Current concept in the diagnosis,treatment and rehabilitation of patients with congestive heart failure
Ivana Sopek Merkaš,Ana Marija Slišković,Nenad Lakušić
Ivana Sopek Merkaš,Nenad Lakušić,Department of Cardiology,Special Hospital for Medical Rehabilitation Krapinske Toplice,Krapinske Toplice 49217,Croatia
Ana Marija Slišković,Department of Cardiology,University Hospital Centre Zagreb,Zagreb 10000,Croatia
Nenad Lakušić,Department of Clinical Medicine,Faculty of Dental Medicine and Health Osijek,Osijek 31000,Croatia
Nenad Lakušić,Department of Internal Medicine,Family Medicine and History of Medicine,Faculty of Medicine Osijek,Osijek 31000,Croatia
Abstract Heart failure (HF) is a major public health problem with a prevalence of 1%-2% in developed countries.The underlying pathophysiology of HF is complex and as a clinical syndrome is characterized by various symptoms and signs.HF is classified according to left ventricular ejection fraction (LVEF) and falls into three groups:LVEF ≥ 50%-HF with preserved ejection fraction (HFpEF),LVEF<40%-HF with reduced ejection fraction (HFrEF),LVEF 40%-49%-HF with mid-range ejection fraction.Diagnosing HF is primarily a clinical approach and it is based on anamnesis,physical examination,echocardiogram,radiological findings of the heart and lungs and laboratory tests,including a specific markers of HF-brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide as well as other diagnostic tests in order to elucidate possible etiologies.Updated diagnostic algorithms for HFpEF have been recommended (H2FPEF,HFA-PEFF).New therapeutic options improve clinical outcomes as well as functional status in patients with HFrEF (e.g.,sodium-glucose cotransporter-2-SGLT2 inhibitors) and such progress in treatment of HFrEF patients resulted in new working definition of the term “HF with recovered left ventricular ejection fraction”.In line with rapid development of HF treatment,cardiac rehabilitation becomes an increasingly important part of overall approach to patients with chronic HF for it has been proven that exercise training can relieve symptoms,improve exercise capacity and quality of life as well as reduce disability and hospitalization rates.We gave an overview of latest insights in HF diagnosis and treatment with special emphasize on the important role of cardiac rehabilitation in such patients.
Key Words:Heart failure;Classification of heart failure;Diagnosis of heart failure;Treatment of heart failure;Cardiac rehabilitation;Heart failure rehabilitation
INTRODUCTION
Heart failure (HF) is one of the major public health problems with a prevalence of 1-2% in developed countries (varies by definition and region),and it increases with age(rising to over 10% in people over 70 years of age)[1].Approximately 33% of men and 28% of women at the age of 55 have a lifetime risk of developing HF[1].
The pathophysiology of HF is complex and it is therefore a clinical syndrome characterized by various symptoms and signs,which is caused by structural and/or functional abnormalities of the heart.HF can be a terminal stage of many cardiovascular diseases,including myocardial infarction,heart valve disease,tachyarrhythmias,congenital heart defects,and cardiomyopathies[1].Most commonly,HF develops as a consequence of a myocyte injury caused by coronary artery disease,uncontrolled arterial hypertension,valvular heart diseases and diabetes mellitus,and it is important to consider pulmonary disorders such as chronic obstructive pulmonary disease or pulmonary arterial hypertension as causes that can lead to HF[1-3].
The main pathophysiological mechanisms leading to HF are increased hemodynamic overload,ischemia,myocardial dysfunction and remodelling,excessive neurohumoral stimulation-chronic sympathetic nervous system overactivity as one of the key pathophysiological mechanisms (in the acute phase,this upregulated sympathetic activity is an essential compensatory response initiated in order to compensate for reduced contractility,and cardiac output but in the long-term,it contributes to cardiac dysfunction as it leads to cardiac hypertrophy and cell dysfunction),activation of the renin-angiotensin-aldosterone system (RAAS),excessive or inadequate proliferation of the extracellular matrix,accelerated apoptosis,and genetic mutations[3-6] (Figure 1).HF is associated with a variety of complications,such as frequent hospitalizations,fatal arrhythmias,and death in the advanced stage of the disease.

Figure 1 Pathophysiological mechanisms in chronic heart failure (data from[3]).
CLASSIFICATION
As mentioned,HF is caused by a number of conditions,causing left and right ventricular dysfunction[6-8].The most common classification of HF refers to the left ventricular ejection fraction (LVEF).Accordingly,HF is classified into three groups:with preserved left ventricular ejection fraction LVEF ≥ 50%-HFpEF,with reduced ejection fraction LVEF<40%-HFrEF,and patients with a mid-range ejection fraction are between these two groups LVEF 40%-49%-HF with mid-range ejection fraction (HFmrEF)[1,2].
HF is often consi dered as a left-sided failure when caused primarily by left heart pathologies (e.g.,left ventricular,mitral valve,or aortic valve dysfunction).The ventricle fails in its ability to eject blood,or can do so only at the cost of high filling pressures[6,7].Right-sided or right ventricular HF also has a complex pathophysiology and it is influenced by multiple factors (such as volume status,pulmonary vascular resistance,right ventricular,pulmonic valve,or tricuspid valve dysfunction,left ventricular function) and usually occurs as a result of left ventricular HF[8,9].Left and right ventricular HF may overlap or occur separately and most patients with right HF have some elements of left HF.
Considering the clinical presentation,HF is divided into acute and chronic[1,2].Patients with a low LVEF and no symptoms or signs of HF are characterized as asymptomatic patients with a reduced left ventricular systolic function.Those who have signs and symptoms of HF belong to the group of patients with chronic HF,and those who do not experience worsening of symptoms within at least a month are considered stable patients with chronic HF[6].Acute HF includes the worsening of chronic HF,pulmonary oedema,and cardiogenic shock.There are a number of factors that can trigger acute HF,e.g.,myocardial disfunction,pericardial tamponade,and acute valve insufficiency,which are among the most frequent primary cardiac causes of acute HF[1,2].Decompensation of chronic HF can occur without known precipitant factors,but infection,uncontrolled hypertension,rhythm disturbances,exacerbation of chronic obstructive pulmonary disease,or non-adherence to medicaments can be the cause of decompensation[1,2].
Chronic HF is a complex of multiorgan dysfunction,characterized by the impaired function of the heart,kidney,and skeletal muscles,with increased stimulation of the sympathetic nervous system and numerous humoral and neuroendocrine disorders.The severity of symptoms in chronic HF is classified according to the New York Heart Association (NYHA)[10] and American College of Cardiology/American Heart Association (ACC/AHA)[11] in four stages[2] (Table 1).The NYHA functional classification is an independent predictor of mortality and it is widely used in clinical practice[12].
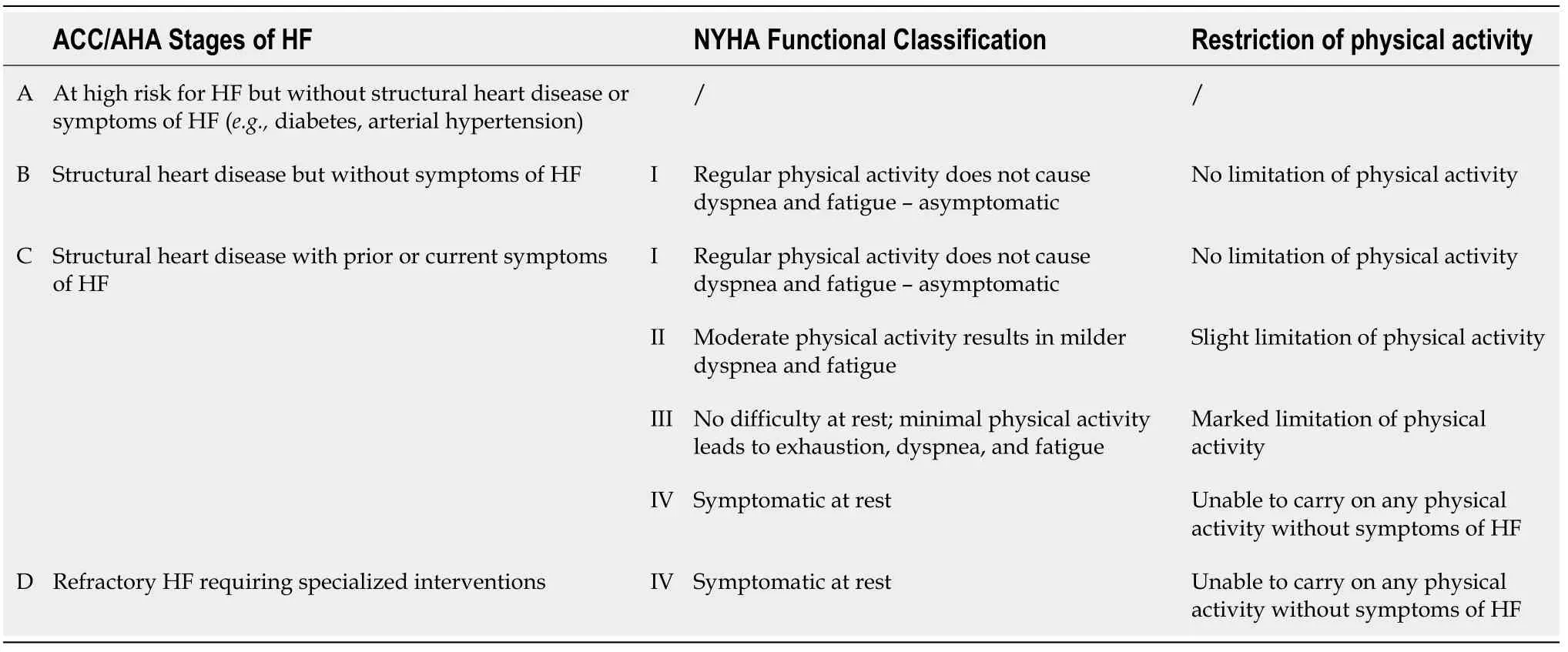
Table 1 Comparison of American College of Cardiology/American Heart Association Stages of HF and New York Heart Association Functional Classifications (data from[2])
CLINICAL PRESENTATION
Clinical signs and symptoms of HF include shortness of breath,dyspnoea (initially with severe physical exertion,and in the advanced stage at rest and worsening in the supine position),orthopnoea (dyspnoea in the supine position),paroxysmal nocturnal dyspnoea (sudden onset of shortness of breath at night),poor mobility,dizziness,lack of appetite,fatigue,and muscle weakness due to early fatigability.Due to compensatory mechanisms,in the early phase of HF patients do not have to present with all the specific symptoms and physical signs,such as those related to fluid retention.In the advanced stages,physical examination and auscultation can reveal abnormal pulmonary phenomena (wheezing,crepitation),the third heart murmur (S3 gallop)that can rarely be heard,presence of an oedema (generalized or localized),and cardiac cachexia (loss of muscle mass).The signs of predominantly right-sided HF are distended jugular veins,ascites,hepatojugular reflux (pressing the hands on the abdomen leads to a more pronounced filling of the jugular veins),and oedema of the legs[1-4].
DIAGNOSIS
The cornerstone in the diagnosis of HF is primarily established by a clinician’s assessment and it is based upon a careful medical history (coronary heart disease,arterial hypertension,diabetes,valve disease,cardiotoxic drugs,irradiation,etc.),a physical examination,an electrocardiogram (ECG),an ultrasound of the heart(echocardiography),radiological findings of the heart and lungs,laboratory tests,including a specific markers of HF-brain natriuretic peptide (BNP) or N-terminal pro-B-type natriuretic peptide (NT-proBNP),as well as other diagnostic tests,in order to elucidate possible aetiologies [e.g.,invasive coronary angiography,magnetic resonance(MR),or computed tomography (CT)][1,6-8].
Even though a decreased left ventricular ejection fraction identifies patients with HFrEF,the echocardiogram alone does not establish or exclude the diagnosis of HF,since approximately half of the patients with HF have a preserved left ventricular ejection fraction.The most common patients with HFpEF are elderly women with hypertension,ischemic heart disease,atrial fibrillation,obesity,diabetes mellitus,renal disease,or obstructive lung disease[13].Echocardiography is important in revealing findings that go along with HF and in verifying possible causes of HF (e.g.,left ventricular diastolic dysfunction,left ventricular systolic dysfunction,valve dysfunction,regional wall motion abnormalities,left ventricular hypertrophy,left atrial enlargement).
Two algorithms,Heavy,2 or more Hypertensive drugs,Atrial Fibrillation,Pulmonary hypertension,Elder age>60,elevated Filling pressures (H2FPEF)[14] and HF Association Pre-test assessment,Echocardiography and natriuretic peptide,Functional testing,Final aetiology (HFA-PEFF)[15] may facilitate a HFpEF diagnosis[13].The H2FPEF score,which relies on simple clinical characteristics and echocardiography,enables the discrimination of HFpEF from noncardiac causes of dyspnoea and assists in the determination of the need for further diagnostic testing[14].Elevated natriuretic peptides support a diagnosis of HFpEF[16] but normal levels do not exclude it.Echocardiography has a relevant role in HFpEF and is used for the non-invasive hemodynamic assessment of high LV filling pressures (indirectly pulmonary capillary wedge pressure,PCWP).Early (E) transmitral filling velocities that are measured at the mitral leaflet tips by pulse wave Doppler,Tissue Doppler echocardiography which is performed to measure early (e′) diastolic tissue velocities at the septal and lateral mitral annulus,and the mean of the septal and lateral E/e′ ratio is used to estimate the PCWP[16,17].Other important measures include the left atrial volume index,the LV mass index,the LV relative wall thickness,tricuspid regurgitation velocity,and the LV global longitudinal systolic strain[15].According to the consensus recommendation from the HF Association (HFA) of the European Society of Cardiology (ESC) and the definition of HFA-PEFF score,the major (2 points) and minor (1 point) criteria were defined from these measures[15].The score has functional,morphological,and biomarker domains (Figure 2).Within each domain,a major criterion scores 2 points or a minor criterion 1 point.If several major criteria within a single domain are positive,this domain still contributes 2 points.If no major but several minor criteria are positive the contribution still is 1 point.Major and minor criteria are not additive in a single domain and points are added only when they come from different domains[15].

Figure 2 Heart failure-classification and criteria in diagnosis (data from[1,15]).
Patients with mild or moderate HF may appear normal on physical examination,with normal vital signs.Euvolemic patients with chronic dyspnoea,symptoms of HF,and normal cardiac filling pressures at rest may have abnormal hemodynamic responses during exercise,suggesting that the chronic symptoms are related to HF[18-21].These patients with normal cardiac output at rest have an inability to increase cardiac output during exercise without an excessive increase in filling pressures,resulting in fatigue and intolerance.Elevated resting E/e′ strongly supports the presence of high PCWP and thus HFpEF,but a normal resting E/e′ does not exclude HFpEF[15,18].Exercise stress echocardiography on a bicycle or a treadmill with imaging during exercise is recommended,but there are no universally adopted protocols[15].Exercise echocardiography should be considered abnormal if the average E/e’ ratio at peak stress increases to ≥15,with or without a peak tricuspid regurgitation (TR) velocity of>3.4 m/s[15].An increase in only TR velocity should not be used to diagnose HFpEF because it might be a result of the normal hyperdynamic response to exercise (with increased pulmonary blood flow) without the LV diastolic dysfunction.For selected patients with suspected HF with uncertain diagnosis despite the evaluation,as described above,the clinical gold standard for the diagnosis of HF is the identification of an elevated PCWP on an invasive hemodynamic test (right heart catheterization with the PCWP assessed at rest or during exercise)[15,18].The patient must have symptoms consistent with HF and PCWP ≥ 15 mmHg at rest or ≥ 25 mmHg during exercise for establishing a diagnosis of HF[18].If these criteria are not fulfilled,further evaluation for other causes of dyspnoea is required.To summarize,echocardiography may help rule out HFpEF,although approaches to exclude HFpEF based solely on data at rest are of questionable accuracy,and furthermore,there is evidence that reinforces the value of exercise testing using invasive and non-invasive hemodynamic assessments to definitively confirm or rule out the diagnosis of HFpEF[18].
TREATMENT
The therapy for HFrEF aims to improve quality of life,reduce the rate of hospitalizations,as well as morbidity and mortality.Care for patients with HFrEF includes an overall approach-the treatment of possible causes and associated conditions (e.g.,hypertension,diabetes mellitus,and thyroid dysfunction,anemia) of HF,pharmacologic therapy,monitoring,education and cardiac rehabilitation,palliative care,device therapy and cardiac transplantation.
Antagonism of neurohormonal activation is the foundation of the modern HF therapy.The pharmacological treatment aims to alleviate the symptoms and to improve the quality of life,prevent complications and the need for hospitalization,and thereby reduce high mortality.Diuretics are used to facilitate the symptoms of congestion.Meta-analyses show that diuretics,compared with placebo,appear to reduce the risk of death and reduce symptoms,and compared with an active control,appear to improve the functional capacity of patients with HFrEF[22,23].The progression of the disease of the heart muscle itself can be prevented by acting on the reflex mechanisms that are activated in the body when HF occurs.
The first line of treatment is angiotensin converting enzyme inhibitors (ACEi) and beta-adrenergic receptor blockers (beta-blockers),regardless of the aetiology of HF[1,2,24].If the patient does not tolerate ACEi or they are contraindicated,then angiotensin receptor blockers (ARBs) are used.ACEi have been shown to reduce morbidity and mortality in patients with HFrEF[25-27],and data suggests that there are no differences among the available ACEi regarding their effects on symptoms or on survival[28].The usage of beta-blockers showed an improvement of LVEF and a reduction of mortality and the number of hospitalizations[29].Unlike ACEi,beta-blockers have no class effect and evidence of beneficial effects in the treatment of HF has been reported for bisoprolol,prolonged-release metoprolol,carvedilol,and nebivolol[30,31].Therapy with ACEi and beta-blockers is complementary and can be started together.Betablockers are recommended as a start for clinically stable patients at a low dose,followed by titration to the maximum tolerated dose[1].
Mineralocorticoid receptor/aldosteron antagonists (MRA)-spironolactone and eplerenone are recommended in all symptomatic patients (who are on ACEi and betablocker therapy) with LVEF ≤ 35%[1,2].Inhibition of aldosterone action results in decreased endothelial inflammation and decreased stimulation of the sympathetic system and RAAS systems with an antifibrotic effect.Studies show reduction of allcause mortality (for spironolactone 30%[32]) and lower rate of hospitalization in patients treated with MRA[33-35].Possible side effects are hyperkalemia,hyponatremia,reversible increase in blood urea and creatinine levels in patients with impaired renal function,hypotension in patients with low blood pressure (although recent study shows that MRA treatment had little effect on systolic blood pressure in patients with HFrEF and therefore low systolic blood pressure is not a reason to withhold MRA therapy in patients with HFrEF[36]).Spironolactone binds to both androgen and progesterone receptors,so men can experience breast enlargement-gynecomastia,while women can develop excessive farsightedness-hirsutism,and postmenopausal bleeding[8].Due to its selective binding to the mineralocorticoid receptor,eplerenone has no endocrine side effects,has a lower risk of hyperkalemia,and it is a better choice in diabetics[8].Caution should be exercised in the use of MRA in patients with impaired renal function and serum potassium level greater than 5.0 mmol/L[1].
If symptomatic HF persists (NYHA class II or III),replacement of ACEi (or ARB if used) by angiotensin receptor neprilysin inhibitor (sacubutril)-ARNI (angiotensin receptor neprilysin inhibitor) is recommended to further reduce morbidity and mortality[1,24,37].Neprylisin inhibits natriuretic peptides (NP),bradykinin,adrenomedullin,and the β-amyloid (Aβ) peptide[37].The combination of the renin-angiotensin system and neprilysin inhibition showed to be superior to a separate approach[38],but in clinical trials,the combination of ACEi and neprilysin was associated with serious angioedema[39,40].Combined molecule LCZ696 consisting of sacubutril and valsartan minimizes the risk of serious angioedema,and the mechanism of action is inhibition of the neprilysin via the active metabolite of sacubitril and blocking of the angiotensin II receptor via valsartan[41,42].There is an increase in the number of peptides that neprilysin degrades,such as A-type natriuretic peptide (ANP) and BNP,which bind to NP receptors.This results in vasodilation,the enhancement of natriuresis and diuresis,increased glomerular filtration,the inhibition of renin and aldosterone release,decreased sympathetic activity,as well as antihypertrophic and antifibrotic effects[43].Considering described mechanism of action,BNP concentrations rise with neprilysin inhibition and the clinical validity of measuring BNP in patients treated with sacubitril/valsartan has been questioned.The use of NT-proBNP is preferred and recommended in assessing the effectiveness of therapy,although either biomarker predicts the risk of major adverse outcomes in patients treated with ARNI[44].Monitoring of blood pressure and renal function is necessary in these patients and accordingly the dose increases to the maximum tolerated dose.The longterm use of ARNI has possible side effects such as amyloid deposition and cognitive disfunction,and these side effects may be associated with specific polymorphisms in individuals[45,46].One small study on healthy individuals showed increase of βamyloid protein in the soluble rather than the aggregable form,which may indicate cerebral safety[47].Long-term safety,especially in patients at risk of Alzheimer disease,needs to be assessed[1,46].
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are an insulin-independent class of oral antihyperglycemic medication that are used in the treatment of type 2 diabetes.They reduce blood glucose by the inhibition of glucose reabsorption at the proximal tubule of the kidney which results in glycosuria and natriuresis[48].Effects in lowering body weight and decreasing systolic blood pressure,as well as side effects such as genital tract infections,lower leg amputations,electrolyte disturbances,bone fractures are noted[48].According to the latest recommendations on HF,empagliflozin should be considered in patients with type 2 diabetes mellitus (T2DM) in order to prevent or delay the onset of HF or prolong life,and canagliflozin and dapagliflozin should also be considered for patients with T2DM and either established cardiovascular (CV)disease or at high CV risk[49].Newest studies show that SGLT2 inhibitors reduce the risk of cardiovascular death or hospitalization in patients with HFrEF regardless of the presence or absence of diabetes[50,51].Beneficial effects of SGLT2 inhibitors may complement and improve the effects of first line HF therapy (increase in natriuresis,decreasing LV wall stress,preload,afterload and interstitial oedema),alongside with clinically important benefit such as improving renal function in HF patients[50,51].Therefore,compelling evidence suggest that SGLT2 inhibitors should be added to the current recommended treatments of HFrEF,even in the absence of diabetes (Figure 3).
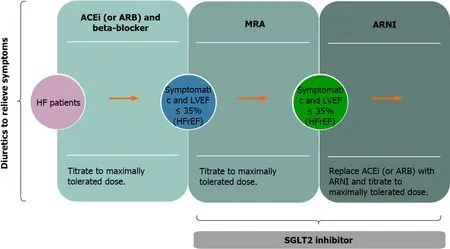
Figure 3 Heart failure medication therapy (data from[1,51]).
In addition to the maximum tolerated dose of betablocker,ACEi (or ARB),and the MRA,ivabradine should also be considered in symptomatic patients with increased heart rate (more than 70 / min),in sinus rhythm and EFLV<35%,to reduce the risk of HF hospitalization or cardiovascular death (or in patients who are unable to tolerate or have contraindications for a beta-blocker)[1,24].
In symptomatic patients (NYHA Class II-IV) with HFrEF who cannot tolerate ACEi nor ARB (or they are contra-indicated),hydralazine and isosorbide dinitrate may be considered to reduce the mortality[1,52].Digoxin may be considered in symptomatic patients in sinus rhythm despite optimal medical therapy to reduce the risk of hospitalizations[1,53].It is also used in patients with HF and atrial fibrillation (AF) to slow a rapid ventricular rate,but it is only recommended when adequate heart rate is not achieved with other therapeutic options[54,55].Optimal ventricular rate for patients with HF and AF has not been well established but resting ventricular rate of 70-90/min is recommended based on current opinion (acceptable up to 110/min),rather than insisting on strict lower ventricular heart rate[56].
Non-dihydropyridine calcium-channel blockers (CCBs) are not recommended for the treatment of patients with HFrEF[1] (diltiazem and verapamil are considered to be unsafe in patients with HFrEF[57]).In case of compelling indications amlodipine and felodipine can be used in patients with HFrEF[1].
Statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) reduce the mortality and morbidity in patients with atherosclerotic disease,but there is no evidence of benefit or improvement of the prognosis in patients with HFrEF[58].Patients who already receive statin because of coronary artery disease or hyperlipidemia should continue to use this therapy[1].The n-3 polyunsaturated fatty acid(PUFAs) is not a routinely used supplement in patients with HFrEF since the randomized trial demonstrated minimal to no benefit[59].
Novel therapeutic approaches
The effect of a novel oral soluble guanylate cyclase stimulator-vericiguat was tested in patients with HFrEF and the result of the new randomized trial showed that the incidence of death from cardiovascular causes or hospitalization for HF was lower among subjects who received vericiguat in comparison to those who received placebo(HF hospitalizations were significantly reduced,while cardiovascular deaths were not significantly diminished)[60].
Omecamtiv mecarbil is a cardiac-specific myosin activator that improves cardiomyocyte contraction which is being studied for a potential role in the treatment of left ventricular systolic HF[61].The latest trial showed a significant reduction of HF event or death from cardiovascular causes in subjects who received omecamtiv mecarbil twice daily in contrast to those who received the placebo[62].
The effect of the anticoagulant therapy in HF patients in sinus rhythm is being assessed since HF is associated with activation of thrombin-related pathways,which predicts a poor prognosis.Studies with rivaroxaban (factor Xa inhibitor) hypothesized that the treatment could reduce thrombin generation and improve outcomes for patients with worsening chronic HF and underlying coronary artery disease[63,64].Rivaroxaban did not reduce HF hospitalization but did reduce the rate of stroke[63].Thromboembolic events occurred frequently in patients with HF,coronary artery disease,and sinus rhythm.A post-hoc analysis revealed that rivaroxaban may reduce the risk of thromboembolic events in this population,but these events are not the major cause of morbidity and mortality in patients with recent worsening of HF where rivaroxaban had no effect[64].Rivaroxaban at dose 2.5 mg twice daily in addition to aspirin may be considered for ambulatory patients with coronary artery disease (CAD)and chronic HF in NYHA class I/II with an LVEF>30%,in order to reduce the risk of stroke and CV death[49,64].For chronic HF patients in NYHA class III/IV and recent HF hospitalization,initiation of treatment with rivaroxaban is not recommended,as no benefit was shown[49].
The treatment of comorbidities that are present in chronic HF patients is an important part of holistic approach and improves outcomes of these patients.Iron deficiency is common in patients with and without anemia and has unfavorable clinical and prognostic consequences in patients with HFrEF.Important clinical trials have been conducted with ferric carboxymaltose (FCM)[65-67],and the treatment with FCM may result in the improvement of functional capacity,symptoms and quality of life (whether FCM is associated with an improved outcome in these high-risk patients needs further study).The trial including iron deficient patients hospitalized for acute HF showed that intravenous FCM compared to placebo was associated with reduction of total HF hospitalizations and CV death[68].
To conclude,there are important advances in the medicament treatment of HFrEF and therapeutic development is accelerated.These new therapeutic options improve clinical outcomes and functional status[69].Accordingly,new working definition of HF with recovered left ventricular ejection fraction (HFrecEF) has been proposed[69,70].HFrecEF is a complex clinical entity and definition includes:(1) Documentation of a LVEF<40% at baseline,combined with (2) a ≥ 10% absolute improvement in LVEF;and (3) A second measurement of LVEF>40%[69,70].The proportion of patients with HFrecEF varies widely (10%-40%) and should be followed every 6 mo to 1 year,with imaging obtained every 3-5 years to monitor LV function[70].HFpEF has a significant morbidity and mortality and so far,no treatment has clearly demonstrated an improvement of outcome in HFpEF,but rather it is limited to symptom relief,which effectively improves the quality of life[13,71,72].The emphasis is on treatment of comorbidities-hypertension,atrial fibrillation,obesity,diabetes mellitus,renal disease,obstructive lung disease,or ischemic heart disease.Regular exercise program is an important part in the treatment of these patients[13].
Device therapy
Implantable cardioverter-defibrillator (ICD) is effective in correcting potentially lethal ventricular arrhythmias.Some antiarrhythmic drugs might reduce the rate of tachyarrhythmias and sudden death,however they do not reduce overall mortality and may even increase it[1].An ICD is recommended in secondary prevention to reduce the risk of sudden death and all-cause mortality in patients who have recovered from a ventricular arrhythmia causing hemodynamic instability,and who are expected to survive for more than one year with good functional status[1,73,74].ICD therapy is recommended for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with LVEF of 35%or less,symptomatic while receiving optimal medical therapy,and who have reasonable expectation of survival for more than one year with good functional status[1,24,75,76].There is no benefit in patients who had an ICD implanted within 40 d after a myocardial infarction[77].Decision about ICD implantation should be made for each patient individually,taking into consideration patient’s opinion and their quality of life,the LVEF (survival benefit) and other diseases that can be cause of death within the following year[1,73,74].ICD therapy is not recommended in patients in NYHA Class IV with severe symptoms refractory to pharmacological therapy if they are not candidates for CRT,a ventricular assist device,or cardiac transplantation[1,78,79].
Cardiac resynchronization therapy (CRT) is recommended for symptomatic patients in sinus rhythm,with left bundle branch block (LBBB) QRS morphology,QRS ≥ 150 ms (and in patients with QRS duration of 130-149 ms) and EFLV ≤ 35% despite optimal medical therapy to improve symptoms and reduce morbidity and mortality[1,2,80-82].For patients with ECG non-LBBB QRS morphology and QRS ≥ 150 msec CRT should be considered and CRT may be considered in patients with QRS 130-149 ms non-LBBB QRS morphology (in sinus rhythm)[1,2,80,83].CRT rather than right ventricular pacing is recommended for patients with HFrEF regardless of NYHA class who have an indication for ventricular pacing in order to reduce morbidity,although no clear effect on mortality was observed (this also includes individualized decision for patients with atrial fibrillation)[84-86].
Patients with severe symptoms despite optimal medical and device therapy are potentially eligible for mechanical circulatory support-mechanical ventricular assist VAD);left-sided (LVAD) or right-sided (RVAD),biventricular (BiVAD)[1,87].Heart transplantation is the last line of treatment for patients with the end-stage chronic HF[1,88].These patients need to be motivated,well informed,emotionally stable,capable of complying with the intensive treatment required postoperatively and in order for transplantation to be successful and increase survival,proper selection criteria need to be applied[88].
ROLE OF CARDIAC REHABILITATION IN PATIENTS WITH CHRONIC HF
Although survival after diagnosis of HF has improved,the prognosis in such patients remains poor and quality of life severely reduced.The meta-analysis (2019),including over 1.5 million all-type HF patients,estimated the 1,2,5 and 10-year survival to be 87%,73%,57% and 35%,respectively[89].Analysis about long-term outcomes among patients hospitalized with HF (including all three groups-HFrEF,HFmrEF,HFpEF;2017) shown very high 5-year mortality rate of 75%,regardless of LVEF[90].
Chronic HF reduces the ability of physical activity in patients,which has detrimental effects on their daily life activities and reduces quality of life.Patients with HF have limited exercise capacity because of dyspnea and fatigue,so these symptoms make patients fearful of being active,moreover because exercise-induced dyspnea can be interpreted as worsening of their disease.In patients with stable HF,exercise training can relieve symptoms,improve the exercise capacity and quality of life,as well as reduce disability,hospitalization and mortality[91-93].The Cochrane systematic review (2014) reported that exercise-based cardiac rehabilitation (CR) compared to no exercise control shows improvement in health-related quality of life (HRQoL)and hospital admission among people with HF,as well as possible reduction in mortality over long term[94].A single large randomized controlled trial (RCT) with medically optimized and stable patients with systolic HF (LVEF ≤ 35%) showed a modest and non-significant reduction in the primary composite outcome of all-cause mortality or all-cause hospitalization,but improvement in self-reported health status compared with usual care without training that persisted over time[95,96].Recent systematic review and meta-analysis (2019)[97],a meta-analysis of randomized trials(2018)[98] and the Cochrane meta-analysis (2019)[99] that included a total of 5783 patients,predominantly HFrEF (NYHA class II and III receiving center-based exercisebased CR programs) but also patients with HFpEF,showed that exercise rehabilitation reduced hospital admissions overall,as well as for HF,and clinically important improvement in HRQoL was shown.In patients with HFpEF clinically relevant improvements in exercise capacity can be achieved,without significant changes in LV function or structure[100,101],regardless of training modality[102].
In practice,it is reasonable to advise patients to avoid stimuli that cause worsening of the disease symptoms.There are many pathophysiological mechanisms of exercise intolerance in HF:cardiac (systolic and/or diastolic dysfunction,reduced stroke volume,elevated filling pressures,secondary pulmonary hypertension and right ventricle disfunction,mitral regurgitation,reduced chronotropic reserve),ventilatory system (exaggerated minute ventilation relative to CO2production,ventilation/perfusion mismatch,alveolar edema),skeletal muscle (reduced muscle mass,reduced enzymes for oxidative metabolism and generation of ATP),endothelial function(reduced nitric oxide,increased reactive oxygen compounds,reduced vasodilatory response),neurohumoral system (increased sympathetic activity,low vagal activity,increased levels of pro-inflammatory cytokines)[103].However,in chronic HF,poor exercise tolerance and quality of life can be successfully improved by dosed and tailored exercise training (ET)[96,104-107].ET reduces sympathetic tone and increases the influence of the parasympathetic tone at rest,restores baroreflex sensitivity and decreases chemoreflex sensitivity in HF which is important in term of autonomic nervous system imbalance and chronic sympathetic nervous system overactivity as one of the key pathophysiological mechanisms in HF leading to vasoconstriction,altered renal blood flow and adverse remodeling-hypertrophy and cell disfunction.ET in HF also results in reduction of reactive oxygen species and a concomitant increase in nitric oxide signaling,a reduction in Angiotensin II type 1 receptor signaling and a restoration of the imbalance of Angiotensin converting enzyme (ACE)and ACE2 expression,as well as a decrease in circulating pro-inflammatory cytokines,all of which contribute to the improvement of autonomic imbalance[103,108].
The guidelines of the European Society of Cardiology[1],American College of Cardiology/American Heart Association[2] and Canadian Cardiovascular Society[109]have included evidence-based recommendations for the use of exercise training in the management of chronic HF.Exercise training (or regular physical activity) is safe and effective in the improvement of symptoms and functional capacity (Class I,level of evidence A)[1,2,109],the reduction of the risk of hospitalization from HF (Class I,level of evidence A)[1] and in the improvement of exercise duration,HRQoL and reduction of mortality (Class IIa,level of evidence B)[2].
A consensus document of the HF Association and the European Association for Cardiovascular Prevention and Rehabilitation[110] emphasizes that cardiac rehabilitation program for patients with HF should include multiple components such as medical evaluation and baseline patient assessment,appropriate evaluation of many risk factors associated with such patients (e.g.,concomitant diseases-anaemia,valvular heart disease,renal function,patients age),education concerning medication adherence,psychosocial support,as well as exercise training and physical activity counseling.In addition to adopting a change in lifestyle that includes daily life activities (e.g.,housework,gardening,walking,recreation,proper nutrition),conducting structured physical activity and ET is important for further maintenance of stable condition of these patients.Implementation of ET requires appropriate patient selection,training protocol identification,intensity level determination,and progression monitoring.ET is recommended for stable New York Heart Association (NYHA)class I-III HF patients[110].Early mobilization of patients after an episode of acute HF is also recommended.At this stage,gradual mobilization,respiratory exercises,and small muscle groups exercises is needed to establish clinical stability and help patients to achieve a sufficient level of functional capacity and trust prior to conducting a symptom-limited cardiopulmonary exercise test (CPET) and initiating regular ET.Exercise modalities are known to be safe for HF patient when given at the right intensity and duration.The overall concept in ET is to be done gradually and individualized.When clinical stabilization is achieved,it is necessary to assess whether there are contraindications for conducting rehabilitation (Table 2).This includes reassessment of the patient’s condition and functional evaluation (history,clinical examination,electrocardiogram,ultrasound of the heart and CPET,and if the patient is unable to perform,then six-minute walking test)[110].
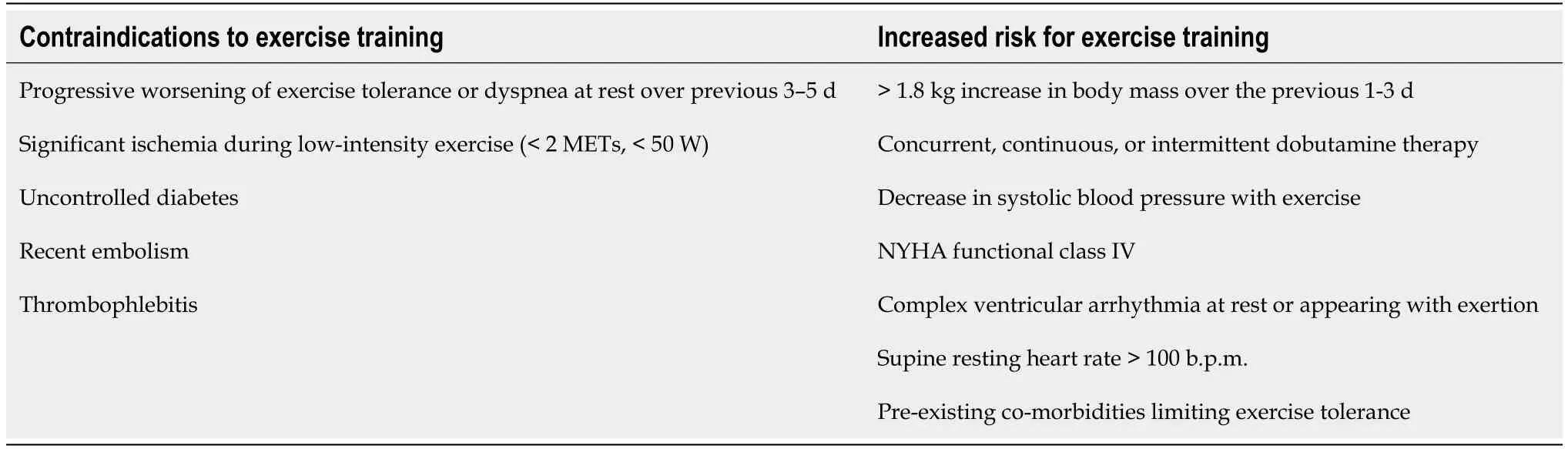
Table 2 Contraindications for exercise training and screening for increased risk for exercise training (data from[110])
The choice of exercise modality should take into account patients associated diseases,work habits,preferences and abilities,limitations as well as the availability of rehabilitation itself.Determining the appropriate level of ET intensity is key in achieving the desired effects,while simultaneously having control over the potential risks associated with these patients.There is no general agreement on modalities of exercise,instead an individual approach is recommended,with careful clinical assessment,taking into consideration patient’s preferences[110,111].Exercise protocols can be different depending on the variables:intensity (aerobic and anaerobic),type(endurance,resistance,strength),method (continuous and intermittent/interval),application (systemic,regional area,respiratory muscles),control (supervised and non-supervised),setting (hospital and rehabilitation center or home-based).Three exercise modalities in different combinations have been proposed[110,111].
Aerobic/endurance training
Metabolic function can be assessed by maximum oxygen uptake which depends on the ability of the respiratory and cardiovascular systems to deliver oxygen from the atmosphere to the muscle and the ability of the working muscles to utilize oxygen.The volume of oxygen (VO2) measured in patients with chronic HF at the end of the exercise test is not the maximum VO2 value because such patients cannot reach it.Instead of the term VO2max we use the term VO2peak,by symptom-limited CPET.The CPET will give insight into the degree of cardiac impairment and will objectively measure VO2peak and help to determine training intensity and perform training adjustments[110].The most used and evaluated exercise modality,the cornerstone of cardiac rehabilitation programs,is moderate continuous exercise (MCE)[112-114].The intensity of training is thus usually prescribed relative to VO2peak,and the recommended intensity is 40%-50% at the beginning,with an increase during the exercise process to 70%-80% of VO2peak[110].CPET is not always available in everyday clinical practice,so indirect methods have been proposed to assess the intensity of ET.In practice,heart rate (HR) reserve (HRR)-the difference between the basal and peak HR (the training in the range of 40%-70% HRR is recommended),and rating of perceived exertion (RPE) (training of 10/20-14/20 of the Borg RPE is recommended)are used.The intensity of physical training of 60% VO2peak corresponds to RPE from 12 to 13,and from 85% VO2peak corresponds to RPE 16[110].High-intensity interval training (HIIT) programs have been considered as a valuable exercise modality for low-risk HF patients[111,115].HIIT is not superior to moderate continuous training(MCT) in changing left ventricular remodeling or aerobic capacity[115] but the recent meta-analysis showed that improves VO2peak and should be considered as a component of care of HFrEF patients[116].Aerobic training dominates among cardiac rehabilitation programs,as in patients with chronic HF because it has the highest level of evidence,and proven beneficial effects for this type of activity[110,111].
Resistance/strength training
Muscle contraction is performed against a specific opposite force and thus generating resistance (e.g.,lifting weights).It gradually overloads the musculoskeletal system,strengthens and tones the muscles and it is suggested as an anabolic intervention due to the risk of muscle mass loss[110].A meta-analysis showed that resistance exercise as a single intervention can increase muscle strength,aerobic capacity,and quality of life in HFrEF patients,and may offer an alternative approach,especially for those unable to participate in aerobic training[117].It can be used as an adjunct to aerobic training which is the mainstay in HF patients.
As HF patients suffer from easy fatiguability,the initiation of a resistance/strength training (RST) program must be individually adjusted to the patient under medical supervision and each patient must be individually introduced into the training regimen.The amount of cardiovascular stress expected during RST depends on the magnitude of the resistance [% of one repetition maximum (% 1-RM)],the size of the working muscle mass and the relation between the duration of the muscle contraction and rest period between repetitions[110].The minimum recommendations for implementation of an RTS in three progressive steps are:1.“Instruction phase”-pretraining to learn and practice slow conduction,without or at very low resistance (RPE<12,<30% 1-RM).2.“Resistance/endurance phase”-start of training with a high number of repetitions and a low intensity (RPE 12-13,30%-40% 1-RM).3.“Strength phase”-higher intensity (RPE<15,40%-60% 1-RM),increasing muscle mass[110].Surveillance over each step is necessary because of the possibility of abdominal straining and consequent blood pressure elevations so prescribing the appropriate level of training according to the patient’s clinical stability,motivation,and experience with RST is of great importance.
Respiratory training
The review of trials using inspiratory muscle training in patients with chronic HF suggested that such an intervention may improve the functional capacity and quality of life,especially in those with inspiratory muscle weakness[118].Such additional exercises in combination with standard aerobic training might be useful.
There are limited data about ET for a special group of patients with chronic HF and implanted ICD or CRT.Evidence show that physical activity and exercise can be safely applied with adequate supervision[119,120] and it was confirmed in the larger RCT analysis of patients with ICD and HF[121].It has been shown that physical activity can almost double the improvement in functional capacity and quality of life in CRT patients[122,123] and ET resulted in reduction of a number of ICD activations in the exercise group[120,121].Moreover,non-sustained ventricular tachycardia in the presence of an ICD is not a contraindication for aerobic training[121].Patients with an ICD should begin training under medical supervision,and the HR must be monitored if it is possible to reach a HR close to the programmed intervention zone of the device.Patients who have symptomatic arrhythmias or ICD discharge should be directed to exercise modalities in which brief loss of consciousness due to ICD discharge may be less harmful (e.g.,avoiding swimming or climbing)[110].Medical staff caring for such patients should be specially educated in understanding the possible challenges and problems associated with such patients.
Although progress has been made in rehabilitating patients with chronic HF,further RCT with large number of patients are needed to assess the effect and benefit from each training modality.Education of medical staff on the beneficial effects of physical exercise in patients with chronic HF and involvement of more patients in cardiac medical rehabilitation programs are crucial in order to provide complete care to chronic HF patients.A small part of patients with chronic HF participates in cardiac rehabilitation programs (the data vary,only 2.6% retrospectively[124],and in one observational study only 10% of eligible HF patients received cardiac rehabilitation referral at discharge after hospitalization for HF[125]),and this is partly due to the fact that chronic HF is not yet an indication for rehabilitation in many countries,at least not as a first diagnosis.Developing adequate and effective training methods and highlighting the beneficial effects of such an approach will result in improving the quality of life and providing better medical care to patients with chronic HF.
CONCLUSION
New diagnostic methods and treatment options of HF are evolving rapidly.Accordingly,the number of patients with recovered LVEF (HFrecEF) and improved functional status is increasing.Beside medicament options to maintain future stable state of the patients with HF,cardiac rehabilitation is an important part of care,ET is proved to be safe in HF patients and should be implemented as a part of overall approach.Nowadays it is important to emphasize the role of cardiac rehabilitation in patients with chronic HF,raise consciousness that HF is not yet an indication for rehabilitation in many countries,at least not as a first diagnosis,and nurture a holistic approach to patients with HF.
 World Journal of Cardiology2021年7期
World Journal of Cardiology2021年7期
- World Journal of Cardiology的其它文章
- Sliding with the sines-fatal hyperkalemia:A case report
- Modes of failure with fractional flow reserve guidewires:Insights from the manufacturer and user facility device experience database
- Effect of trabeculated myocardial mass on left ventricle global and regional functions in noncompaction cardiomyopathy
- Large eustachian valve fostering paradoxical thromboembolism:passive bystander or serial partner in crime?
