melatonin application protects against heat-induced testicular damage via activation of melatonin receptors and inhibition of ERS (Supplementary Figure S1).
MATERIALS AND METHODS
Ethics approval and consent to participate
All animal experiments were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals(Ministry of Science and Technology of the People’s Republic of China,Policy No.2 006 398) and were approved by the Animal Care and Use Center of Northwest A& F University,Shaanxi,China (approval No.201902A299).
Animals
Experiments were performed on male ICR mice (30–35 g,9 weeks old) obtained from the Laboratory Animal Center of the Fourth Military Medical University,China.The mice were maintained at 20±2 °C and 50%–70% humidity under a 12 h:12 h light:dark cycle.All experimental animals and procedures were approved by the Institutional Animal Care and Use Committee of Northwest A& F University.
Induction of testicular heat stress
Healthy male ICR mice (Dashuo,China) were exposed to a single heat stress treatment at 42 °C.Specifically,the mice were anesthetized,and the lower part of the body,including the hind legs and scrotum,were submerged in a 42 °C thermostatic water bath for 20 min.Following treatment,the mice were placed in an animal room and provided with normal water and mouse chow for recovery over 6–24 h.Finally,the treated mice were euthanized,and testicular samples were collected.The mice were divided into four groups (control and heat-treated groups).For heat treatment,15 mice were divided into three groups (6,12,and 24 h) for one independent experiment,with the treated mice analyzed at each time point. Three independent experiments were performed.
To explore whether melatonin pretreatment can protect mice from the effects of heat-induced testicular damage,the mice were injected with 20 mg/kg melatonin for 7 consecutive days before heat treatment.In the heat treatment group,10 male mice were divided into two groups (con and pre-mel) in one independent experiment;in the no heat stress group,10 male mice were also divided into two groups (con and premel).The testes were extracted after 12 h of recovery.Three independent experiments were conducted.
Histological analysis
For histological analysis,testes were fixed in 4%paraformaldehyde (PFA) solution and testicular sample sections were embedded in paraffin and stained with hematoxylin and eosin (H& E).The integrity and structural modifications of tubule sections were evaluated semiquantitatively,Briefly,a score between 0 and 5 was given for(i) nuclear and (ii) epithelial alterations,with 0 representing the complete absence of alteration and 5 representing the most severe alterations (Dumont et al.,2015;Milazzo et al.,2008).
Cells
The mouse spermatocyte cell line (GC-2) and human embryonic kidney epithelial cell line (293T) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM,Gibco,USA)with 2 mmol/L L-glutamine (Invitrogen,Thermo Fisher Scientific,USA),80 U/mL streptomycin,100 U/mL penicillin(Invitrogen,Thermo Fisher Scientific,USA),and 10% fetal bovine serum (Gibco,Thermo Fisher Scientific,USA).The cells were cultured at 37 °C in a humidified 5% CO2incubator and passaged with a 1:5 subculture ratio.Trypsin-EDTA solution (2–3 mL) was added to the flask and cells were observed under an inverted microscope until the cell layer was dispersed.The cells were dissociated by 0.25% trypsin-EDTA(Invitrogen,Thermo Fisher Scientific,USA) and inoculated to the culture dish,with the medium exchanged every 24 h.
Thermal stress induction in cells
Cells (5×103) were seeded in each well of a 48-well plate.After the cells adhered to the wall for 24 h,they were incubated at 42 °C for 2 h.The cells were then cultured normally for 4 h.
Reverse transcription
We used a FastKing RT Kit (Tiangen Biotech,China) for reverse transcription.Firstly,we used 2 μL 5×gDNA buffer and 1 μg RNA,with water to 10 μL,at 42 °C for 3 min.Secondly,we used 2 μL 10×King RT buffer,1 μL FastKing RT enzyme mix,and 2 μL FQ-RT primer mix,with water to 10 μL,at 42 °C for 15 min and 95 °C for 3 min.
Quantitative real-time polymerase chain reaction (qRTPCR)
We used 10 μL 2×SuperReal Color PreMix (Tiangen Biotech,China) for qRT-PCR,0.2 μL reverse primer,0.2 μL forward primer,1 μL cDNA,and 8.6 μL distilled water.The qPCR instrument was purchased from BIO-RAD (CFX Connect Optics Module,Serial NO.788BR08727,Singapore).The reaction steps were:95 °C for 15 min,followed by 40 cycles at 95 °C for 10 s,60 °C for 30 s,and 72 °C for 30 s,then 72 °C for 10 min,and 4 °C for permanent preservation.All datasets are expressed as fold-change relative to the control,i.e.,delta Ct usingGapdhorβ-actinas the housekeeping genes.The primers used for qRT-PCR are shown in Supplementary Table S1.Each primer was localized to the extron.
Immunofluorescence staining
The preparation of the cell and testis samples is described above. Immunofluorescence staining was performed as reported recently (Xu et al.,2020).All antibody details are given in Supplementary Table S2.
Western blot (WB) analysis
Protein was extracted from testicular tissue and cells using RIPA lysis buffer (Beyotime,China) with phosphatase inhibitor and phenylmethane sulfonyl fluoride (PMSF) (Beyotime,China).Details on WB analysis are described in our previous article (Yang et al.,2021).The antibodies used are shown in Supplementary Table S2.Semi-quantification of WB was performed via Image J.
TUNEL assay
Cell and testis sample treatment is described above.Cells were fixed with 4% PFA for 30 min and washed three times with phosphate-buffered saline (PBS).We added 0.3% Triton X-100 (Beyotime,China) to penetrate the cell membrane for 10 min,then washed the cells twice using PBS.The samples were added with 50 μL TUNEL assay solution (Beyotime,China) and incubated at 37 °C for 60 min,while protected from light.The cells were incubated with 1 μg/mL Hoechst 33 342 dye solution at room temperature for 5 min in the dark,then washed with PBS.The samples with apoptotic cells were visualized by fluorescence microscopy.
Testis sections were subjected to the following reagents in sequence:xylene (1) for 10 min,xylene (2) for 10 min,absolute ethanol for 3 min,90% alcohol for 3 min,70% alcohol for 3 min,and distilled water for 2 min.We added 20 μg/mL DNase-free proteinase K to the sections,which were then incubated at 25 °C for 20 min and washed three times with PBS. The slides were incubated with terminal deoxynucleotidyl transferase (TdT) enzyme for 1 h at 37 °C,followed by counterstaining with Hoechst 33 342 for 5 min.Finally,the sections were observed by fluorescence microscopy.
Flow cytometry
An Annexin V-FITC/PI apoptosis kit (Multisciences,China)was used to assess cell apoptosis distribution.Cells (1×105)were seeded in a 6-well plate and preprocessed with melatonin for 24 h.According to the description above,cells were induced by heat stress.The cells were then collected in 1.5 mL tubes and resuspended in 500 μL cold 1×binding buffer.To each tube was added 5 μL Annexin V-FITC and 10 μL PI,followed by incubation for 5 min at room temperature in the dark.Cells were analyzed on an EPICS ALTRA flow cytometer (Beckman Altra,USA).Annexin V-FITC (Ex=488 nm;Em=530 nm) was detected using the FITC detection channel and propidium iodide (PI) (Ex=535 nm;Em=615 nm)was detected using the PI detection channel.The cells were single stained,with the negative control group set to 0.1%–0.3%.Data were analyzed using Expo32 v1.2.
Knockdown of PERK and ATF6 in GC2 cells
Primer sequences for down-regulating PERK and ATF6 short hairpin RNAs (siRNAs) were designed using the Contech siRNA designer as follows:
siRNAs-PERK: GATCCGCAGGTCCTTGGTAATCATCTCAA GAGGATGATTACCAAGGACCTGCTTTTTTg
siRNAs-ATF6:AATTCAAAAAAAAGAGCAGATCGAAGTCAT ACTCTTGATATGACTTCGATCTGCTCTTg
The siRNAs (Sangon,China) were inserted into the CD513B-U6 vector to obtain sh-PERK-CD513B-U6 and sh-ATF6-CD513B-U6 eukaryotic expression plasmids. The inserts were verified and sequenced by Sangon Biotech(China).The inserts were verified using PCR and double restriction enzyme digestion with EcoR I and BamH I.The 293-T cells were used for lentiviral packaging.In total,1×105GC2 cells were cultured in a 6-well plate before infection with the lentivirus for 12 h.The infected cells were screened by puromycin and detected by qRT-PCR.
Statistical analysis
All data are presented as mean±standard deviation (SD) and were analyzed using unpaired two-tailed Student’st-tests.Pvalues of <0.05 and <0.01 were considered statistically significant and highly statistically significant,respectively.
RESULTS
Heat stress causes testicular damage in male mice
To identify the effects of hyperthermia,mouse testes were exposed to heat by immersing the scrotum into a 42 °C thermostatic water bath for 20 min.The mice were examined at 6,12,and 24 h after heat treatment.Based on H& E staining,heat-stress exposure caused obvious testicular damage at 12 h (Figure 1A;Supplementary Figure S2A).Although there was no significant change in testicular size,the testicular weight/body weight ratio decreased at 12 h after heat treatment (Figure 1B,C).Based on TUNEL assay,apoptosis was found in the testicular tissue at 12 h after heat treatment (Figure 1D;Supplementary Figure S2B).The mRNA expression levels ofCaspase3,Caspase9,Caspase12,andHsp 70indicated that heat stress and apoptosis both occurred in the male mouse testes (Figure 1E).Hyperpyrexia enhanced the expression of HSP70 and cleaved CASPASE3 at the protein level (Figure 1F–G).Statistical analysis verified the above results.Hence,our results confirmed that heat stress can cause testicular apoptosis and damage.
Atf6 and Perk signaling pathways are enhanced by heat stress in mouse testes
To explain why heat stress induced testicular damage and apoptosis,we extracted mRNA from the heat-treated mouse testes and performed qRT-PCR for markers of ERS (Grp78)and key factors of the three signaling pathways (ATF6,IRE1,PERK).Results showed that the expression levels of ATF6,GRP78,and PERK were significantly higher in heat-treated mice (Figure 2A,B).Importantly,based on TUNEL and BOULE co-immunostaining,most apoptosis-positive cells were located in the spermatocytes (Figure 2C).Immunofluorescence staining revealed that the heat-exposed testes mainly activated the ATF6 and p-PERK proteins rather than p-IRE1 (Figure 2D–F;Supplementary Figure S2C–E).Hence,we speculated that hyperthermia may promote activation of theAtf6andPerksignaling pathways,with spermatocytes being the most severely affected cells.
Heat stress induces spermatocyte apoptosis and ERS
We used the mouse spermatocyte cell line GC2 to address whether hyperthermia affected spermatocyte proliferationin vitro.qRT-PCR showed that the expression levels ofHsp70andHsp27,members of the heat shock protein family expressed in large quantities when stimulated by heat,were up-regulated at each time point (0,2,4,and 6 h) after 42 °C exposure (Figure 3A).At 4 h after exposure to 42 °C,the mRNA expression levels ofGrp78,Chop,andCaspase3and the ERS signaling pathways were significantly increased(Figure 3B,C).These results implied that heat stress promoted spermatocyte apoptosis and ERS,but the changes to different ERS signaling pathways were not consistent.Western blotting and statistical analysis showed that the expression levels of p-PERK and ATF6 increased significantly at different time points,while the phosphorylation level of IRE showed no significant or consistent change.The ER apoptotic protein CASPASE12 was also slightly up-regulated(Figure 3D,E). To verify the above results,immunofluorescence and TUNEL staining were used on GC2 cells after 2 h of heat treatment and 4 h of recovery.The number of TUNEL-positive cells increased significantly after heat stress (Figure 3F;Supplementary Figure S2F).The activation of HSP27 and HSP70 indicated that exposure to 42°C caused heat stress in spermatocytes,while the high expression of GRP78 in the cytoplasm and localization of CHOP in the nucleus indicated the presence of ERS(Figure 3G,H;Supplementary Figure S2G).Moreover,the marked changes in the expression levels of p-PERK and ATF6 in comparison with IRE1 demonstrated that the three different ERS signaling pathways play different roles in heatinduced spermatocyte damage (Figure 3I;Supplementary Figure S2G).Thus,our results indicated that p-PERK and ATF6 play dominant roles in spermatocyte damage induced by high temperature.
Knockdown of ATF6 and PERK relieves heat-induced damage
To address whether ATF6 and PERK induced GC2 cell apoptosis,two cell lines were established,i.e.,cells with ATF6 knocked down (si-ATF6) and cells with PERK knocked down(si-PERK).qRT-PCR showed an~80% decrease in the expression levels ofAtf6andPerkin cells following ATF6 and PERK knockdown (Figure 4A;Supplementary Figure S3A).As an internal control,western blotting and statistical analysis showed that the expression levels of ATF6 and p-PERK were reduced in si-RNA (Figure 4B,C).Both cell lines were exposed to 43 °C for 2 h individually,then incubated at 37 °C for 4 h.When the expression levels of ATF6 and PERK decreased in the GC2 cells,the expression levels of apoptotic genesCaspase12,Caspase3,andChopalso subsequently decreased (Figure 4D–F;Supplementary Figure S3B).Taken together,these results indicate that hyperthermia can induce spermatocyte apoptosis and ERS,while inhibition of ATF6 and PERK can significantly alleviate cell damage.

Figure 1 Heat stress causes testicular damage in male mice
Melatonin reduces spermatocyte damage caused by heat stress via ATF6 and PERK
Melatonin is reported to regulate ERS,autophagy,and apoptosis in different cell types,such as tumor cells,tumor microenvironment (TME) cells,and liver cells (Fernández et al.,2015;Mortezaee et al.,2019).Therefore,we further explored the effects of melatonin on relieving spermatocyte damage induced by heat stress through ERS signaling pathways.Flow cytometry was used to determine the occurrence of cell apoptosis in melatonin and heat-treated spermatocytes.We found that the level of apoptosis with 10−6mmol/L melatonin (6.4%) pretreatment was significantly lower than that in the heat-stress group (16.6%) 4 h after exposure (Figure 5A).The rate of apoptosis in the 10−6mmol/L melatonin-treated cells was lower than that in the heat-stress group,as determined by PI staining (Figure 5B,C).We detected the expression levels of markers of ERS and apoptosis in the testes of the four heat-treated groups at 4 h after heat treatment.Results showed thatCaspase3andCaspase12,which are marker genes for apoptosis,decreased at the mRNA level following melatonin pretreatment(Figure 5E; Supplementary Figure S3C). The mRNA expression levels ofPerkandAtf6were also inhibited by 10−7melatonin pretreatment (Figure 5D;Supplementary Figure S3C).Western blot analysis showed that the changes in the protein levels of ATF6,p-PERK,c-CASPASE3,and CASPASE12 were consistent with the mRNA levels(Figure 5F–H).Our results suggest that melatonin inhibits theAtf6andPerksignaling pathways,and thus plays a protective role against heat-induced damage in spermatocytes.

Figure 2 Atf6 and Perk signaling pathways are enhanced by heat stress in testes
MT1/MT2 receptors are essential for melatonin to relieve heat stress in spermatocytes
Melatonin activates two G protein-coupled receptors,MT1 and MT2,to exert beneficial actions in a variety of physiological processes (Liu et al.,2016).To evaluate the importance of melatonin receptors in inhibiting ERS and apoptosis,we performed western blotting to detect the expression levels of MT1 and MT2 in spermatocytes pretreated with melatonin.Results confirmed that MT1 and MT2 were activated in the melatonin-pretreated spermatocytes (Figure 6A,B).Immunofluorescence staining also showed that the expression of MT1 and MT2 increased significantly upon the addition of melatonin (Figure 6C; Supplementary Figure S2H).Furthermore,when luzindole was used as an inhibitor against MT1 and MT2 expression,suppression was observed (see Figure 3),and the protective effects of melatonin on spermatocyte damage caused by heat stress were eliminated(Figure 6D,E).These data suggest that the effects of melatonin on spermatocytes are mediated by the MT1 and MT2 receptors.
Melatonin alleviates apoptosis of testicular spermatocytes caused by heat stress in vivo
To verify the potential of melatonin as a clinical drug to alleviate heat stress,we conducted a complementary experiment in male mice.Before 42 °C heat treatment to the testes,mice were exposed to consecutive injections of melatonin (20 mg/kg bw/day) for 7 days,with testicular tissue sections subsequently examined by H& E staining.In the group without melatonin pretreatment,clumps of cells appeared in the lumen and the tubules showed vacuolization after heat treatment.In contrast,in the melatonin pretreatment group,numerous tubules were seen with normal seminiferous epithelium and vacuolization was rarely observed (Figure 7A).TUNEL staining was used to further examine the antiapoptotic effects of melatonin on the testes in heat-stressed mice.The rate of apoptosis in testes treated with melatonin was significantly lower than that in the heat-stress treatment group (Figure 7B;Supplementary Figure S2I).To evaluate the effects of melatonin on inhibiting ERS,we used western blotting to detect the expression levels of PERK and ATF6 in testes pretreated with melatonin.Results confirmed that PERK and ATF6 were activated in the melatonin-pretreated group(Figure 7C).Immunofluorescence staining showed that the expression of ATF6 was significantly reduced in the melatonin-pretreated group (Figure 7D).Cumulatively,our data indicate that melatonin can protect the testes from damage caused by heat stress.
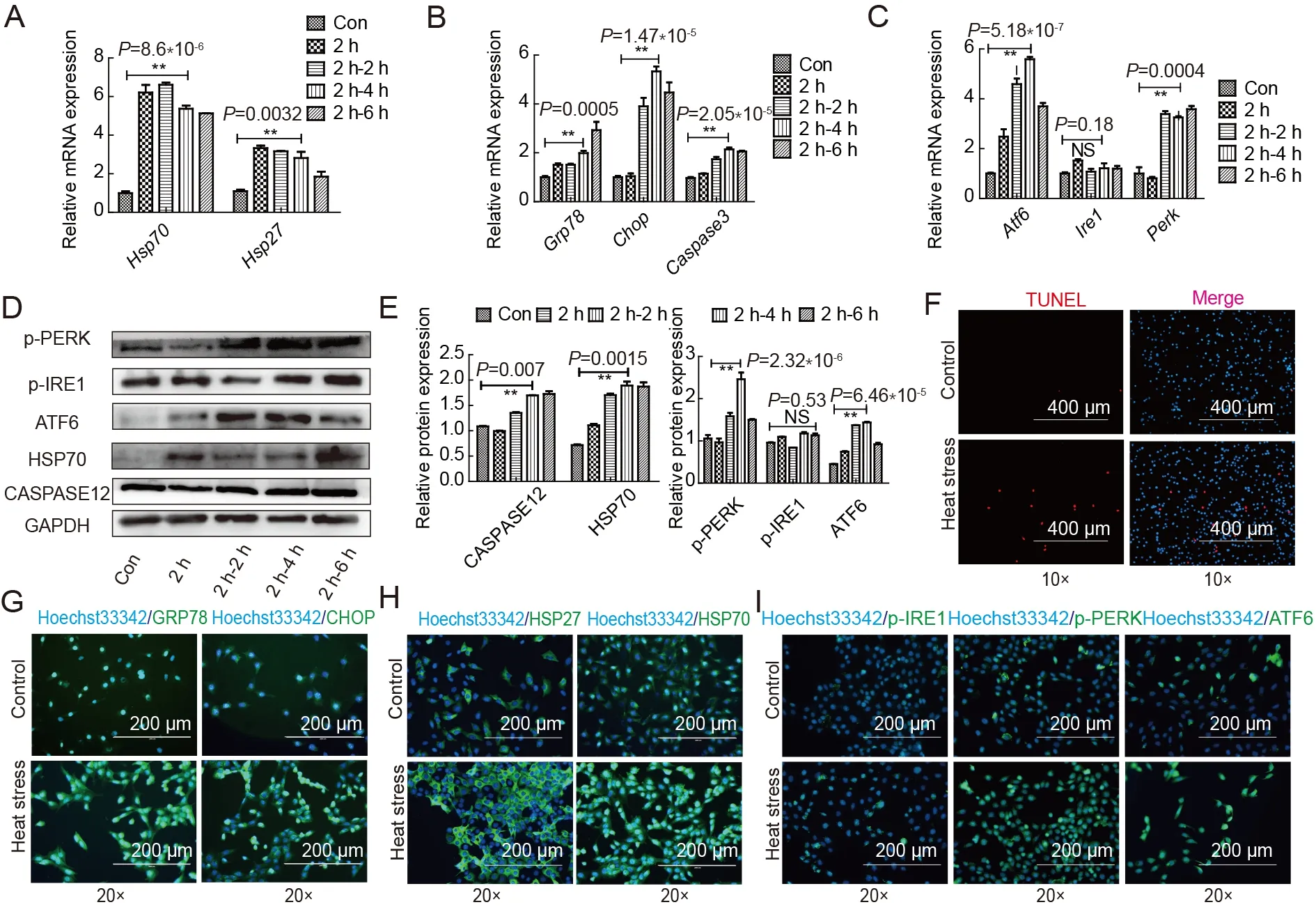
Figure 3 Heat stress induces spermatocyte apoptosis and endoplasmic reticulum stress

Figure 4 Knockdown of ATF6 and PERK relieves heat-induced damage
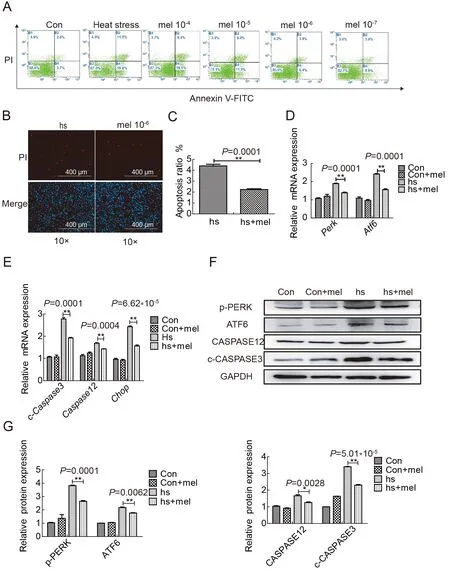
Figure 5 Melatonin relieves spermatocyte damage caused by heat stress through ATF6 and PERK

Figure 6 MT1/MT2 receptors are essential for melatonin to relieve heat stress in spermatocytes
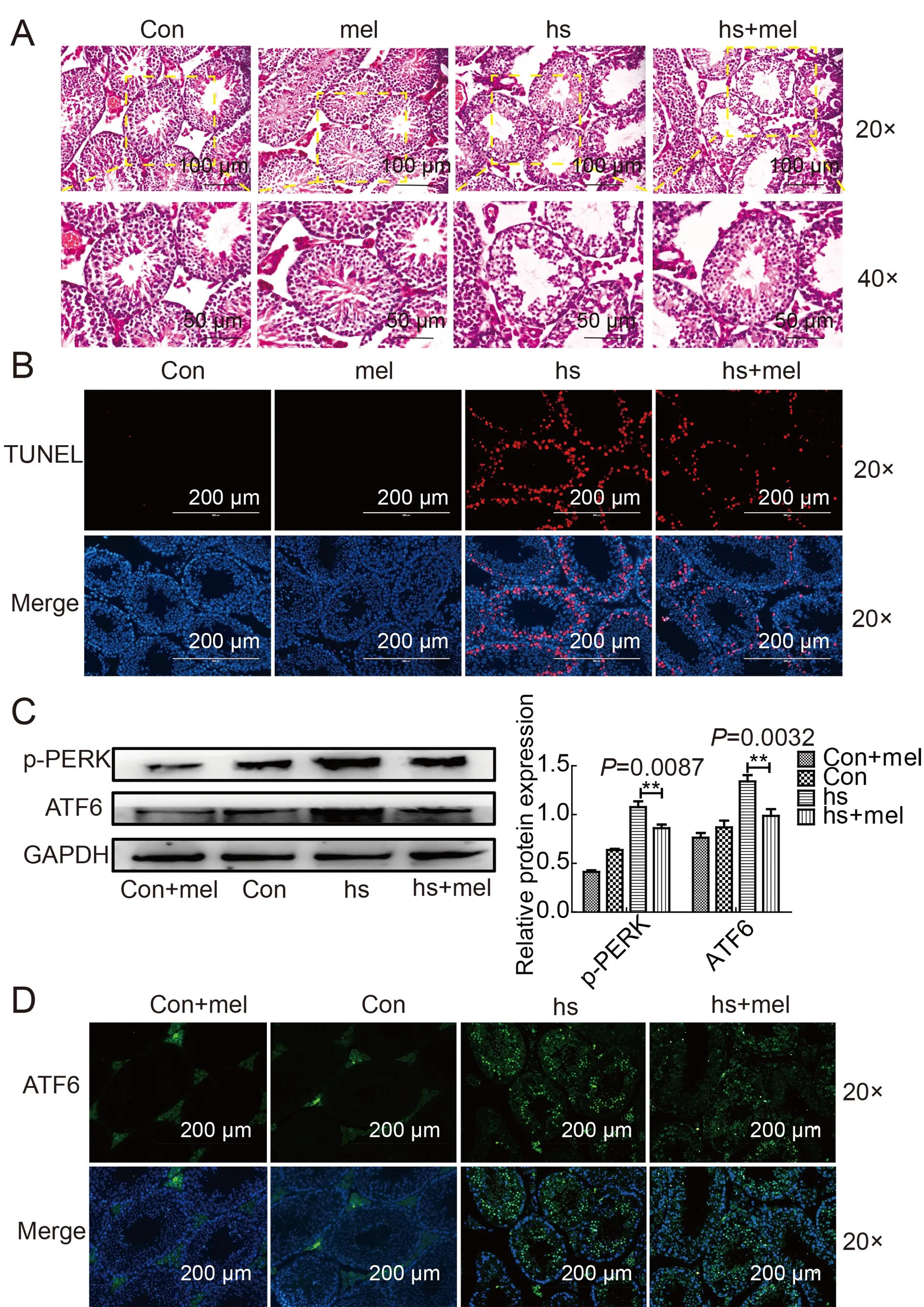
Figure 7 Melatonin alleviates apoptosis of testicular spermatocytes caused by heat stress in vivo
DISCUSSION
As global temperatures rise,heat stress has become an important factor affecting the reproductive performance of mammals and increases the risk of subfertility/infertility in humans (Leyk et al.,2019).Thus,research on methods that effectively retain fertility during heat stress is crucial.In the current study,we explored the use of melatonin as a potential target for remedying heat-induced testicular damage and spermatocyte apoptosis,and elucidated the underlying protective mechanism of melatonin,which acts via modulation of the ATF and p-PERK signaling pathways.
During spermatocyte meiosis and sperm cell formation,thick spermatocytes and round spermatids are particularly sensitive to high temperature (Yadav et al.,2018).This was evident from our experiments,which showed an increase in the expression of HSP70 after scrotal heat treatment for 20 min at 42 °C.The testes also showed a series of injuries,such as testicular atrophy and tubule vacuolation.Spermatocyte apoptosis was induced in the testes shortly after heat treatment.Studies have shown that heat stress induces ERS in the spermatocytes of mice and activates the UPR,leading to apoptosis (Kim et al.,2013).Chemicals such as BIS phenol-α and diethylstilbestrol disrupt ER homeostasis by inducing IRE1 phosphorylation and CHOP expression in rats,which has a toxic effect on testicular spermatogenesis (Jiang et al.,2016).Different ERS pathways play different roles in response to heat stress.P-EIF2 can reduce the pathological thermal sense of the dorsal root ganglia and reduce cell damage and maintain cell homeostasis (Khoutorsky et al.,2016).In the present study,we demonstrated the relationship between the ERS signaling pathways and spermatocyte apoptosis in mouse testes exposed to heat stress.We also evaluated the response of ERS-related genes and found that theAtf6andPerksignaling pathways play important roles in thermal stress in mouse testes.In contrast,IRE1 was not found to play an obvious role in thermal stimulus recovery.Previous studies have shown that heat stress in HeLa cells can lead to down-regulation of IRE1,activation of autophagy,and activation of the UPR negative feedback system through the regulation of the IRE1-XBP1 axis (Homma & Fujii,2016).Therefore,the role of IRE1 as a special target in heat stress needs to be further explored.The activation ofChopis known to induce ERS and apoptosis (Kim et al.,2020).Our results further substantiated that heat stress can activate ERSassociated apoptotic genes,such asCaspase12andChop.Thus,heat stress appears to influence spermatocyte homeostasis,potentially causing male subfertility and inferior sperm quality.
Melatonin is a hormone synthesized and secreted by the pineal gland and the testes.As a powerful antioxidant,melatonin plays a direct physiological role in the regulation of sperm function and an important protective role in testicular development and testosterone synthesis.As a local regulator,melatonin directly affects the synthesis and secretion of androgens by mesenchymal cells and regulates the production of steroid hormones by supporting cell proliferation and energy metabolism,thus affecting spermatogenesis (Yu et al.,2018).Melatonin can also regulate apoptosis and autophagy homeostasis.Under this state,phosphorylated BECLIN1 binds to BCL2,damages the BCL2 and BAX complex,increases free BAX in cells,and causes mitochondria-dependent apoptosis (Pan et al.,2018).Melatonin can reduce reproductive damage caused by heat stress and improve the tight connections of supporting cells by reducing apoptosis and oxidative stress (Zhang et al.,2020).Our results are consistent with previous reports indicating that melatonin can effectively alleviate different ERS signaling pathways and reduce spermatocyte apoptosis (Cui et al.,2017).The effects of melatonin are dose dependent,with excessively high concentrations not only failing to relieve apoptosis caused by heat stress,but also aggravating cell death;in contrast,excessively low concentrations show no significant effects (unpublished data).In the present work,a melatonin concentration of 10-7was found to be highly effective for spermatocyte apoptosis remission,as detected by flow cytometry.Melatonin inhibited theAtf6andPerksignaling pathways,down-regulated the expression ofChopandCaspase12,and reduced the apoptosis of spermatocytes caused by high temperature.In mammals,melatonin activity is often mediated through its interactions with the G proteincoupled MT1 and MT2 receptors (Dubocovich et al.,2010).In the spermatocyte cell line GC2,the role of melatonin was dependent on both MT1 and MT2.This was confirmed using specific melatonin inhibitors to inhibit the two receptors,which eliminated the protective effects of melatonin on apoptosis and subsequently on ERS.In vivostudies were also carried out to test the clinical potential of melatonin using continuous intraperitoneal injections of melatonin.Results confirmed our hypothesis that melatonin could prevent testicular injury caused by thermal stimulation and reduce testicular cell apoptosis.
In conclusion,melatonin showed considerable potential as an agent to reduce spermatocyte apoptosis and testicular damage caused by hyperthermia.By inhibiting theAtf6andPerksignaling pathways,we found that the three different ERS signaling pathways play different roles in heat stress(Figure 8).This study offers valuable support for the (pre-)clinical practice of melatonin treatment,which could help protect the testes from damage caused by heat stimulation and thus improve male reproductive health.
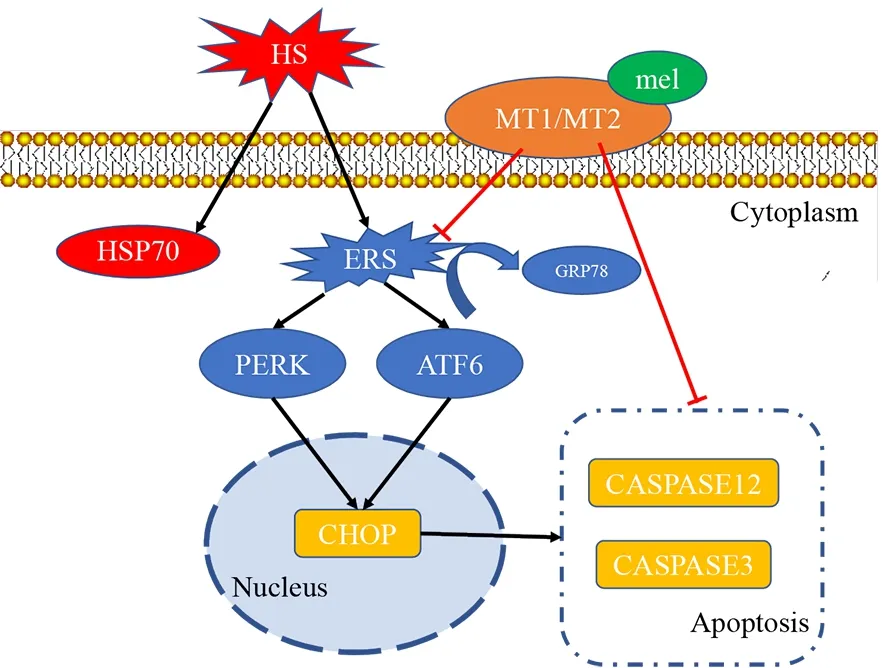
Figure 8 Illustration of melatonin relieving heat-induced spermatocyte apoptosis in mouse testes
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
D.Z.Q.,J.L.H.and S.P.conceived and designed the study.D.Z.Q.,H.C.,D.H.Y.,W.L.H.,C.H.,J.S.and B.L.L.performed the experiments. D.Z.Q. performed statistical analyses.D.Z.Q.,H.C.,and S.P.wrote and edited the manuscript.All authors read and approved the final version of the manuscript.
- Zoological Research的其它文章
- Genome and population evolution and environmental adaptation of Glyptosternon maculatum on theQinghai-Tibet Plateau
- 3DPhenoFish:Application for two-and threedimensional fish morphological phenotype extraction from point cloud analysis
- A new snake species of the genus Gonyosoma Wagler,1828 (Serpentes:Colubridae) from Hainan Island,China
- Inhibition of mTOR signaling by rapamycin protects photoreceptors from degeneration in rd1 mice
- A bright future for the tree shrew in neuroscience research:Summary from the inaugural Tree Shrew Users Meeting
- PINK1 gene mutation by pair truncated sgRNA/Cas9-D10A in cynomolgus monkeys

