Comparison of serum ALT levels in mice with autoimmune hepatitis induced by ConA: a meta-analyses
Ke-Hui Zhang, Meng-En Zhou, Meng-Xing Cao, Yong Li
1Shanghai Municipal Hospital of Traditional Chinese Medicine ,Shanghai University of Traditional Chinese Medicine, Shanghai 200071,China; 2Shanghai University of Traditional Chinese Medicine,Shanghai 200071,China.
Abstract Background: Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease, and with few major advances in therapy methods over the last years.It's hard to verify the pathogenesis of AIH and effective novel treatments for AIH because of lacking proper animal models, nor to accurately reproduce the human condition.To find clues to overcoming the therapeutic insufficiency, preclinical animal models are needed.Concanavalin A-mediated AIH model has been widely used in the experiences.Generally, serum ALT levels were tested to assess the animal models.The purpose of this study is to reveal the correlation between ALT serum levels in ConA-induced AIH mouse models and ConA dose and duration.Methods: Animal studies in eight databases (PubMed, Cochrane's library, Excerpta Medica Database (Embase), Web of Science, China National Knowledge Internet (CNKI),Wan fang database, Weipu Data-base (VIP) and China Biomedical Literature Database (CBM)) were searched up to May 2020.This study performed a meta-analysis by comparing the ALT serum level in the AIH animal models.We did register our research in PROSPERO.(https://www.crd.york.ac.uk/ PROSPERO/) The ID is CRD42020182967.Result: 11 studies with 265 mice were included in our meta-analysis.The serum level of ALT of the AIH model animals (mean difference (MD) 4.83, P< 0.001) was higher than that of the control group.Conclusion: This study found that ALT levels could be elevated after caudal vein injection of ConA.Different time points also affect ALT serum levels.More experiences studies are need to further illustrate the effect of Con-A administration in experimental models.
Keywords: Animal model, ALT, ConA, Meta.
Introduction
Autoimmune hepatitis (AIH) is characterized by progressive destruction of the liver parenchyma and a chronic fibrosis [1].It can be detected the elevation of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and the appearance of autoantibody in the blood [1], which is also true in some animal models.Knowledge gained from experience animal models will help to identify novel therapies [2].The current treatment of autoimmune hepatitis is still largely dependent on the administration of corticosteroids and cytostatic drugs [3,4].Due to the lack of understanding immunopathogenic mechanisms of AIH and the absence of valid animal models, the treatment process has been hindered for a long time.
To further understand the pathological mechanisms and hepatic immunology of autoimmune hepatitis,several animal models were accomplished.But it is not easy to establish an experimental model that accurately reflects the chronic nature of human immunological hepatitis, partly because of liver immune tolerance,including T cell inactivation, apoptosis and regulatory dendritic cell differentiation.There was no spontaneous model that can serve as the "gold standard" model for the disease.In the early 1970s, Meyer [5]stimulated hepatitis by injecting liver homogenates of heterologous origin combined with various activating adjuvants into mice and found prolonged flares of hepatitis but were not sufficient to induce disease.And then, Kuriki et al.injected mice with syngeneic liver homogenate combine with the polysaccharide of Klebsiella pneumonia 03:k1 as an adjuvant to induce"experimental autoimmune hepatitis" in the 1980s[6].Subsequently, some researchers established gene knockout models of AIH, such as NTxPD-1-deficient mice [7], TGF-β gene knockout [8] and a triple knockout mouse model for the receptor tyrosine kinases Tyro3, Axl and Mer [9].Among them, NTXPD-1 deficient mice [7], namely programmed death (PD-1) deficient mice when neonatal thymectomized caused spontaneous and fatal AIH.The pathological manifestations included the appearance of the antinuclear antibody (ANA), elevated serum aminotransferase levels, severe portal inflammation and piecemeal necrosis.Transforming growth factor plays an important role in immune homeostasis, and TGF-β-/- mice are particularly prone to spontaneous development of large areas of hepatitis and hepatocyte necrosis, with elevated serum ALT levels [8].However,knockout mice tend to multiorgan inflammatory lesions and early death, limiting their potential use as preclinical models.Then in 2010, Zierden et al.[10]established a spontaneous chronic liver inflammation in Alb-HA/CL4-TCR double transgenic mice, and used the transgenic technology to stabilize the expression of a specific antigen in the liver [10].Mice expressing influenza virus hemagglutinin (HA) under the control of the liver albumin promoter, expressing HA-specific TCR on most CD8T cells, and expressing neoantigens specifically in the liver developed spontaneous chronic hepatitis, liver inflammatory necrosis, and elevated serum ALT levels in these mice [10].
In contrast to the above approach, most researchers used external inflammatory stimuli to induce AIH-like disease models, for example, α-Galactosylceramide(α-GalCer) [11], which is a synthetic glycolipid which can be recognized by invariant NKT cells through CD1d, and Concanavalin A purified from Canavalia brasiliensis [12].The most commonly used is the injection of Concanavalin A causing non-specific T-cell and macrophages in a dose-dependent manner to induce acute immune-mediated liver injury and severe liver damage, rather than chronic [13].Con-A drove and activated the recruitment of CD4+T cells and NKT cells through the interaction between lymphocytes on the surface of sinusoidal endothelial cells and macrophages, secrete inflammatory cytokines such as IFN-γ and TNF-α, who have been identified as the final effector cytokines required for Con-A induced hepatitis [14,15], and then subsequently recruited and activated other cells and induced apoptotic cell death of LSECs and hepatocytes.Within a short time after injection, liver inflammation was damaged and ALT was increased, but autoantibodies were not produced.Above all, Con-A induced hepatitis is a valuable model for highly specific activation of T cells and has been eligible to extensively apply for investigating the immune mechanisms in AIH.Generally, we test the ALT serum levels to assess the animal models in order to find an optimal model to understand the underlying mechanisms and to explore potential treatment options.
Methods
This meta-analysis was managed based upon on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplementary Materials).We registered the research in PROSPERO.(https://www.crd.york.ac.uk/ PROSPERO/) The ID is CRD42020182967.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: 1) The study objects were murine of any species; 2) The mice were given a single intravenous injection of Con A through tail vein at a dose of 20 mg/ kg or 15 mg/kg body weight and were sacrificed at various time points(8h,12h or24 h) in AIH mice models.3) subjects in the control group were intravenously injected with an equal volume of saline at different time points, eg.8h,12h, 24h; 4) assessed the serum level of ALT and reported in the studies.The exclusion criteria included the following: 1) It's a human or other species study;2) trials were performed in vitro or intraperitoneal injection; 3) Combined with other drugs, nor an AIH animal models; 4) studies did not provide primary outcomes; 5) Studies did not provide original data; 6)dates were duplicated in another study.
Data Sources and Search Methods
Animal studies in eight databases (PubMed, Cochrane's library, Embase, Web of Science, CNKI, Wan fang database, VIP and CBM) were electronically searched up to May 2020.The search was independently conducted by two authors (Zhang and Zhou).There were no language or date of publication restrictions.We searched based on the following keywords:'Autoimmune Hepatitis' or 'Autoimmune Hepatitides'or 'Hepatitides, Autoimmune' or 'Autoimmune Chronic Hepatitis' or 'Autoimmune Chronic Hepatitides'or 'Chronic Hepatitides, Autoimmune' or 'Chronic Hepatitis, Autoimmune' or 'Hepatitides, Autoimmune Chronic' or 'Hepatitis, Autoimmune Chronic' in combination with 'Concanavalin A' or 'Receptor,Concanavalin A' or 'Concanavalin A Binding Sites' or'Concanavalin A Receptor*'.
Selection of Studies
After excluded duplicates, the selection of studies was based on the title and abstract, and then all irrelevant studies were excluded.Then we evaluated the full text of potentially relevant articles and excluded certain articles based on inclusion and exclusion criteria.Both were conducted by two independent authors (Zhang and Zhou), and any disagreement was discussed with a third author (Cao).
Data Extraction and Management
For each study, the following data were extracted:sample size; gender, age and weight of rats; Con-A dosage in mg/kg; the route of drug administration; the time points of treatment; and outcomes of interest (ALT levels).When the data regarding data extraction were not sufficient for meta-analysis or lost, the original authors would be contacted.If didn’t get a response, the study was excluded.If remained disagreement between reviewers, the third review author was consulted to resolve that.
Assessment of Risk of Bias in Included Studies
The risk of bias assessment was managed by two review authors independently of all included studies on the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) risk of bias tool [16],including similar baseline characteristics, random sequence generation, allocation concealment, random outcome assessment, random housing,intervention blinding, incomplete outcome data, selective outcome reporting, assessment blinding, other sources of bias.
Statistical Analysis
All statistical analyses were carried out by using reporting, assessment blinding, other sources of bias.
Statistical Analysis
All statistical analyses were carried out by using Review Manager (RevMan) software version 5.3 and Stata16 software.We enforced meta-analyses to compare the serum level of ALT between AIH animalmodels and vehicle group.Considered that the main outcomes were continuous dates, the pooled effect sizes were evaluated by calculating the mean differences (MDs) or standardized mean differences(SMDs), and 95% confidence intervals (CIs).When involved studies were the same measure unit, MD was used; If not, SMD was performed.The chi-squared test and the I2statistic were used to value heterogeneity.IfP>0.1 orI2>50%, the fixed-effect model was performed.IfP≤0.1 orI2≥ 50%, we explored potential sources of heterogeneity and used random-effect model.We performed sensitivity analysis by re-evaluating the remaining studies and then removing articles in turn to assess the stability of the results.Furthermore, we implemented subgroup analyses according to the dose and duration of ConA on mice in each group.P≤0.05 was considered to be statistically significant.It was considered statistically significant whenP≤0.05.
Results
Study Selection
The flow chart of the screening studies is exhibited in Fig.1 We initially identified 453 records from eight databases (PubMed n=126, Embase n=161, Cochrane n=3, Web of science n=33, CNKI n=54, CBM n=8,and Wan fang date n=56, and VIP n=12) based on the above keywords.After excluding duplicated records(n=126), 327 articles remained.After screened the abstracts and titles of the remaining records, 177 articles were removed.Furthermore, the rest of articles we read the full-text, and as a result, 138 articles were excluded, which including 1.Non-AIH animal models(n=6); 2.in vitro study (n=2) 3.exact data deficiency(n=75); 4.different experience design (n=50); 5.system review (n=5).In most of the articles, ALT serum levels are expressed in histogram without providing raw data,which results in the inability to obtain accurate data such as mean values and variances.We tried to contact the corresponding author by email or phone, but there was no response.Finally, according to the preset inclusion criteria, eleven studies were included in the meta.
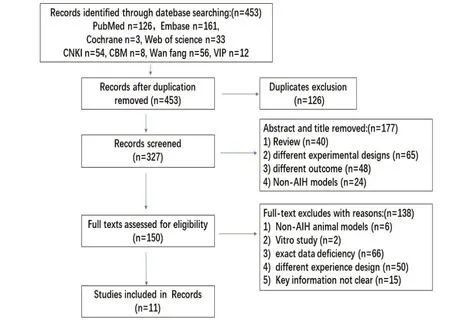
Figure 1 Flow diagram showing the selection process of the studies in the meta-analysis
Study Characteristics
Tab.1 summarized the characteristics of the eleven included studies.A total of 265 mice were included in this meta-analysis [17-27].Considering that the mice conjected different concentrations of Con-A in various time points, 265 animals were included in the study.The sample sizes of the included studies ranged from 5 to 10 animals.The age was from 5weeks to 10 weeks.This review included 11 articles, which contained 26 groups, and divided into experimental group and control group.The experimental group was injected with concanavalin-A through the tail vein,and the control group was injected with normal saline of equal volume.There were ten groups of mice were given Con-A intravenously at 20mg/kg body weight, and 14 groups were given 15mg/kg body weight, remained two with 10mg/kg.Eight studies included only female animals,two studies included only male animals [22,23], one study did not report the sex.After injection, serum was extracted at different time points, ranging from 6 hours to 48 hours.Different methods were used to detect serum alanine aminotransferase (ALT).Four studies were used an automated chemistry analyzer[18,19,24,27], three studies used assay kits [17,20,21],one used colourimetric kits for measuring[26], two send the samples to the hospital laboratory department[22,23], and remaining one didn’t mention it [25].All studies used SPSS software program to analyze the data presented as mean ± standard deviation (SD).

Table 1 Basic characteristics of the included studies
Assessment of Risk of Bias in Included Studies
Each study supplied similar baseline characteristics of animals.All studies made reference to randomization,but no one detailed the method of random sequence generation.Only two studies mentioned assessment blinding [22,23].No one study supported information of intervention blinding.There was no report exhibited the allocation of concealment and random housing.No selective reporting, incomplete outcome data, and other sources of bias in all studies.The risk of bias of included studies are listed in Tab.2.The disagreements between the two reviewers were resolved by consensus or a third review author.
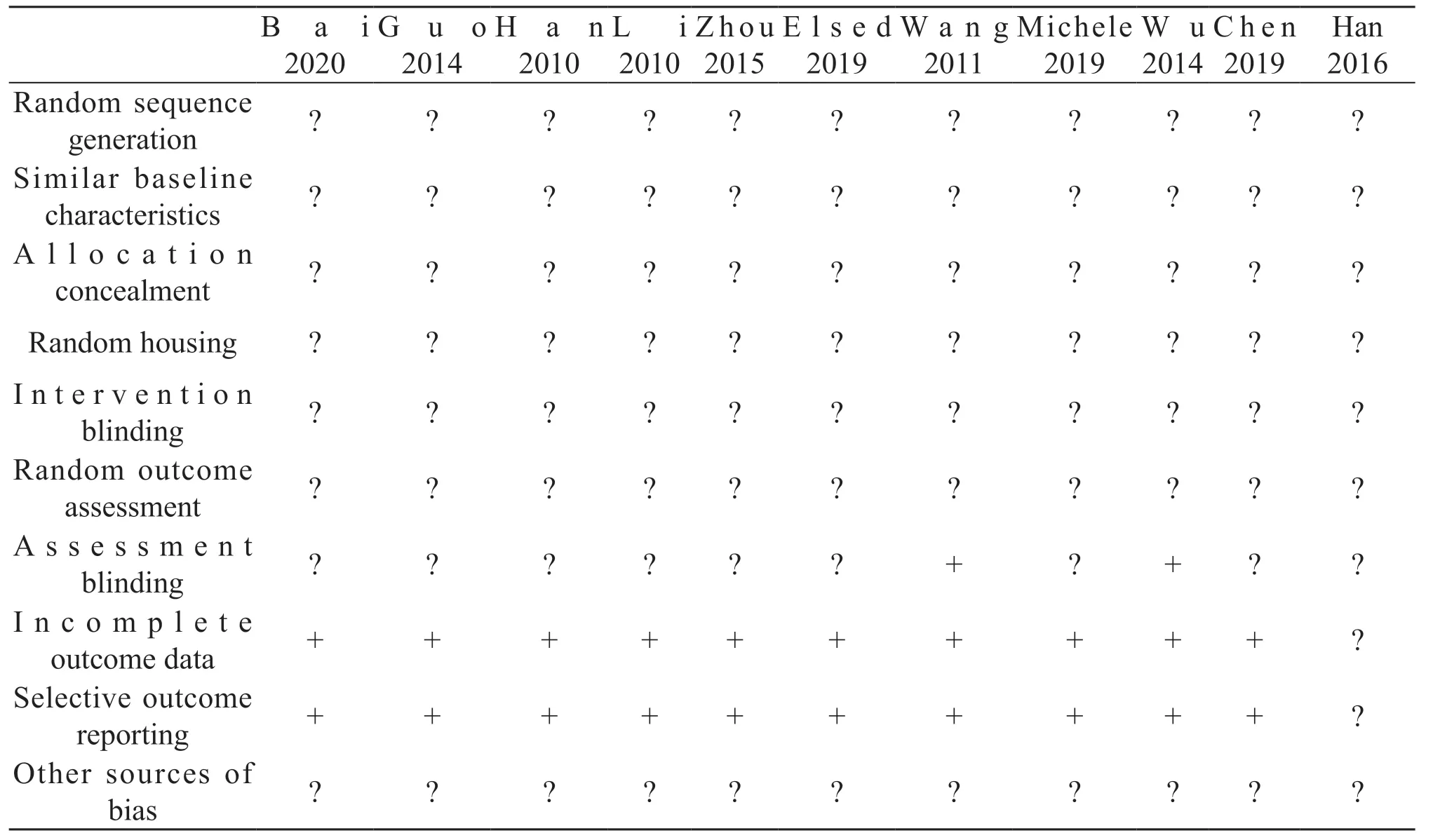
Table 2 Risk of bias of included studies
Meta-Analyses
All studies exhibited the serum ALT data as the outcome indicator, and compared the results between the control group and therapy group.Then, we summarize the whole data and found that ALT level of the ConA-treated group was higher than that of the blank control group (SMD 4.83, 95%CI: 3.61 to 6.06,P < 0.00001; heterogeneity chi2= 196.98,P< 0.00001,I2= 87%, Fig.2).Thus, the result should be interpreted prudently.We conducted a sensitivity analysis.One or several studies were randomly removed to observe the change of heterogeneity, and it was found that when each item was removed one by one, the change of the results of the meta-analysis, particularly the heterogeneity, were not significant.When the studies in the 7 articles were removed, heterogeneity was significantly reduced (SMD 2.36, 95%CI: 1.52 to 3.20,P 0.00001; heterogeneity chi2=14.59,P=0.04, I2=54%,Fig.3), Which suggests that heterogeneity is associated with the differences between the included studies.After the heterogeneity was reduced, only 4 references remained [19,21,22,24].

Figure 2 Forest plot for the serum ALT levels
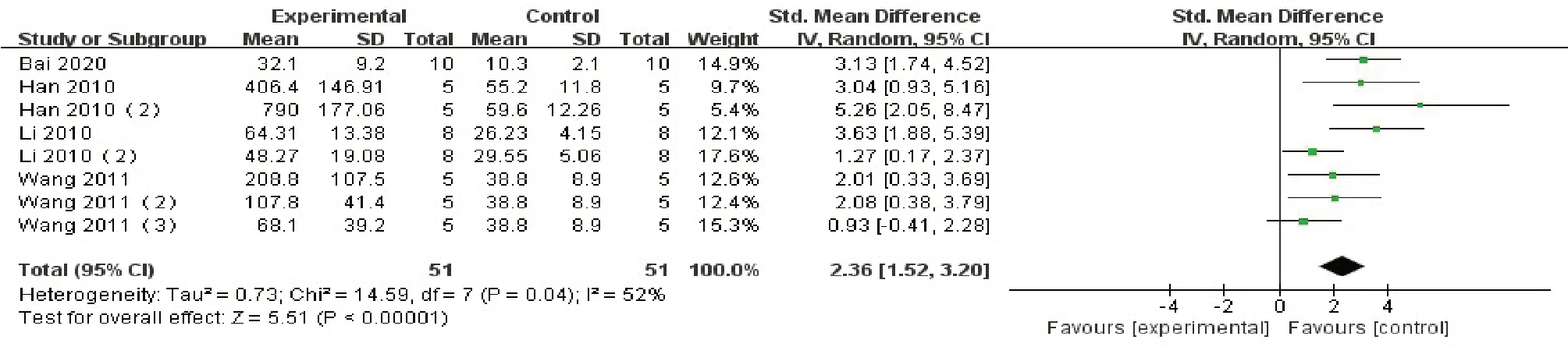
Figure 3 Forest plot for the serum ALT levels, Heterogeneity was reduced after the exclusion of certain included studies
Generally, meta-analysis that includes 10 or more studies need to assess publication bias.Therefore, due to the small number of included studies, the funnel plot was not used to assess the publication bias in our study.We consider the duration and dose of Concanavalin A as covariates.However, in the included studies, each covariable model could not guarantee more than 10 observation results, so there were not enough studies to include regression analysis.Secondly, there was an interaction between the two covariables.Due that, no regression analysis was conducted.
Subgroup Analysis
We performed a subgroup analysis according the dose and duration.Subgroup analysis showed that the duration of action of Con-A were different, so that the ALT data were different, but all of them were higher than the blank control group.When the duration of action is 24h, the SMD is 7.12 (95%CI: 2.68 to 11.55,P< 0.00001; heterogeneity chi2= 46.37,P< 0.00001,I2= 91%, Fig.4); when is 12h, the SMD is 4.38,(95%CI: 1.09 to 7.66,P<0.00001; heterogeneity chi2= 34.23,P<0.00001, I2= 91%, Fig.4); and when it is 8h, the SMD is 5.99 (95%CI: 4.27 to 7.71,P<0.00001; heterogeneity chi2= 30.32,P< 0.00001, I2=77%, Fig.4).At the same time, different concentrations of drugs had different effects on liver enzymes whether at 15mg/kg body weight (SMD 5.87, 95%CI: 3.88 to 7.87,P< 0.00001; heterogeneity chi2= 131.51,P<0.00001, I2= 90%, Fig.5).or at 20mg/kg (SMD 2.62,95%CI:1.63 to 3.61,P<0.00001; heterogeneity chi2=21.86,P<0.00001, I2= 63%, Fig.5), whose values were higher than those of the control group.
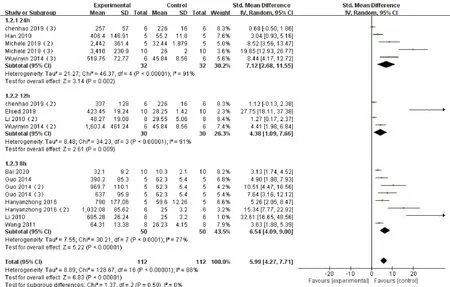
Figure 4 Subgroup analysis: Forest plot of comparison according to the duration

Figure 5 Subgroup analysis: Forest plot of comparison according to the Con-A concentration
Discussion
Summary of evidence
Tail vein concanavalin A injection is a common modelling method for acute liver injury and autoimmune hepatitis in mice at present.Generally,Con-A was treated with multiple time points and different concentrations respectively, and then liver enzyme levels and anti-nuclear antibodies were detected.However, there is no uniform standard to specify the dosage and duration.To explore specific modelling methods, we collected all relevant animal experiments' studies and completed this meta-analysis comparing serum ALT levels in animal models constructed at different time points and drug doses.We included 11 studies to compare ALT levels.It was found that the serological response of animals injected with the drug was higher than that of the control group,indicating that con-A injection by tail vein could cause liver inflammation, which suggested that We could treat mice with Con-A through the tail vein to induce the autoimmune hepatitis model, furthermore,to explore the pathogenesis and treatment about AIH,and verify the effectiveness and pharmacological action of the potential drug and therapy.However, due to the differences in measurement methods, species, sex, age and feeding of mice, the baseline level of the measured values in the control group was also different, and there was great heterogeneity after the data were combined.Through subgroup analysis, we combined ALT levels at 8h, 12h, and 24h of Con-A injection in mice.In this study, combined values showed that ALT levels at 8h and 24h were higher than 12h.The combined SMD data of 15mg/kg body weight was higher than that of 20mg/kg, but the results of subgroup analysis showed high heterogeneity.
Limitations
There were several limitations in our study.Considering the existence of a series of differences among measuring tools, methods, units, species,operations and design protocols in animal experiments,meta-analysis about animal trials tends to be more heterogeneity.Not to mention the exploratory nature of animal experiments and the differences between experiment.
Conclusions
Through the research literature, we have learned that Con-A induced liver injury in mice presented A timedose dependence to A certain extent, and the effects of the duration and dose of the drug were cross-sectional.For example, serum ALT levels may peak at 24h for 15mg/kg [28-30], and liver enzyme levels peak at 12 h for 20mg/kg [31-33], and at higher doses, the mice died, but the specifics are inconclusive [34,35].In addition, since we only included published studies,the possibility that negative results were not reported could not be ruled out, so the experimental results may be overestimated.Furthermore, the number of eligible references that met the characteristics of animal experiments and the inclusion criteria was small, and the results were less robust.All the above factors may affect the implementation of the Meta-analyses and cause some potential bias in our research.In the future,we need more samples and well-designed preclinical experimental studies to probe into the inflammatory effect of Con-A on the liver, clarify its rational dose and drug action time, to better understand the pathogenesis and treatment mechanism of the disease, and achieve the best, safest and waste-free effect.
 Medical Theory and Hypothesis2021年2期
Medical Theory and Hypothesis2021年2期
- Medical Theory and Hypothesis的其它文章
- Study on traditional Chinese medicine syndrome of esophageal cancer based on Delphi method
