芒果葡萄座腔菌对3种杀菌剂的敏感性测定
赵江 余知和 李其利 唐利华 郭堂勋 黄穗萍 莫贱友 孙明艳
摘要:【目的】明确从我国芒果主产区分离获得的89株葡萄座腔菌对3种杀菌剂的敏感性,以期为葡萄座腔菌引起的芒果病害防控提供参考。【方法】采用菌丝生长速率法对采自广西、海南、云南、四川、广东和福建芒果主产区的89株芒果葡萄座腔菌进行咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性测定,并利用DPS 9.01和SPSS 20分析供试菌株对3种药剂的敏感性及交互抗性。【结果】供试89株芒果葡萄座腔菌对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性存在差异,其中对咪鲜胺的EC50介于0.0048~38.5037 mg/L,平均值为2.8637 mg/L,最大EC50是最小EC50的8021.6倍;对苯醚甲环唑的EC50介于0.0147~8.8935 mg/L,平均值为1.1761 mg/L,最大EC50是最小EC50的605.0倍;对吡唑醚菌酯的EC50介于0.0195~145.0578 mg/L,平均值为8.1939 mg/L,最大EC50是最小EC50的7438.9倍。采自不同芒果产区的芒果葡萄座腔菌株对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性存在差异,其中采自广东的菌株对咪鲜胺和吡唑醚菌酯的平均EC50最大,分别为8.1127和15.7240 mg/L,采自四川的菌株对苯醚甲环唑的平均EC50最大,为1.6730 mg/L。不同属的芒果葡萄座腔菌菌株对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性存在差异,其中毛色二孢属(Lasiodiplodia)菌株对3种杀菌剂的平均EC50均高于葡萄座腔菌属(Botryosphaeria)和新壳梭孢属(Neofusicoccum)菌株的平均EC50。【结论】采自我国不同芒果产区的89株芒果葡萄座腔菌菌株对苯醚甲环唑较敏感,咪鲜胺次之,对吡唑醚菌酯的敏感性最低。供试芒果葡萄座腔菌对3种杀菌剂无交互抗性,可合理混合或交替施用。不同来源、不同属的芒果葡萄座腔菌对3種杀菌剂的敏感性存在差异,生产中应根据具体的区域及病原菌种类合理地科学施用。
关键词: 葡萄座腔菌;葡萄座腔菌属;毛色二孢属;新壳梭孢属;咪鲜胺;苯醚甲环唑;吡唑醚菌酯;敏感性
中图分类号: S436.679 文献标志码: A 文章编号:2095-1191(2021)04-0984-11
Susceptibility of Botryosphaeriaceous isolates from
mango to three fungicides
ZHAO Jiang1,2, YU Zhi-he1*, LI Qi-li2,3*, TANG Li-hua2,3, GUO Tang-xun2,3,
HUANG Sui-ping2,3, MO Jian-you2,3, SUN Ming-yan4
(1College of Life Sciences, Yangtze University, Jingzhou, Hubei 434023, China; 2Institute of Plant Protection, Guangxi Academy of Agricultural Sciences, Nanning 530007, China; 3Guangxi Key Laboratory of Biology for Crop Diseases and Insect Pests, Nanning 530007, China; 4Flowers Research Institute, Guangxi Academy of Agricultural
Sciences, Nanning 530007, China)
Abstract:【Objective】To determine the susceptibility of 89 strains of Botryosphaeriaceous isolate from major mango producing areas in China to three fungicides:prochloraz, difenoconazole and pyraclostrobin, and to provide reference for mango disease control caused by Botryosphaeriaceous isolate. 【Method】Mycelial growth rate method was used to determine the sensitivity of Botryosphaeriaceous isolate collected from main mango producing areas of Guangxi, Hainan, Yunnan, Sichuan, Guangdong and Fujian to prochloraz, difenoconazole and pyraclostrobin, and DPS9.01 and SPSS20 softwares were used to analyze the sensitivity and cross resistance of the strains to the three fungicides. 【Result】 Among the 89 strains tested in this study, there were differences in susceptibility to prochloraz, difenoconazole and pyraclostrobin. The EC50 for prochloraz ranged from 0.0048 to 38.5037 mg/L, with an average value of 2.8637 mg/L, and the maximum EC50 was 8021.6 times of the minimum EC50. The EC50 for difenoconazole ranged from 0.0147 to 8.8935 mg/L, with an average value of 1.1761 mg/L, and the maximum EC50 was 605.0 times of the minimum EC50. The EC50 values for pyraclostrobin ranged from 0.0195 to 145.0578 mg/L, with an average value of 8.1939 mg/L, and the maximum EC50 was 7438.9 times of the minimum EC50. There were differences in the susceptibility of the isolates collected from different mango producing areas to prochloraz, difenoconazole and pyraclostrobin, among which the isolates from Guangdong had the highest average EC50 to prochloraz(8.1127 mg/L) and pyraclostrobin(15.7240 mg/L), and the isolates from Sichuan had the highest average EC50 to difenoconazole(1.6730 mg/L). There were differences in the susceptibility of Botryosphaeriaceous isolates from different genera to prochloraz, difenoconazole and pyraclostrobin. The average EC50 of the isolates in Lasiodiplodia to three fungicides was higher than that of Botryosphaeria and Neofusicoccum. 【Conclusion】All the 89 strains of Botryosphaeriaceous isolates collected from different mango producing areas are more sensitive to difenocona-zole, followed by prochloraz, and have the lowest sensitivity to pyraclostrobin. There is no cross-resistance to the three fungicides, and it can be reasonably mixed or applied alternately. There are differences in the sensitivity of mango Botryosphaeriaceous isolates from different sources and genera to the three fungicides, so it should be rationally and scientifically applied according to specific regions and pathogenic species in production.
Key words: Botryosphaeriaceous isolate; Botryosphaeria; Lasiodiplodia; Neofusicoccum; prochloraz; difenocona-zole; pyraclostrobin; sensitivity
Foundation item: National Natural Science Foundation of China(31600029, 31560526); Guangxi Natural Science Foundation(2016GXNSFCB38000)
0 引言
【研究意义】葡萄座腔菌隶属子囊菌门(Ascomycota)座囊菌纲(Dothideomycetes),其中的葡萄座腔菌属(Botryosphaeria)、新壳梭孢属(Neofusicoccum)、毛色二孢属(Lasiodiplodia)和小穴壳菌属(Dothiorella)等成员可侵染植物叶片、枝干、果实等部位,引起多种果树及林木病害,是一类地理分布、寄主范围广泛的植物病原真菌(Slippers et al.,2005;王璠,2013;吕蕊花等,2020)。芒果是一种重要的经济作物,已成为我国热带及亚热带地区农业主导产业之一,主要分布在我国广西、海南、云南、四川、广东、福建和贵州7个省区。2019年我国芒果种植面积达32.3万ha,产量278.2万t,居世界第三(陈业渊等,2020)。但在芒果生长过程中,由葡萄座腔菌引起的芒果枝枯病、流胶病、蒂腐病等病害严重影响芒果的产量和品质,造成经济损失(Slippers et al.,2005;胡美姣等,2009a)。目前,生产上大量使用多菌灵和甲基硫菌灵等苯并咪唑类杀菌剂(BMZs)、吡唑醚菌酯等甲氧基丙烯酸酯类杀菌剂(QoIs)及苯醚甲环唑、咪鲜胺等甾醇脱甲基类抑制剂(DMIs)防治芒果病害(赵磊等,2017)。杀菌剂的长期大量使用导致病原真菌对杀菌剂的敏感性降低,抗药性风险增加(王萌等,2015;欧阳秋飞等,2019)。因此,对采自不同产区的芒果葡萄座腔菌进行咪鲜胺、苯醚甲环唑及吡唑醚菌酯的敏感性测定,对药剂的抗性风险监测及风险评估具有重要意义。【前人研究进展】潘贤丽和李继勇(1985)首次报道芒果蒂腐病与葡萄座腔菌相关,并将病原鉴定为Diplodia sp.;随后,葡萄座腔菌导致的芒果流胶病和芒果树回枯病在我国被报道(胡美姣等,2012;Li et al.,2014)。其中,芒果蒂腐病在贮运期发病率达10%~40%,造成严重经济损失,是仅次于芒果炭疽病的第二大芒果病害(王璧生等,1994)。可可球二孢[Lasiodiplodia theobromae(syn. Botryodiplodia theobromae)]在我国被认为是芒果蒂腐病的主要病原菌(杨波,2013)。2009年胡美姣等(2009a)首次测定了22种杀菌剂对在海南地区引起芒果蒂腐病的2株可可球二孢菌的毒力,结果表明,咪鲜胺、苯醚甲环唑和吡唑醚菌酯等12种杀菌剂可作为防治芒果蒂腐病的首选药剂。胡美姣等(2009b)首次报道海南芒果蒂腐病菌除对多菌灵产生抗性外,还对甲基硫菌灵、醚菌酯和烯唑醇产生了抗药性。目前,吡唑醚菌酯、苯醚甲环唑和咪鲜胺是芒果病害防治的常用药剂(王萌等,2014;贺瑞等,2018)。但随着这3种杀菌剂的连续大量使用,已有报道芒果炭疽菌对咪鲜胺产生了抗药性(梁凌星,2015;成禄艳,2016)。王萌等(2015)对62株芒果可可球二孢菌进行多种杀菌剂抗药性测定,结果表明,采自海南的芒果可可球二孢菌对苯并咪唑类杀菌剂普遍存在抗药性,特别是吡唑醚菌酯,在海南地区已产生抗药性群体,且以中抗和高抗菌株为优势群体(贺瑞等,2018)。在国外,对芒果病害的防治也主要依靠化学手段(Malik et al.,2016),由于杀菌剂的滥用,引起芒果蒂腐病的可可球二孢菌对甲基硫菌灵、多菌灵、苯醚甲环唑和乙霉威等杀菌剂的敏感性均有所下降(Ateeq et al.,2015)。【本研究切入点】目前除海南芒果产区外,我国其他芒果产区芒果葡萄座腔菌对咪鲜胺和苯醚甲环唑的敏感性以及不同属的芒果葡萄座腔菌对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性鲜有报道。【拟解决的关键问题】对采自广西、海南、云南、四川、广东和福建芒果主产区的89株芒果葡萄座腔菌进行咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性测定,明确各产区芒果葡萄座腔菌菌株对3种供试杀菌剂的敏感性差异,为葡萄座腔菌引起的芒果病害化学防治提供科学依据。
1 材料与方法
1. 1 试验材料
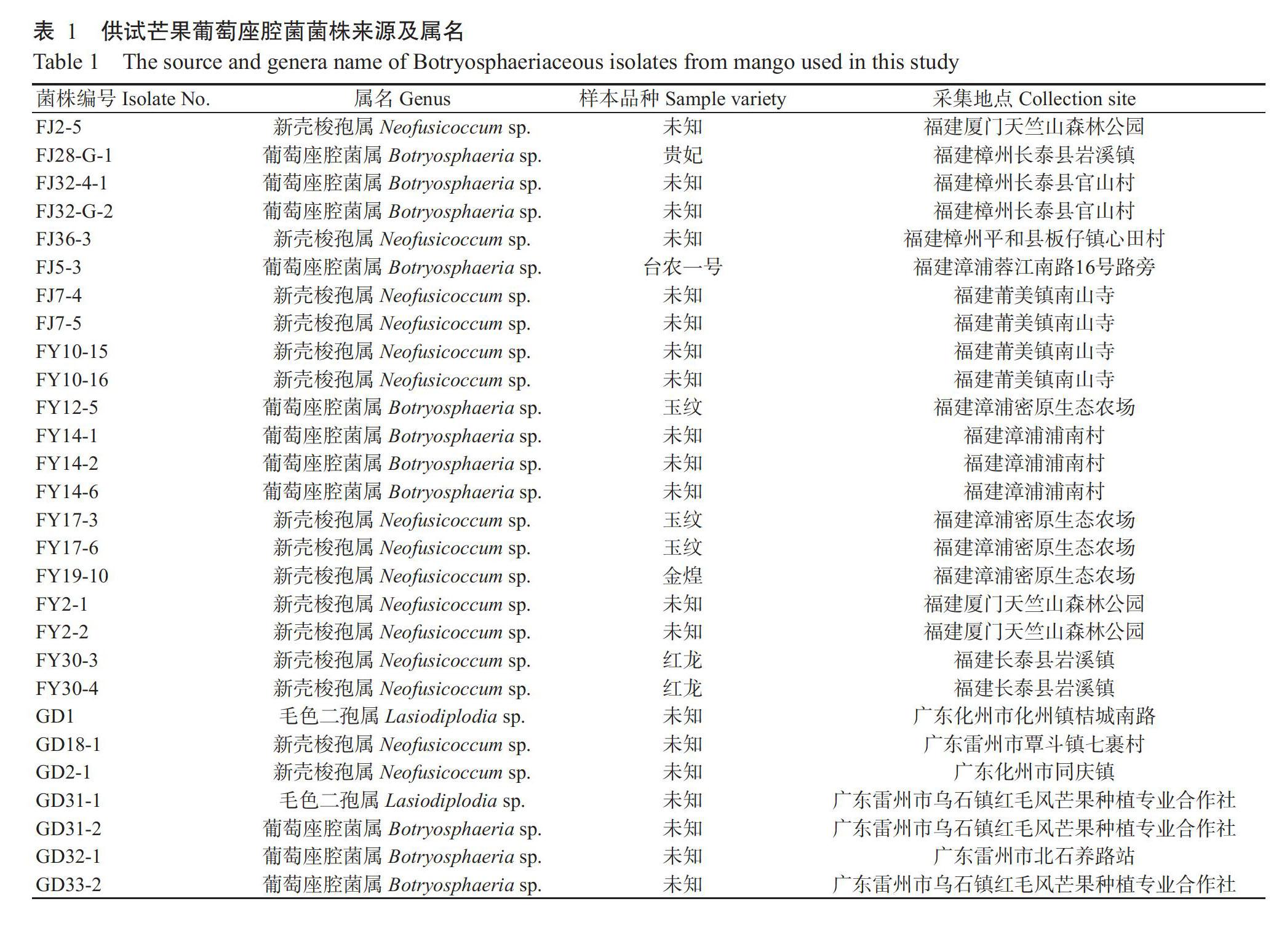
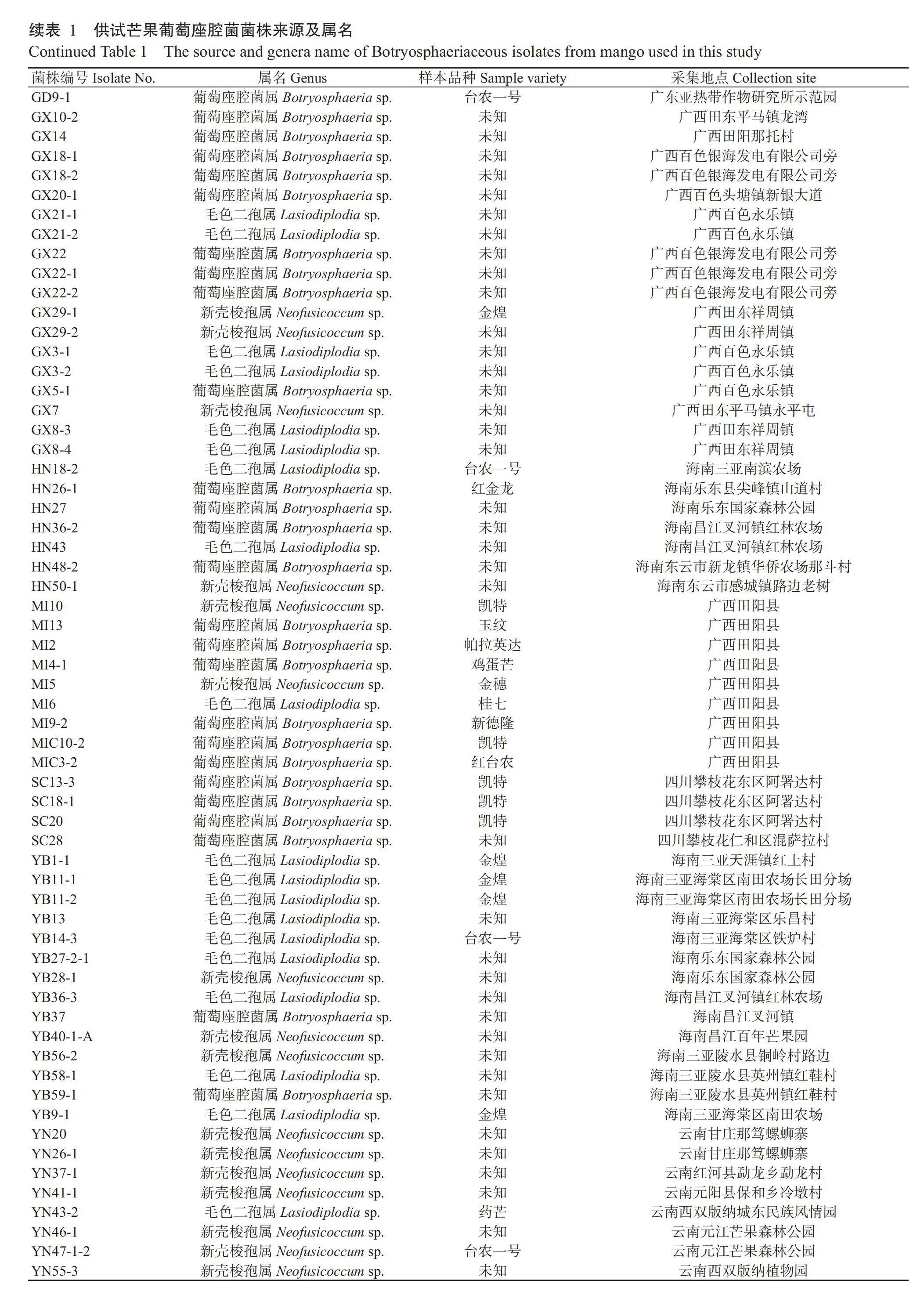
1. 1. 1 供试菌株 2016年7月—2017年8月分别从海南、广东、广西、云南、四川和福建芒果产区采集玉纹芒、凯特芒、台农一号、桂七等14个芒果品种的叶斑及蒂腐病样本313份,经组织分离、鉴定,共获得89株葡萄座腔菌,其中广西27株、海南21株、福建21株,广东8株,云南8株、四川4株(表1)。所有分离菌株保藏于广西农业科学院植物保护研究所果树病害实验室。不同菌株对3种杀菌剂的敏感性测定于2020年5月—2021年2月在广西农业科学院植物保護研究所果树病害实验室内进行。
1. 1. 2 供试药剂 98.3%咪鲜胺(Prochloraz)原药、97%苯醚甲环唑(Difenoconazole)原药和97.8%吡唑醚菌酯(Pyraclostrobin)原药(广西田园生化股份有限公司)。
1. 2 试验方法
1. 2. 1 含药培养基制备 准确称取一定量的杀菌剂原药,用丙酮溶解咪鲜胺和吡唑醚菌酯、甲醇溶解苯醚甲环唑,分别配制成10 mg/mL母液。取4 mL母液溶解于6 mL无菌水中,稀释为4 mg/mL的药剂溶液。取4 mg/mL的药剂溶液5 mL加入到冷却至50 ℃左右195 mL的PDA培养基中,充分混匀后用移液枪吸取14 mL培养基倒入直径9 cm的培养皿中,制成含杀菌剂浓度为100 mg/L的培养基。对4 mg/mL的药剂溶液进行梯度稀释,分别制成含杀菌剂浓度为10.00、1.00、0.10和0.01 mg/L的培养基。以相同样方法制备等量丙酮或甲醇稀释液的PDA培养基为对照。
1. 2. 2 芒果葡萄座腔菌对3种杀菌剂的敏感性测定
敏感性测定参照张成玲等(2019)的菌丝生长速率法。供试菌株转至PDA培养基,置于28 ℃培养3 d后,用直径5 mm的打孔器在菌落边缘打取菌饼,移至含不同浓度杀菌剂的培养基中央,28 ℃下黑暗培养。每个浓度处理4次重复。待对照培养基上菌落长满整个平皿时,采取十字交叉法测量菌落直径,以浓度对数(x)为横轴,菌落生长抑制率的几率值(y)为纵轴求杀菌剂对芒果葡萄座腔菌菌株的毒力回归方程y=ax+b,并计算抑制菌体生长的有效中浓度(EC50)及相关系数。用以下公式计算抑菌率(梁凌星,2015)。
抑菌率(%)=(对照菌落直径-处理菌落直径)/
对照菌落直径×100
1. 3 统计分析
采用Excel 2013进行试验数据整理分析,利用DPS 9.01求出毒力回归方程、EC50和相关系数,并运用LSD法进行差异显著性检验,利用SPSS 20以SW法(Shapiro-Wilk)进行敏感频率分布正态性检验及交互抗性分析。
2 结果与分析
2. 1 芒果葡萄座腔菌对3种杀菌剂的敏感性测定结果
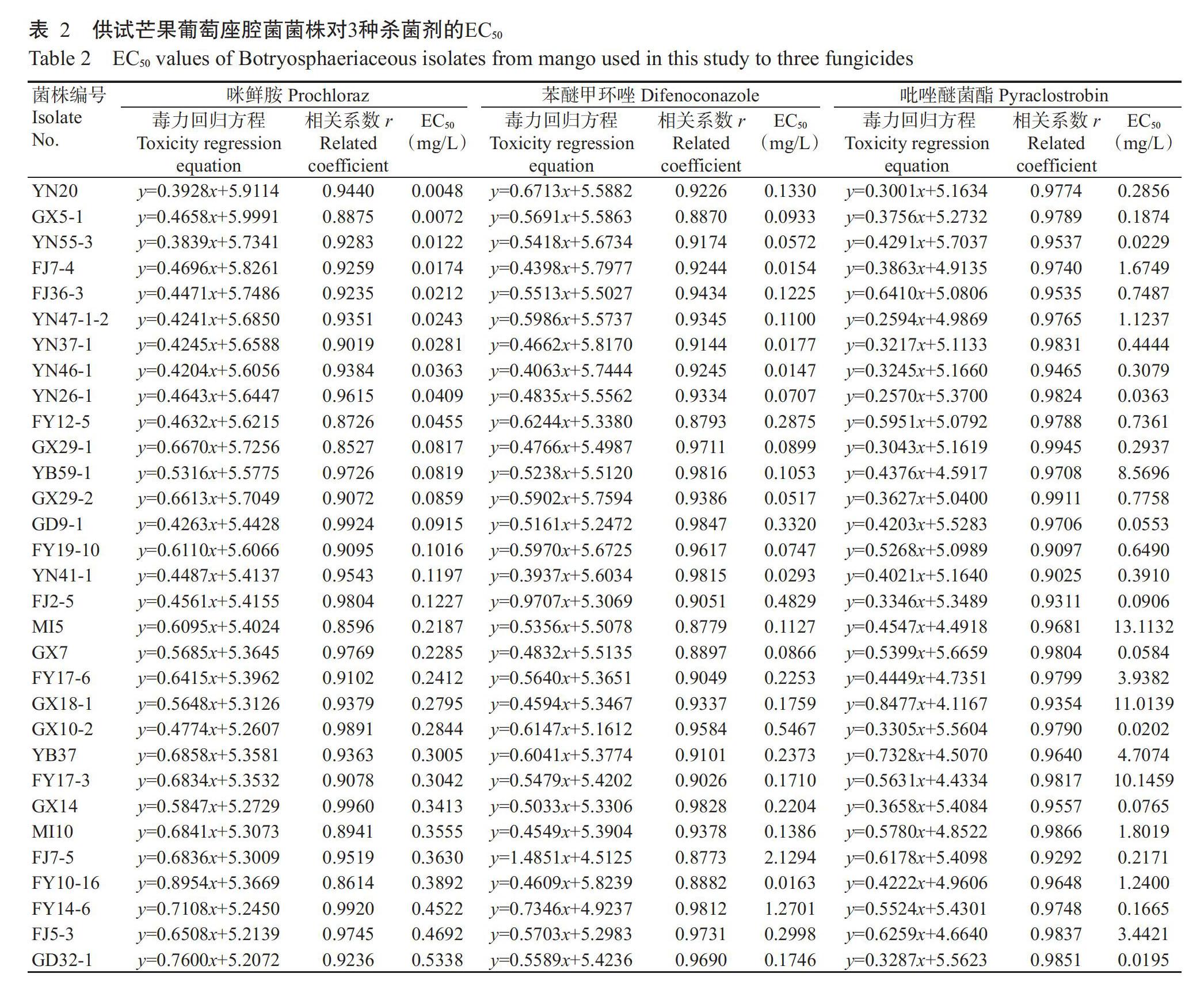
敏感性测定结果(表2)显示,供试89株芒果葡萄座腔菌菌株对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性存在差异,其中对咪鲜胺的EC50介于0.0048~38.5037 mg/L,平均值为2.8637 mg/L,最大EC50约是最小EC50的8021.6倍;对苯醚甲环唑的EC50介于0.0147~8.8935 mg/L,平均值为1.1761 mg/L,最大EC50是最小EC50的605.0倍;对吡唑醚菌酯的EC50介于0.0195~145.0578 mg/L,平均值为8.1939 mg/L,最大EC50约是最小EC50的7438.9倍。供试89株芒果葡萄座腔菌菌株对吡唑醚菌酯的平均EC50显著高于咪鲜胺和苯醚甲环唑(P<0.05,下同),为咪鲜胺的2.9倍、苯醚甲环唑的7.0倍。经Shapiro-Wilk法测得咪鲜胺的W值为0.5270 mg/L,P值为0.00(<0.05);苯醚甲环唑和吡唑醚菌酯的W值分别为0.6860和0.4400 mg/L,P值为0.00(<0.05)。病原菌敏感性频率分布图(图1)显示,89株芒果葡萄座腔菌菌株对3种杀菌剂的敏感性频率分布不符合正态分布(P<0.05),且右侧有拖尾,说明大部分芒果葡萄座腔菌菌株对3种杀菌剂较为敏感,其EC50主要集中在正态分布区间内,但有少数菌株对3种杀菌剂敏感性显著降低,具有较高的抗性风险。
2. 2 不同来源芒果葡萄座腔菌菌株对3种杀菌剂的敏感性差异分析结果
分析来自不同地区的89株芒果葡萄座腔菌菌株对3种杀菌剂的敏感性,结果(表3)表明,不同来源的芒果葡萄座腔菌菌株对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性存在一定差异。采自广西、云南、福建、广东、海南和四川的芒果葡萄座腔菌菌株对咪鲜胺的平均EC50分别为2.4622、1.0770、0.9012、8.1127、4.3862和0.9605 mg/L,就平均EC50而言,采自广东的菌株最高,其次为海南、广西、云南和四川,福建的最低;对苯醚甲环唑的平均EC50分别为1.4404、0.3274、0.6987、1.1103、1.5676和1.6730 mg/L,就平均EC50而言,采自四川的菌株最高,其次为海南、广西、广东和福建,云南的最低;对吡唑醚菌酯的平均EC50分别为10.8843、1.2669、2.6955、15.7240、11.5022和0.3253 mg/L,就平均EC50而言,采自广东的菌株最高,其次为海南、广西、福建和云南,四川的最低。
2. 3 不同属葡萄座腔菌菌株对3种杀菌剂的敏感性差异分析结果
通过LSD法分析隶属于3个属(葡萄座腔菌属、新壳梭孢属和毛色二孢属)水平的89株芒果葡萄座腔菌菌株对3种杀菌剂(咪鲜胺、苯醚甲环唑和吡唑醚菌酯)的敏感性,结果(表4)表明,不同属的芒果葡萄座腔菌菌株对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性存在明显差异,就EC50平均值而言,毛色二孢属的菌株显著高于葡萄座腔菌属和新壳梭孢属的菌株,后两者间无显著差异(P>0.05,下同)。
2. 4 芒果葡萄座腔菌对3种杀菌剂敏感性的交互抗性
进行供试89株芒果葡萄座腔菌菌株对3种杀菌剂EC50的对数值两两回归分析,结果(图2)显示,咪鲜胺与吡唑醚菌酯的相关方程为y=0.3893x-0.1268,相关系数为0.4686;咪鲜胺与苯醚甲环唑的相关方程为y=0.8567x+0.1669,相关系数为0.7055;2个方程的相关系数均小于0.7500,说明咪鲜胺与吡唑醚菌酯、苯醚甲环唑无交互抗性。苯醚甲环唑与吡唑醚菌酯的相关方程为y=0.2830x-0.3458,相关系数为0.4137,小于0.7500,說明苯醚甲环唑与吡唑醚菌酯无交互抗性。
3 讨论
咪鲜胺和苯醚甲环唑同属于甾醇脱甲基化抑制剂,具有广谱、高效、低毒的特点,已成为芒果田间病害及果实采后贮存期病害防治的主要药剂之一(杨石有等,2015)。而吡唑醚菌酯作为芒果真菌病害的主要防治药剂之一(Zhao et al.,2018),由于使用量及使用年限的增加,在海南省已出现部分中抗和高抗菌株(贺瑞等,2018)。尽管咪唑类杀菌剂具有特异的作用位点,不同病菌对咪唑类杀菌剂抗药性风险较小,但由于长期、大量使用,已有芒果炭疽病菌对其产生抗药性的报道(杨石有等,2015),但尚未有不同来源的葡萄座腔菌对咪鲜胺或苯醚甲环唑敏感性的报道。随着芒果可可球二孢菌对甲基硫菌灵、醚菌酯和烯唑醇等苯并咪唑类杀菌剂逐渐产生抗药性,甚至出现高抗菌株(贺瑞等,2018),加之咪鲜胺和苯醚甲环唑在芒果病害防治中广泛使用,因此对其抗性监测十分必要(成禄艳,2016)。
2009年胡美姣等(2009a)首次對咪鲜胺对海南芒果蒂腐病主要病原菌可可球二孢菌的毒力进行测定,EC50平均值为0.35 mg/L,最大值为0.99 mg/L;随后,师超等(2010)测定了咪鲜胺对2株采自三亚市的芒果可可球二孢菌的毒力,EC50平均值为0.54 mg/L,最大值为0.99 mg/L。本研究中采自海南的芒果葡萄座腔菌菌株对咪鲜胺的EC50平均值为4.3862 mg/L,最大值为18.9241 mg/L,明显高于胡美姣等(2009a)、师超等(2010)的测定值,表明随着咪鲜胺使用年限的增加,海南芒果葡萄座腔菌菌株对咪鲜胺的敏感性逐渐降低。采自四大传统芒果产区(海南、广西、广东和云南)的芒果葡萄座腔菌对咪鲜胺的敏感性均低于福建和四川。咪鲜胺对采自广东的毛色二孢属葡萄座腔菌GD1的EC50最大,高达38.5037 mg/L,为最小EC50的8021.6042倍,高于前人研究的测定值(师超等,2010),存在较高的抗性风险。本研究是首次报道不同地理来源葡萄座腔菌属和新壳梭孢属芒果葡萄座腔菌对咪鲜胺的敏感性。
2009年胡美姣等(2009a)首次对吡唑醚菌酯对海南芒果可可球二孢菌的毒力进行测定,EC50平均值为0.67 mg/L,最大值为0.95 mg/L,研究认为吡唑醚菌酯可作为防治芒果蒂腐病的首选杀菌剂。但随着吡唑醚菌酯的大量使用,吡唑醚菌酯对海南芒果可可球二孢菌的毒力逐渐下降。2018年,贺瑞等对95株海南芒果可可球二孢菌对吡唑醚菌酯的抗药性进行测定,发现66.67%的菌株的EC50高于100.00 mg/L,最大EC50为1525.43 mg/L,中高抗菌株成为优势群体。本研究采自海南的芒果葡萄座腔菌菌株对吡唑醚菌酯的EC50平均值为11.5022 mg/L,最大值为65.2708 mg/L,低于贺瑞等(2018)的测定值。另外,采自广东的菌株对吡唑醚菌酯的EC50平均值高于采自海南的菌株。本研究89株芒果葡萄座腔菌菌株对吡唑醚菌酯的EC50值介于0.0195~145.0578 mg/L,平均值为8.1939 mg/L,最大EC50是最小EC50的7438.9倍,存在较大的抗性风险。
苯醚甲环唑作为防治芒果蒂腐病的首选药剂,在芒果生产上大量使用(王萌等,2014)。本研究首次报道我国不同芒果产区葡萄座腔菌对苯醚甲环唑的敏感性。本研究中89株芒果葡萄座腔菌菌株对苯醚甲环唑的平均EC50为1.1761 mg/L,EC50最大值为8.8935 mg/L,均低于咪鲜胺和吡唑醚菌酯。采自不同芒果产区的葡萄座腔菌菌株对苯醚甲环唑较为敏感,咪鲜胺次之,对吡唑醚菌酯的敏感性最低。但89株芒果葡萄座腔菌菌株对苯醚甲环唑的最大EC50是最小EC50的605.0倍,部分菌株对苯醚甲环唑的敏感性显著下降,提示有抗药性风险存在。另据报道,苯醚甲环唑在巴西(Junior et al.,2009)、巴基斯坦(Ateeq et al.,2015)和印度(Shukla et al.,2018)等国对芒果病害均具有良好的防效。
前人研究表明,苯醚甲环唑和吡唑醚菌酯对芒果炭疽菌无交互抗性(张令宏等,2009);咪鲜胺和苯醚甲环唑对田藤仓镰孢菌无交互抗性(毛程鑫等,2020);咪鲜胺和吡唑醚菌酯对拟轮枝镰孢菌无交互抗性(杨石有等,2020)。本研究中89株芒果葡萄座腔菌菌株对咪鲜胺、苯醚甲环唑和吡唑醚菌酯无交互抗性,与前人研究一致。不同属的芒果葡萄座腔菌对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性存在明显差异,就EC50平均值而言,毛色二孢属的菌株远高于葡萄座腔菌属和新壳梭孢属的菌株,后两者间无显著差异。本研究结果在一定程度上反映了我国主要芒果产区的葡萄座腔菌对咪鲜胺、苯醚甲环唑和吡唑醚菌酯敏感性的实际情况。
杀菌剂仍是目前防治芒果病害的主要手段(成禄艳,2016)。加强药剂的抗性风险监测、评估,对合理使用药剂,采取轮换或混合使用药剂等有效规避药剂抗性风险具有指导意义(郑肖兰等,2011)。本研究明确了我国不同芒果产区的芒果葡萄座腔菌对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性,为3种杀菌剂的合理使用提供参考。
4 结论
采自我国不同芒果产区的89株芒果葡萄座腔菌菌株对苯醚甲环唑较敏感,咪鲜胺次之,对吡唑醚菌酯的敏感性最低。供试芒果葡萄座腔菌对咪鲜胺、苯醚甲环唑和吡唑醚菌酯无交互抗性,可合理混合或交替施用。不同来源、不同属的芒果葡萄座腔菌对咪鲜胺、苯醚甲环唑和吡唑醚菌酯的敏感性存在差异,生产中应根据具体的区域及病原菌种类合理地科学施用。
参考文献:
陈业渊,党志国,林电,胡美姣,黄建峰,朱敏,张贺,韩冬银,高兆银,黄媛媛. 2020. 中国杧果科学研究70年[J]. 热带作物学报,41(10):2034-2044. doi:10.3969/j.issn.1000-2561.2020.10.010. [Chen Y Y,Dang Z G,Lin D,Hu M J,Huang J F,Zhu M,Zhang H,Han D Y,Gao Z Y,Huang Y Y. 2020. Mango scientific research in China in the past 70 years[J]. Chinese Journal of Tropical Crops,41(10):2034-2044.]
成禄艳. 2016. 芒果炭疽病菌对几种杀菌剂的抗药性研究[D]. 广州:华南农业大学. [Cheng L Y. 2016. Study on the resistance of mango anthracnose pathogen Colletotrichum glosoporioides to several fungicides[D]. Guangzhou:South China Agricultural University.]
賀瑞,赵磊,符瑞,陈芷岑,林晓翠,杨叶. 2018. 海南芒果蒂腐病菌对吡唑醚菌酯的抗药性测定[J]. 植物保护,44(4):188-193. doi:10.16688/j.zwbh.2017419. [He R,Zhao L,Fu R,Chen Z C,Lin X C,Yang Y. 2018. Resistance of Botryodiplodia theobromae caused mango stem end rot to pyraclostrobin in Hainan[J]. Plant Protection,44(4):188-193.]
胡美姣,安勇,师超,张强,李敏,高兆银,杨凤珍. 2009a. 22种杀菌剂对芒果蒂腐病菌的毒力测定[J]. 农药,48(3):215-217. doi:10.16820/j.cnki.1006-0413.2009.03.022. [Hu M J,An Y,Shi C,Zhang Q,Li M,Gao Z Y,Yang F Z. 2009a. Toxicological test of 22 fungicides to Botryodiplodia theobromae Pat. caused mango stem end rot[J]. Agrochemicals,48(3):215-217.]
胡美姣,师超,安勇,李敏,杨凤珍,高兆银. 2009b. 杧果蒂腐病菌对多菌灵的抗药性测定及其杀菌剂筛选[J]. 果树学报,26(5):671-677. doi:10.13925/j.cnki.gsxb.2009.05. 021. [Hu M J,Shi C,An Y,Li M,Yang F Z,Gao Z Y. 2009b. Resistance of Botryodiplodia theobromae to carbendazim and the fungicides screening using stem end rot of mango fruit as a control[J]. Journal of Fruit Science,26(5):671-677.]
胡美姣,杨冬平,杨波,李敏,高兆银,张正科,蒲金基. 2012. 芒果树回枯病病原菌鉴定及其生物学特性研究[J]. 热带作物学报,33(1):122-126. doi:10.3969/j.issn.1000-2561.2012.01.025. [Hu M J,Yang D P,Yang B,Li M,Gao Z Y,Zhang Z K,Pu J J. 2012. Identification and bio-logical characteristics of the blight pathogen of mango tree[J]. Chinese Journal of Tropical Crops,33(1):122-126.]
梁凌星. 2015. 抗咪鲜胺杧果胶孢炭疽菌突变的CYP51基因功能探究[D]. 福州:福建农林大学. [Liang L X. 2015. The function study of mutated CYP51 in Colletotrichum glosoporioides for resistance to prochloraz[D]. Fuzhou:Fujian Agriculture and Forestry University.]
吕蕊花,林枞,白崇旭,方亚妮,冯昭中,史琳娜,吕瑞华,张喜荣. 2020. 猕猴桃贮藏中常见病原菌的分离鉴定及抗菌中药的筛选[J]. 河南农业科学,49(10):78-84. doi:10. 15933/j.cnki.1004-3268.2020.10.011. [Lü R H,Lin C,Bai C X,Fang Y N,Feng Z Z,Shi L N,Lü R H,Zhang X R. 2020. Isolation,identification of common fungi during kiwifruit storage and screening of antifungal chinese me-dicine[J]. Journal of Henan Agricultural Sciences,49(10):78-84.]
毛程鑫,陆强,周小军,张书亚,张宇,张传清. 2020. 浙江省稻田藤仓镰孢霉对咪鲜胺的敏感性及抗药性菌株的适合度与交互抗性[J]. 农药学学报,22(3):432-438. doi:10.16801/j.issn.1008-7303.2020.0071. [Mao C X,Lu Q,Zhou X J,Zhang S Y,Zhang Y,Zhang C Q. 2020. Sensitives,fitness and cross-resistance of Fusarium fujikuroi resistant to prochloraz from rice fields in Zhejiang Pro-vince[J]. Chinese Journal of Pesticide Science,22(3):432-438.]
欧阳秋飞,杨建波,马博,陆家明,骆建宇. 2019. 一株芒果蒂腐病病原菌的鉴定及中草药对其抑制作用分析[J]. 江西农业学报,31(12):54-59. doi:10.19386/j.cnki.jxnyxb. 2019.12.11. [Ouyang Q F,Yang J B,Ma B,Lu J M,Luo J Y. 2019. Identification of pathogen causing stem end rot of mango and analysis of inhibitory effect by Chinese herbal medicine[J]. Acta Agriculturae Jiangxi,31(12):54-59.]
潘贤丽,李继勇. 1985. 芒果果实病害的发生及防治[J]. 热带作物研究,(1):42-44. [Pan X L,Li J Y. 1985. Occurrence and control of mango fruit diseases[J]. Chinese Journal of Tropical Agriculture,(1):42-44.]
师超,胡美姣,李敏,高兆银,杨凤珍,涂锡茂,邓新平. 2010. 多种杀菌剂对抗多菌灵的芒果蒂腐病菌菌株的毒力测定[J]. 农药研究与应用,14(3):30-34. [Shi C,Hu M J,Li M,Gao Z Y,Yang F Z,Tu X M,Deng X P. 2010. Toxicity of 21 fungicides to carbendazim-resistant Botryodiplodia theobromae Pat.,pathogen of mango stem end rot[J]. Agrochemicals Research & Application,14(3):30-34.]
王璧生,刘朝祯,戚佩坤. 1994. 芒果蒂腐病病原菌的鉴定及采后药剂试验[J]. 华南农业大学学报,(3):55-60. [Wang B S,Liu C Z,Qi P K. 1994. Identification of mango stem-end rot pathogens and its control in post-harvest[J]. Journal of South China Agricultural University,(3):55-60.]
王璠. 2013. 葡萄座腔菌属(Botryosphaeria)引起的果树病害及研究进展[J]. 植物保护,39(6):7-13. doi:10.3969/j.issn.0529-1542.2013.06.002. [Wang F. 2013. Occurrence of and progress in fruit tree diseases caused by Botryosphaeria spp.[J]. Plant Protection,39(6):7-13.]
王萌,陈小莉,杨叶,徐润,杨妮,周海珍. 2015. 海南芒果蒂腐病对8种杀菌剂的抗药性测定[J]. 农药,54(5):384-386. doi:10.16820/j.cnki.1006-0413.2015.05.024. [Wang M,Chen X L,Yang Y,Xu R,Yang N,Zhou H Z. 2015. Resistance of mango stem-end rot to eight fungicides in Hainan[J]. Agrochemicals,54(5):384-386.]
王萌,杨叶,潘晓威,张宇,范咏梅. 2014. 苯醚甲环唑对采后杧果两种真菌的毒力测定及防腐保鲜试验[J]. 中国南方果树,43(1):52-53. doi:10.13938/j.issn.1007-1431.2014. 01.007. [Wang M,Yang Y,Pan X W,Zhang Y,Fan Y M. 2014. Toxicity determination and anticorrosive and fresh-keeping test of difenoconazole against two kinds of fungi of postharvest mango fruit[J]. South China Fruits,43(1):52-53.]
杨波. 2013. 海南芒果采后真菌病害调查及病原鉴定[D]:海口:海南大学. [Yang B. 2013. Survey and identification of fungal disease for mango fruits in Hainan[D]. Haikou:Hainan University.]
楊石有,刘倩宇,万亚美,郭聪聪,庞民好,刘颖超,董金皋. 2020. 拟轮枝镰孢对多菌灵的敏感性及抗性菌株生物学性状和交互抗性[J]. 农药学学报,22(3):439-446. doi:10.16801/j.issn.1008-7303.2020.0079. [Yang S Y,Liu Q Y,Wan Y M,Guo C C,Pang M H,Liu Y C,Dong J G. 2020. Sensitivity of Fusarium verticillioides to carbenda-zim and biological characteristics of resistant isolates and cross resistance[J]. Chinese Journal of Pesticide Science,22(3):439-446.]
杨石有,周慧珍,张贺,黄雅,刘晓妹,蒲金基. 2015. 116株芒果炭疽病菌对咪鲜胺的敏感性测定[J]. 植物保护,41(3):201-204. doi:10.3969/j.issn.0529-1542.2015.03.040. [Yang S Y,Zhou H Z,Zhang H,Huang Y,Liu X M,Pu J J. 2015. Susceptibility of mango anthracnose pathogen to prochloraz[J]. Plant Protection,41(3):201-204.]
张成玲,孙厚俊,赵永强,杨冬静,徐振,马居奎,谢逸萍. 2019. 甘薯根腐病病原分子鉴定及防治药剂筛选[J]. 江西农业学报,31(8):46-51. doi:10.19386/j.cnki.jxnyxb. 2019.08.08. [Zhang C L,Sun H J,Zhao Y Q,Yang D J,Xu Z,Ma J K,Xie Y P. 2019. Molecular detection of sweet potato root rot disease and screening of fungicides[J]. Acta Agriculturae Jiangxi,31(8):46-51.]
张令宏,李敏,高兆银,杨凤珍,杨叶,谢艺贤,胡美姣. 2009. 抗多菌灵的芒果炭疽病菌的杀菌剂筛选及其交互抗性测定[J]. 热带作物学报,30(3):347-352. [Zhang L H,Li M,Gao Z Y,Yang F Z,Yang Y,Xie Y X,Hu M J. 2009. Screening and cross-resistance analysis of alternative fungicides against carbendazim-resistant Colletotrichum gloeosporioides Penz. from mango(Mangifera indica L.)[J]. Chinese Journal of Tropical Crops,30(3):347-352.]
赵磊,杨叶,王萌,贺瑞,陈绵才. 2017. 海南省芒果可可球二孢对多菌灵的敏感性及菌株适合度研究[J]. 农药学学报,19(3):298-306. doi:10.16801/j.issn.1008-7303.2017. 0039. [Zhao L,Yang Y,Wang M,He R,Chen M C. 2017. Sensitivities of carbendazim-resistance and fitness of Botryodiplodia theobromae strains from mango in Hainan[J]. Chinese Journal of Pesticide Science,19(3):298-306.]
郑肖兰,傅帅,郑服丛,李锐,吴伟怀,贺春萍. 2011. 植物病原菌抗药性研究进展[J]. 热带农业科学,31(1):86-90. [Zheng X L,Fu S,Zheng F C,Li R,Wu W H,He C P. 2011. Research advances on fungicide resistance in plant pathogens[J]. Chinese Journal of Tropical Agriculture,31(1):86-90.]
Ateeq R U,Ummad D U U,Hasan N S A,Rana L M,Aleem K S,Tariq M M,Shoaib F. 2015. Emerging resistance against different fungicides in Lasiodiplodia theobromae as the cause of mango dieback in Pakistan[J]. Archives of Biological Sciences,67(1):241-249. doi:https://doi.org/10.2298/ABS140904030R.
Junior R S,Nunes G H S,Lima L L D,Guimaraes I M,Morais P L D D. 2009. Chemical control stem rot caused by Lasiodiplodia theobromae on mangoes fruits[J]. Revista Brasileira de Fruticultura,31(3):907-910. doi:https://doi.org/10.1590/S0100-29452009000300039.
Li Q L,Deng T J,Huang S P,Gao T X,Mao JY,Hsiang T. 2014. First report of gummosis of mango trees caused by Neofusicoccum parvum in Sichuan,Southwest China[J]. Journal of Plant Pathology,96(S4):113-131. doi:10.4454/ JPP.V96I4.023.
Malik M T,Ammar M,Ranan M,Rehman A,Bally I S E. 2016. Chemical and cultural management of die back disea-se of mango in Pakistan[J]. Acta Horticulture,(1111):363-368. doi:10.17660/ActaHortic.2016.1111.52.
Shukla P K,Bhattacherjee A K,Dikshit A. 2018. Field efficacy of difenoconazole against shoulder browning disease of mango and its residue analysis for safety evaluation in fruit[J]. Indian Phytopathology,71(1):1-5. doi:10.1007/s42360-018-0008-0.
Slippers B,Johnson G I,Crous P W,Coutinho T A,Wingfield B D,Wingfield M J. 2005. Phylogenetic and morphological re-evaluation of the Botryosphaeria species causing diseases of Mangifera indica[J]. Mycologia,97(1):99-110. doi: 10.1080/15572536.2006.11832843.
Zhao F F,Liu J K,Xie D F,Lü D Z,Luo J H. 2018. A novel and actual mode for study of soil degradation and transportation of difenoconazole in a mango field[J]. RSC Advances,8(16):8671-8677. doi:10.1039/C8RA00251G.
(責任编辑 麻小燕)

