Recent advances of biomimetic nano-systems in the diagnosis and treatment of tumor
Anning Li,Jiawei Zhao,Jingru Fu,Jia Cai,Peng Zhang
Wuya College of Innovation,Shenyang Pharmaceutical University,Shenyang 110016,China
Keywords:Biomimetic Nano-systems Tumor Therapy
ABSTRACT The lack of effective methods of diagnosis and treatment presents a major barrier to combat against tumor.The biomimetic concept is an emerging field that expresses great application potential in tumor fighting.Strategy for combining nano-systems with biomimetic technology has gained increasing attention that is proved bioinspired,environmentally benign,and promising.Herein,we provide an up-to-date review of biomimetic nanosystems as well as their applications in tumor therapy.In addition,the challenges and future directions of biomimetic nano-systems to achieve clinical translation are also pointed out.
1.Introduction
Tumor,especially the malignancy,also known as cancer,is a kind of malignant disease that has been plaguing human health for a long time [1].The inducing factors of tumor include chemical,physical and genetic factors,etc.The occurrence of tumor is extremely complicated and there are many theories about it.Essentially,tumor or cancer belongs to a kind of genetic disease.Therefore,early diagnosis and timely treatment are of great relevance.More importantly,the cure rate will be improved significantly.In the past decades,various diagnostic and therapeutic tools for cancer have been developed [2].Chemotherapy,radiotherapy and surgery are routine treatments for cancer in clinic [3].Recently,gene therapy [4],immunotherapy [5],photothermal therapy[6](PTT) and photodynamic therapy [7](PDT) are acquiring increasing importance in the field of cancer.However,the flourishing development of innovative treatment methods are limited by various factors,such as the immaturity of new technology,the absence of their applications in vivo [8].Hence,radiotherapy and chemotherapy are still the most commonly used anti-cancer methods in the clinic.Indeed,the traditional methods have many defects (Fig.1 ),such as poor cargo loading capacity and short effects.Worse still,they usually cause certain damage to normal tissues and cells due to the lack of targeting.Therefore,the toxic and side effects are obvious,and repeated administration also leads to drug resistance even multi-drug resistance (MDR),etc.[9].

Fig.1–Shortcomings of traditional cancer therapy,chemotherapy,radiotherapy and surgical resection can treat cancer to some extent,but they bring serious side effects.
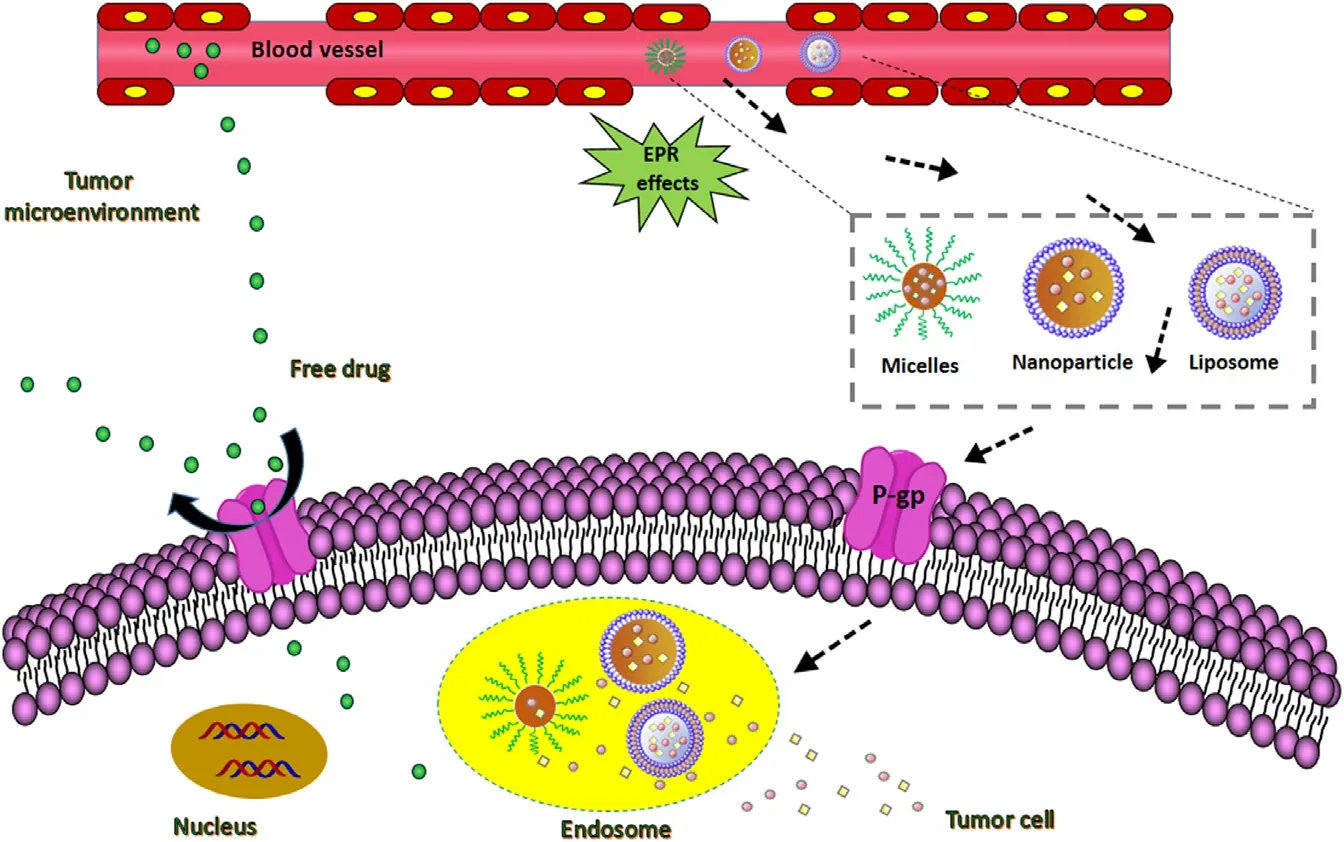
Fig.2–Drug delivery mechanism of various unmodified conventional nanocarriers,they mainly rely on the EPR effect to reach tumor cells,and most drugs are excreted outside the cell by the P-gp.
In recent decades,the vigorous development of nano drug delivery systems has provided a new application platform for many traditional chemotherapeutic drugs.Particularly,nanocarriers,such as liposomes,micelles,nanogels,and nanoparticles,as powerful alternatives for traditional radiotherapy and chemotherapy are widely used in anticancer drug delivery (Fig.2 ).However,these nanocarriers also have many defects,including poor targeting [10],drug leakage,poor stability and biocompatibility,short blood circulation time [11]and so on.Worse still,some physical and biological obstacles such as the endothelium and the mononuclear phagocyte system (MPS) hinder the drug delivery to tumors,resulting in a decrease in the administration efficiency and an unsatisfactory therapeutic effect.Therefore,it is greatly warranted to develop a novel diagnostic and therapeutic method that breaks the traditional model.
In recent years,biomimetic nano-systems have received more and more attention due to their potential in tumor applications.In the field of medical,bionics refers to three aspects,(1) extracting,separating and purifying endogenous substances from human,animal or microorganism directly;(2) synthesizing products that are similar to the endogenous substances in structure and function; (3) building a platform that imitates the microenvironment of disease.The purpose of these strategies is to reproduce the natural pathway of action of biological structures in living organisms as much as possible.The widely used biomimetic materials consist of protein (including albumin,lipoprotein,etc.),cell and cell membranes,bacteria,virus and other pathogens,phospholipids,cholesterol,apatite,exosomes in usual.The major advantages and applications of the aforementioned biomimetic materials are shown in Fig.3 .
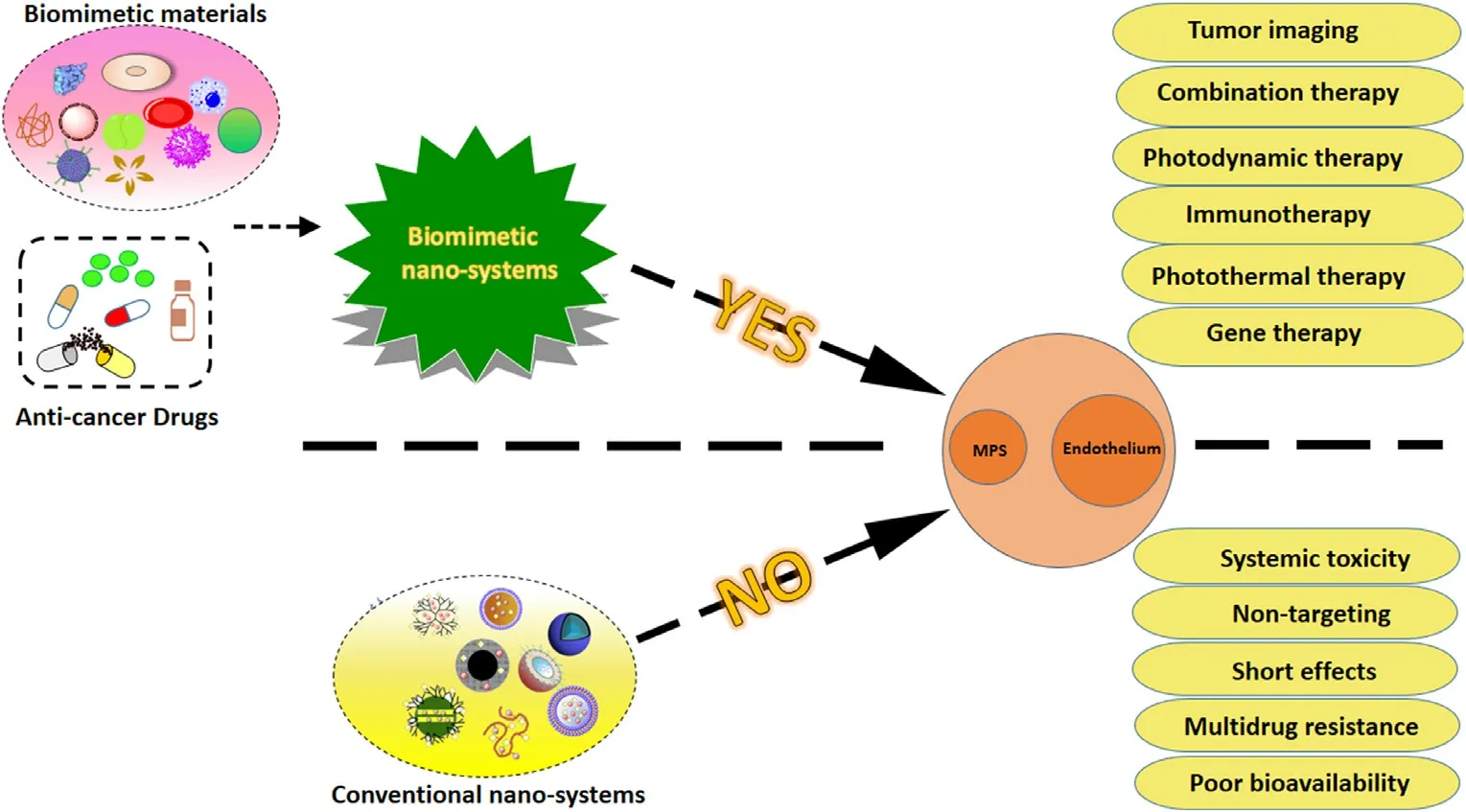
Fig.3–The major advantages and applications of biomimetic nano-systems in tumor therapy.As the picture shows,biomimetic nano-systems combine biomimetic materials with anti-cancer drugs.Compared with nano-systems without biomimetic materials,biomimetic nano-systems can overcome MPS and endothelial systems.
Recently,biomimetic nanocomposites contained endogenous components or synthetic endogenous component analogues,such as pathogens,proteins,cells and cell membranes [12],exosomes [13],phospholipids and cholesterol,have been extensively used for the novel diagnosis and treatment technology of tumors.They displayed many advantageous properties in comparison with other nanomaterials due to the good biocompatibility,excellent targeting ability,low immunogenicity or biological toxicity and degradability in living tissues.Briefly,the therapeutic outcome was improved markedly by combining biomimetic nanocomposites with the traditional or the novel therapy compared with using the original chemotherapy or radiotherapy alone.In this review article we highlight the advances in biomimetic nano-systems in recent years and postulate the challenges and future in this field.
2.Protein-based biomimetic nanocarriers
A protein-mediated nanocarrier means that the nanocarrier is modified by a protein or the nanocarrier is composed entirely of protein components.As a biosafety carrier,protein-based biomimetic nanocarriers have opened up a new treatment modality of tumor.They have shown great success in the co-delivery of anticancer drugs,the gene therapy of tumors,especially in tumor imaging performance.The protein-based biomimetic materials and their applications are concisely summarized in Table 1 .
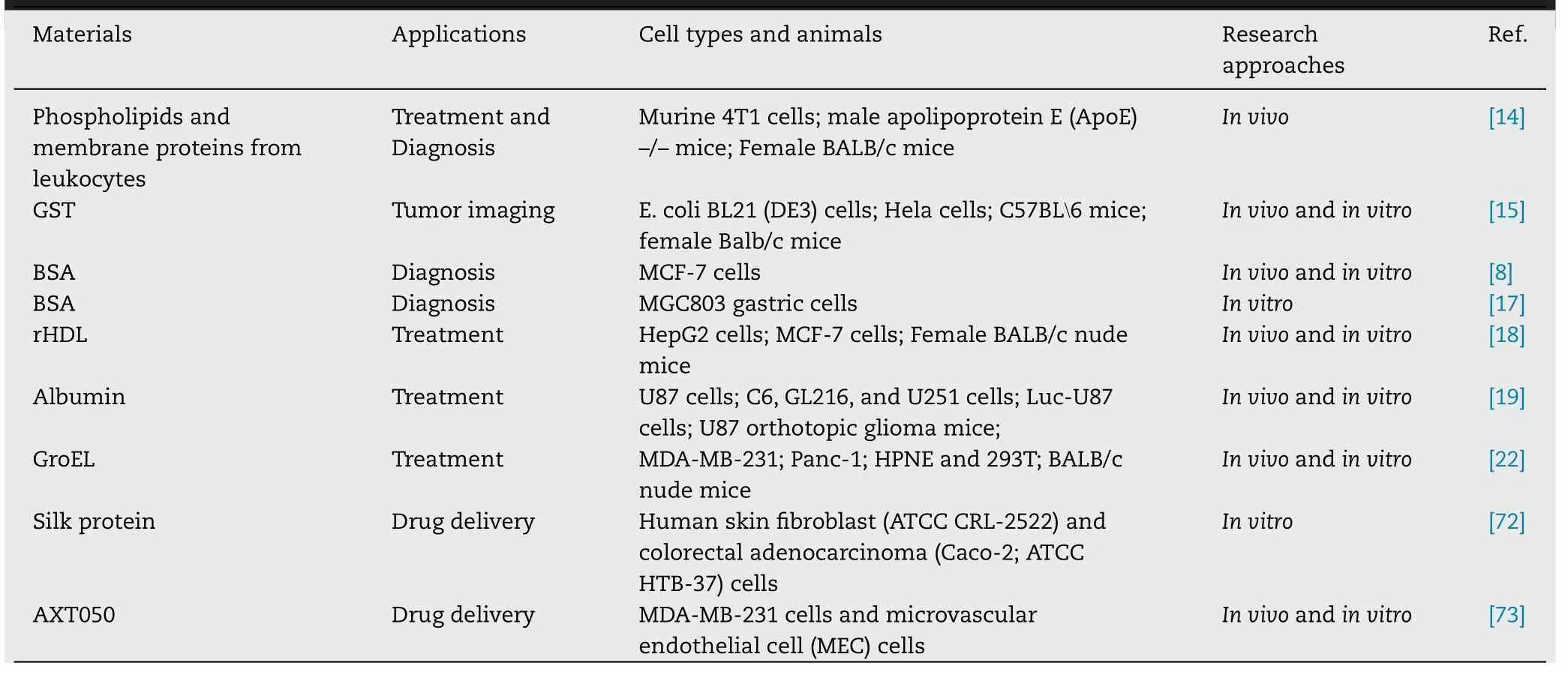
Table 1–Protein-based biomimetic materials and current application status.
2.1.Application of protein-based biomimetic nanocarriers in tumor imaging
One of the ways to improve the cure rate of cancer patients and prolong their survival time is the early diagnosis.There are a series of traditional diagnosis methods,including X-ray diagnosis,endoscopy diagnosis,ultrasound diagnosis,cytology diagnosis,blood biochemical diagnosis,etc.However,these traditional diagnosis methods cannot effectively detect all kinds of tumors due to the complexity of tumor.Therefore,it is necessary to develop a new kind of diagnostic method to overcome the shortcomings of traditional methods.In recent years,biomimetic nanocarriers have gained increasing attention due to their obvious advantages.The use of nanocarriers as diagnostic agents has great potential in the clinical application of tumors.In the early stages of any diseases,there will be a series of Cor111responding abnormal behaviors in the body.The vascular inflammation occurs in the early stages of diseases and then promotes the recruitment,adhesion,and trans-endothelial extravasation of circulating leukocytes.Based on this principle,a leukosomes nanoparticles which integrated leukocyte-derived membrane proteins into synthetic phospholipid bilayers was developed by Martinez et al.[14].The obtained nanoparticles was able to activate endothelial cells specifically and showed significant tumor and vascular lesions.Contrast agents can be flexibly added to the nanoplatform to make magnetic resonance imaging possible.As shown in Fig.4,Yang et al.[15]synthesized the AuNPs@GSTagent with the human endogenous protein GST and gold nanoparticles.The superiority of the prepared nanostructures in imaging performance in tumor-bearing mice was demonstrated through a series of experiments.It was reported that a novel computed tomography (CT) contrast agent with the coexistence of gold and iodine and Au@BSA-diatrizoic acid(DTA) was prepared by Zhao et al.[8].The biocompatibility of the nanocomposite was confirmed by MTT viability and hemolysis assay.This new nanoparticle had superior imaging performance and provided new ideas for the future CT imaging applications.Combined lipoprotein with naturally derived porphyrin molecules,a novel porphyrin lipoprotein(PLP) was developed.The platform acquired a variety of imaging and therapeutic functions.At the same time,it could accurately detect primary and metastatic tumors in other clinically relevant animal models [16].Lin et al.[17]developed BSA-Au nanocomplexes,which displayed no cytotoxicity to MGC 803 gastric cancer cells and expressed exceptional biocompatibility.The addition of folic acid molecules enabled the nanostructures with enhanced tumor targeting.The novel biomimetic nanocomposites may be an excellent candidate for tumor-targeted imaging and therapy.The new nano-diagnosis and treatment strategies developed by these researchers have potential applications in cancer.
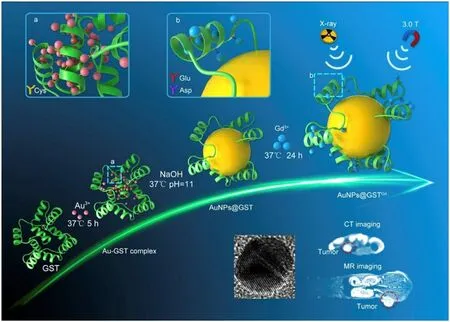
Fig.4–Schematic illustration of GST-mediated biomimetic chemistry of AuNPs@GSTGd; and biomedical imaging functions of AuNPs@GSTGd as a MR/CT dual-modal imaging contrast agent.(Reproduced with permission from [15].Copyright 2018 Elsevier).
Importantly,biomimetic carriers constructed from different basic nanostructures have unparalleled advantages in the treatment and diagnosis of tumor.For example,liposomes and nanoparticles have different physical and chemical properties and the sorts of drugs they deliver during the treatment of tumors are different.Some of these constructed nanocarriers focus on delivery or combination therapy of different solubility chemotherapeutic drugs(including hydrophilic,hydrophobic,and amphipathic drugs),some focus on enhancing the stability of drugs.These new methods can produce or enhance imaging effects compared with the conventional imaging techniques or contrast agents.Most of the proteins in these biomimetic nano-structures are directly derived from organisms.Therefore,they have excellent biocompatibility,minimally toxic and side effects,low immunological rejection and can effectively alleviate or overcome the drug resistance which are undesired during the treatment of tumor.
2.2.Application of protein-based biomimetic nanocarriers in combination therapy
In tumor cells,apoptosis and many proteins that repair mutations and maintain genomic stability are inactivated in varying degrees.Tumor cell produces different mutations in each round of replication,thus forming the heterogeneity of tumor.The heterogeneity makes chemotherapeutic drugs selectively kill only part of the tumor cells.In addition,there are still some frequent and bothersome problems in cancer therapy,such as the incompleteness and complexity of cancer treatment caused by the unpredictability of tumor development,the individual differences among different patients,the toxic and side effects of chemotherapy drugs.One of the most common and efficient solutions to solve the aforementioned issues is the co-delivery of several anticancer drugs.In recent years,the emergence of proteinbased biomimetic nanocarriers has broadened the path for the combined application of chemotherapeutic drugs from different sources and chemical structures.
Paclitaxel (PTX) and doxorubicin (DOX) are widespread used anti-cancer drugs for clinical treatment.However,their drawbacks,such as short effects,severe side effects and MDR,present a major barrier to the clinical applications and the treatment progress of tumors when they are used alone,respectively.Recently,new progress have been made by some research groups in using protein-based biomimetic nanocarriers to deliver chemotherapeutic drugs in combination,which injected vitality into the strategy that used the biomimetic design for drug delivery.As shown in Fig.5,Rui et al.[18]used PTX and DOX as model drugs to be loaded into the liposome simultaneously.And then the liposome was reconstructed by self-assembly of apolipoprotein A-I to form recombinant high-density lipoprotein (rHDL) nanoparticles.The novel biomimetic nanoparticles developed were tested by a series of experiments in vitro and in vivo,respectively.It was revealed that the biomimetic rHDL nanoparticles further stimulated the development of drug delivery which was mainly attributed to their enhanced cytotoxicity,improved therapeutic efficacy and low systemic toxicity.In addition,an enhanced albumin nanoparticle system co-encapsulated with PTX and fenretinide (4-HPR) was synthesized by Lin et al.[19].The nanocarrier utilized the hydrophobicity of PTX and 4-HPR to induce the self-assembly of albumin nanoparticles,overcame the poor solubility of hydrophobic drugs,and enhanced affinity of nanoparticle for cancer cells.The targeted penetration ability of drugs was enhanced owing to the addition of the cell penetrating peptide low molecular weight protamine (LMWP).The nano-systems offer a novel avenue to combination therapy in antiglioma and make a great impact to the drug delivery technology.
As a nanovehicle,the protein-based biomimetic nanocarriers have many superior capabilities such as excellent biocompatibility,low toxic and side effects,enhanced chemotherapy effect.In addition,the proteins used in biomimetic nanostructures can serve as not only targeting ligands but drug carriers [19].This is an irreplaceable advantage of protein-based biomimetic nanocarriers compared with other types of vehicles in the combined application of chemotherapeutics,which significantly improved the targeting ability of anti-cancer drugs and the efficiency of treatment.Moreover,most of the developed nanocarriers based on the similar biomimetic principle could utilize the specific physicochemical properties of proteins or chemotherapy drugs to achieve self-assemble,avoid the complex synthesis process of traditional nanocarriers,and more importantly,improve the stability of nanoparticles significantly.However,in spite of the numerous advantages biomimetic nanocarriers bears,its clinical application is restrained by some potential issues.For instance,the protein may give rise to some undesired changes caused by intense physical or chemical process during the fabrication of the nanocarriers.To conclude,further investigations are needed to overcome the aforementioned barriers.
2.3.Protein-based biomimetic nanocarriers for other applications
In addition to the above applications,protein-based biomimetic nanocarriers also display other advantageous properties,such as achieving high cargo loading and exhibiting great potential in gene therapy.Ren et al.[20]redesigned a caged protein scaffold and introduced phenylalanine into the cavity of the scaffold.DOX was able to be stably stored to achieve an increase in drug loading.The situation that the function of the protein material is limited due to the lack or location of specific chemical conjugate sites is improved successfully by this innovative strategy.rHDL,a kind of biomimetic nanocarrier was developed by Ding et al.for targeted cholesterol-conjugated siRNA (Chol-siRNA)delivery and Pokemon gene silencing therapy.The anti-tumor efficacy of the Chol-siRNA-Pokemon gene complex in vitro and in vivo was significantly improved which demonstrated the great potential of this vector in the clinical application of tumor gene therapy [21].Excitingly,Nie’s team [22]utilized the natural protein molecular chaperone GroEL in bacteria and DOX to develop a natural protein nanomachine with the property of tumor targeting and tumor microenvironmental responsiveness,which was driven by ATP to achieve precise and controlled release of DOX.The important discovery has infused new hope and possibility for the treatment of malignant tumors.
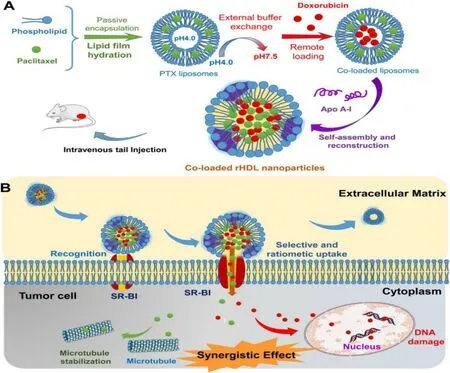
Fig.5–(A) schematic diagram of the preparation method and composition of the coloaded rHDLa; (B) mechanism of the codelivery of rHDL nanoparticles.(Reproduced with permission from [18].Copyright 2016 American Chemical Society).
3.Cell membrane-based biomimetic nanocarriers
The serious side effects,poor bioavailability and low delivery efficiency diminished therapeutic outcome of cancer chemotherapy [23].The emergency of various drug carriers alleviated these problems to a certain extent.Especially,nanocarriers are extensively used for cancer therapy due to the intrinsic structural characteristics such as small particle size and easy surface modification.The recent developments consist of specific targeted delivery,high cargo loading,increased drug half-life in the body,long circulation and improved bioavailability of anticancer drugs,which have expanded the repertoire of nanocarriers.However,after systemic administration,nanocarriers cannot reach tumor cells successfully due to physical and biological barriers,such as endothelial and MPS.Although the enhanced permeability and retention effect (EPR) can overcome endothelial dysfunction to some extent,rely on EPR effect alone is not enough [14].In recent years,the biomimetic cell membranebased drug delivery systems have received increasing attention for the development of the intelligent nanoparticles[24].The biomimetic nanocarriers can avoid immune system obstacles such as macrophage uptake and system clearance,specifically target to the tumor and effectively enter the tumor tissue to achieve the purpose of killing the tumor cells [25].The cell membrane-based biomimetic materials and their applications are concisely summarized in Table 2 .

Table 2–Cell membrane-based biomimetic materials and current application status.
3.1.Cancer cell membrane-based biomimetic nanocarriers
Cell-derived nanoparticles attract more and more attention due to their ability to imitate many of the natural characteristics of source cells [26].Similarly,cancer cell membrane-based nanoparticles are developed and expected to exploit their talent in the diagnosis and treatment of tumors.Li et al.[7]skillfully used persistent luminescent nanoparticles (PLNPs)functionalized with silicon phthalocyanine (Si—Pc) (an optical material with long-lasting luminescence after stopping excitation),hollow silica middle layer,and cancer cell membrane to build a biomimetic nanoplatform which in terms of the different functional features of these three parts.DOX was selected as a model drug to explore anticancer effect of the nanoplatform in vivo .A series of experimental results showed that the nanoplatform had high drug loading and could prevent the premature release of drugs in the blood and specifically target to the metastatic tumors at the same time,which provided guidance for precise controllable metastasis theranostics.Chen’s group[27]mimicked the homologous cancer cells,extracted cell membranes of MCF-7 and used them to encapsulate indocyanine green (ICG)/poly (lactic-co-glycolic acid) (PLGA)-loaded nanoparticles to form a new type of biomimetic nanoparticles.The morphological,photothermal and bimodal imaging capabilities were characterized through a series of experiments.The results showed that the novel nanocarriers had the ability of homologous targeting and specific targeted to the cellular and animal level,respectively.Similarly,Sun et al.[28]developed a novel type of biomimetic nanoparticles which were coated with cancer cell membranes extracted from 4T1 breast cells.The nanocarrier enabled targeted delivery of drugs to primary and metastatic tumors due to the retaining of the cell-membrane proteins presenting on the surface of 4T1 cells.The drug showed prolonged blood circulation time in model mice and the biomimetic drug delivery vehicle had a significant inhibitory effect on the tumor.The discovery not only improves the efficiency of drug delivery in tumor therapy but also has a higher specificity for metastatic tumors,which alleviates the complexity of tumor treatment.During the treatment of tumor,drug resistance has a direct impact on the therapeutic effect,and even directly leads to the failure of treatment.Therefore,it is highly desired that an ideal solution is developed,which is expected to address the issue.Tian et al.[29]constructed a biomimetic oxygen nanoparticles (DHCNPs) which overcame the hypoxia-induced chemoresistance and were highly specific for homologous cancer cells by encapsulating Hb and the model drug DOX in PLGA as the core and the cell membrane of MCF-7 cells as the shell (Fig.6 ).The most exciting thing was that this vector could down-regulate the expressions of hypoxia-inducible factor-1 α (HIF-1 α),multi-drug resistance gene 1 (MDR1) and P-glycoprotein(P-gp) simultaneously.Hence,it was able to reduce the efflux of DOX significantly,alleviate the drug resistance during chemotherapy effectively and improve the efficiency of treatment.
The biomimetic strategy based on cancer cell membranes is the hot topic in the research of biomimetic nanocarriers.The research teams constructed two kinds of nanocarriers in which the cancer cell membranes were selected as the outer membrane,the nanoprobe [30]and the upconversion nanoprobe [31]were selected as the core,respectively.Both of them performed excellently in the imaging of tumors in vivo .Jia et al.[32]took advantage of a biomimetic strategy based on cancer cell membranes and constructed a highly penetrating liposome nanopharmaceutical with good tumor targeting and penetration characteristics in mice.In addition,cancer cell membrane-based biomimetic nanocarriers have been proven to be efficient in the anti-cancer vaccines [26],the PTT [33]and the treatment of breast cancer [24],etc.
3.2.Erythrocyte membrane-based biomimetic nanocarriers
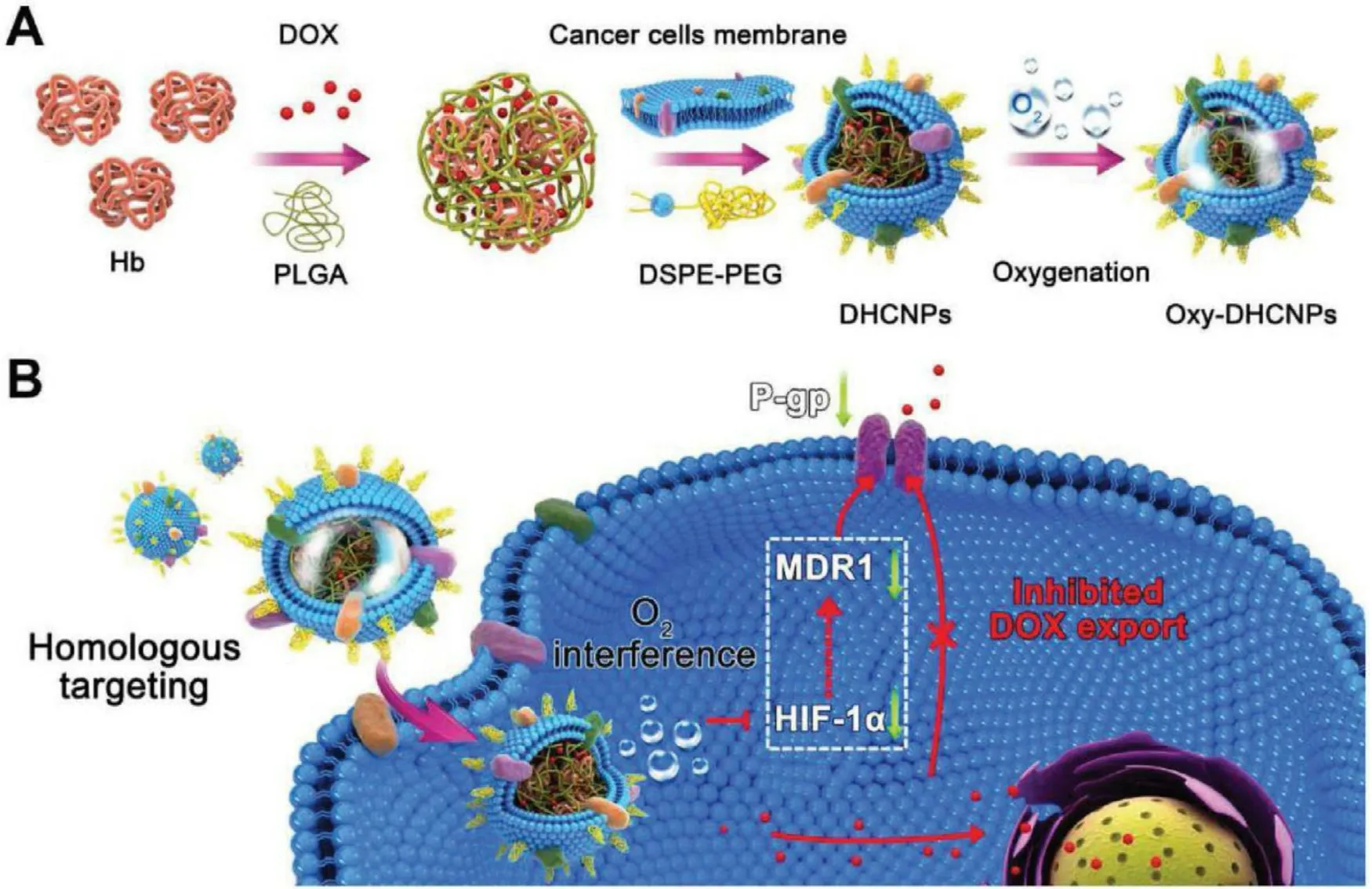
Fig.6–The design and function of DOX/Hb loaded PLGA-cancer cell membrane nanoparticles (DHCNPs) for homologous targeting and O 2 interference.(Reproduced with permission from [29].Copyright 2017 WILEY-VCH Verlag GmbH & Co.KGaA,Weinheim).
The red blood cells are critical for transporting oxygen in the body.Therefore,the erythrocyte membrane has a better permeability to oxygen and has immune escape ability in the body due to its immune functions[34].Importantly,the red blood cell membrane has excellent biocompatibility and safety in vivo because it is derived from natural red blood cells.Zhang et al.[35]prepared a biomimetic nanocarrier by cloaking PLGA-based polymeric nanoparticles with erythrocyte membrane and using gambogic acid (GA) as the model drug.The results showed that the stability,water solubility,bioavailability and biosecurity of GA-loaded nanoparticle were improved obviously compared with free GA.Ding et al.[36]used the natural erythrocyte membrane to improve the therapeutic effect of PDT.A novel kind of upconversion nanoparticles (UCNPs)-based erythrocyte membrane (RM)-coated biomimetic PDT agents was developed which were modified with folate (FA) and triphenylphosphonium (TPP)cation on the surface.The precise targeted delivery of photosensitizers as well as efficient production and release of active oxygen were achieved at the same time compared with the common PDT agents.Some new progress have been made in the application of biomimetic nanocarriers based on natural red blood cell membranes for photothermal therapy of tumors.Jiang et al.[6]combined nanomedicine technology with the tumor microenvironment,wrapped erythrocyte membranes on gold nanorods (GNRs) to obtained the biomimetic gold nanorods (MGNRs).The blood perfusion was effectively improved through the intellectual strategy which can be used for the photothermal treatment of pancreatic ductal adenocarcinoma (PDA).It has been well-documented that MGNRs has displayed prominent in biological stability,improving photothermal therapy efficiency and long circulation in vivo compared with GNRs.As shown in Fig 7,Wang et al.[37]constructed the photothermal polypyrrole nanoparticles coated with red cell membrane in a similar way,which used endothelin A (ET A ) receptor antagonist mediated vasodilatation to improve tumor site blood perfusion and achieved ideal therapeutic effects on model mice.This strategy has been proven to be efficient in tumor photothermal treatment.
PTT and PDT are advanced cancer treatment techniques that are superior to conventional chemotherapy.They can alleviate the side effects caused by chemotherapy and break down a variety of limitations of chemotherapy in cancer treatment.However,the limited transmission capacity of the laser leads to the poor treatment of an internal tumor.Photosensitizers and thermosensitizers lack the ability to target to tumor specifically,which further limits their clinical applications.The biomimetic nano-systems based on cell membranes addressed these issues to some extent and accelerated the process of replacing traditional chemotherapy with new therapies,which brought new hope for the treatment of cancer.At the same time,the combination of cell membrane-based nanocarriers and new therapies lacks large-scale prospective randomized trials to confirm that it is superior to traditional therapies,and there are still a lot of work that need to do.
3.3.Leukocyte membrane-based biomimetic nanocarriers
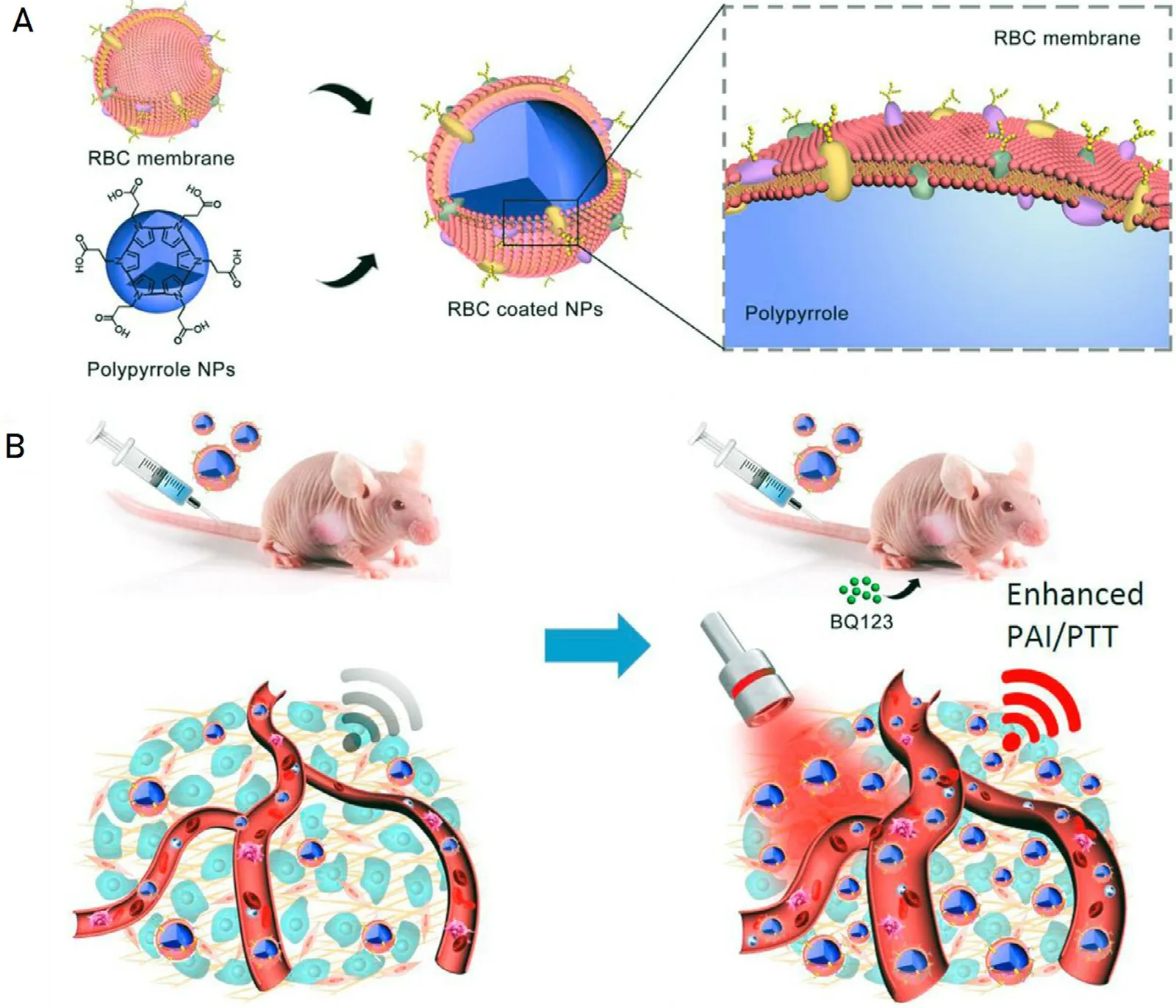
Fig.7–A schematic illustration of improved delivery of long-circulation biomimetic PPy NPs to tumor sites for enhanced PTT through selectively dilating tumor vasculature.(Reproduced with permission from [37].Copyright 2017 Elsevier).
White blood cells are important for curing body damage,preventing invasion of pathogens and immunity to diseases.The leukocyte membrane contains a variety of proteins and antigens that retain some of the functions of white blood cells.For example,membrane proteins play a crucial role in various cellular processes such as intercellular communication and signal transmission between cells [38].Therefore,white blood cell membrane is one of the suitable materials for constructing biomimetic nano-systems.Palomba’s team[39]obtained the Leukolike Vectors (LLV) by modifying the surface of nanoporous silicon particles with leukocyte membrane which retained their biological properties both in vivo and in vitro .The in vivo studies showed that nanocarriers after functionalization enhanced tumor targeting efficiency and increased tumor perfusion due to the overexpression of intercellular adhesion molecule-1 (ICAM-1) in tumorassociated blood vessels.When inflammation occurs,ICAM-1 interacts with leukocytes,which in turn promotes the accumulation of LLV at the tumor issues.Corbo et al.[38]performed proteomic analysis on LLV nanocarriers,further confirming the scientificity and feasibility of the method,and providing new ideas for the development of biomimetic nanocarriers based on other materials.Circulating tumor cells (CTCs) are tumor cells fell into the blood circulation,which are perceived as a significant biomarker for cancer diagnosis in the initial stages.Monitoring the changes of CTCs in the blood can be used to confirm the presence of the tumor,the process of the disease,the prognosis of the patient,the improvement of the clinical condition and the drug resistance after the treatment and so on [40].Xiong et al.[41]developed a biomimetic immunomagnetosomes (IMSs) system consisting of leukocyte membrane and FeOmagnetic nanoclusters (MNCs) for the enrichment of CTCs.The resulting IMS system had good biocompatibility and superior recognition efficiency to CTCs and overcame a series of obstacles of traditional detection methods.The result of this research is of great significance for improving the diagnosis and treatment efficiency of tumors.
3.4.Platelet membrane-based biomimetic nanocarriers
Platelets play an important role in hemostasis,inflammatory response and wound healing.Nano-systems based on platelets and platelet membranes which are involved in the development and metastasis of cancer have gained an increasing interest in the diagnosis and treatment of cancer.The targeted delivery of drugs can be achieved by using platelets as a drug carrier in combination with appropriate ligands.Ying et al.[42]have made it possible to deliver small molecule drugs over long distances using platelet-derived vesicles.The therapeutic effect of antitumor drugs and antibacterial drugs was improved successfully by synthesized nanocarriers which had excellent targeting ability.Jing et al.[43]synthesized platelet-camouflaged nanococktail by using RGD peptides (c (RGDyC)) modified nanoplatelet vesicles (RGD-NPVs) to encapsulate melanin nanoparticles (MNPs) and DOX.The new nanocarriers provided an inspiration for overcoming the MDR in cancer therapy,which realized the dual target to cancer cells and tumor blood vessels,inhibited the growth of drug-resistant tumors effectively.Meanwhile,it has attracted attention of researchers that the interaction between platelets and CTCs provides an opportunity to prevent tumor metastasis[44].The biomimetic particle based on platelet membrane,which reduced tumor metastasis by binding to the tumorspecific apoptosis-inducing ligand cytokine TRAIL was synthesized [45].Platelet membrane-based biomimetic nanocarriers have many superiorities,such as better biocompatibility,reduced immunogenicity and enhanced targeting.At present,it has become possible to prepare composite membrane nanocarriers by fusing erythrocyte membranes with platelet membranes [46],which provides ideas for the development of multi-membrane nanoparticles.
3.5.Other cell membrane-based biomimetic nanocarriers
Cancer cell membranes,erythrocyte membranes,leukocyte membranes and platelet membranes are commonly used materials in cell membrane-based biomimetic strategy.In addition,many other types of cell membranes display great potential in biomimetic nano drug delivery systems,such as natural killer (NK) cell membranes,some biological macromolecules with the characteristics of the biomembrane system.Arunkumar Pitchaimani’s team [47]developed a novel nanodrug system "NKsome" with NK cell membrane fusion cationic liposome.The "NKsome" was tested in vitro and in vivo with DOX as the model drug.It had a good tumortargeting effect and showed good anti-tumor potential to human breast cancer cell MCF-7.The drug delivery system was non-immunogenic and had excellent biocompatibility and stability.Zhang et al.[48]prepared a novel micelle that imitated the structure of cell membranes by using a crosslinkable amphiphilic random copolymer containing a phosphorylcholine group.The simulated cell membrane structure had the potential to prolong the circulation time of the drug in the blood.Therefore,it is a promising anticancer drug carrier.
The cell membranes as a kind of endogenous substance have different physiological characteristics due to their different sources which have expanded the repertoire of cell membrane-based nano-systems in the clinical diagnosis and treatment of tumor.Specifically,anticancer drug carriers based on cancer cell membranes can not only achieve personalized treatment of tumors,but also directly avoid many problems that traditional chemotherapy cannot overcome,such as severe drug resistance,poor stability,hard to metabolize and poor bioavailability.Although cell membrane-mediated biomimetic nanocarriers have been shown to perform well in the diagnosis and treatment of tumors,there are still many potential issues.Firstly,it is difficult to ensure the structure of cell membrane without any damage during the extraction process.Therefore,the stability of cell membrane in vivo may not be as stable as that of the natural cell membrane,which results in its weak effect in the nano-systems.Secondly,some changes take place in the physiological characteristics and functions of the extracted cell membrane due to the loss of intact structure,which further lead to some unexpected reactions in the complicated internal environment.Faced with these obstacles,fortunately,the microfluidic method developed by Molinaro et al.[49]has made the controlled synthesis of nanoparticles a reality.This method may be a reproducible,scalable tool that provides hope for highefficiency preparation of biomimetic nanoparticles.However,it also has some inevitable limitations,for the preparation of cell membrane-based biomimetic nanoparticles,the wide variety,complex structures and different properties of biomimetic materials,the control of temperature,types and proportions of water phase and organic phase in microfluidic technology may affect the fluidity and the particle structure of the cell membrane even the activity of the enzymes on the cell membrane,these are still the issues we need to solve.Therefore,a large number of data in vivo are needed to elucidate the delivery and release mechanism of biomimetic nanocarriers for drugs.Finally,the genes of cancer cells are prone to mutations,and the characteristics of cancer cells vary with the development of the cancer.Therefore,it is difficult to find an ideal homologous cancer cell membranes.In conclusion,although cell membrane-based biomimetic nano-systems have shown great vitality in cancer therapy,people face many inevitable problems and tremendous challenges,and much effort is required to realize the clinical practice of biomimetic nano-systems.
4.Pathogen-based biomimetic nano-systems
Bacteria belong to pathogen,and the idea of using bacteria to treat tumors dates back to the 19th century [50].Proof of that bacterial infection may have a therapeutic effect on tumors for the first time occurred 150 years ago [51].Immunotherapy improves the immune function of the body through various means,so as to curb the growth or spread of cancer.It has been given serious attention due to the reduced side effects,but it is difficult to cure the tumor.Therefore,it is an auxiliary treatment for tumor.Bacteria-mediated tumor therapy is an immunotherapy [52].Stern et al.[53]demonstrated the importance of bacteria-mediated immune responses in anti-tumor.On this basis,researchers combined biomimetic methods with bacteria to treat tumors and obtained some new achievements.SipA is a Salmonella type III secreted effector responsible for regulating P-gp levels via the caspase-3 pathway.Based on this mechanism,a gold nanoparticle system coated with SipA was developed by Mercado-Lubo et al.[54],and DOX was used to study the mechanism of action of SipA on P-gp.The synthetic nanoparticles can alleviate the multidrug resistance of cancer cells,increase the sensitivity of tumors to drugs,and have the potential to improve the efficiency of tumor treatment.Felgner et al.[52]designed and optimized the lipopolysaccharide-modified attenuated Salmonella carrier,which had a better antitumor effect and improved biosafety in vivo .Therefore,combining bacteria with chemotherapeutic drugs and using bacteria to deliver drugs to tumor tissues is a promising therapeutic approach.Felgner et al.[50]summarized the carrier of bacteria as a tumor-targeting therapeutic molecule into two strategies.The first strategy is to use a prodrugconverting enzyme produced by bacteria to convert a prodrug into a drug with anti-tumor activity at a specific site.Another strategy is to utilize a bacterial secretion system in which combines therapeutic compounds with the signaling molecules that are necessary for delivery of the compounds through a specific secretory system,allowing the bacteria to produce therapeutically active compounds which are delivered to tumor tissue during the colonization of tumor.
The occurrence and development of cancer are related to the changes of the intracellular genes.Though gene therapy has great potential,many links and problems in gene therapy technology still plague scientists.Many key problems remain to be solved,such as the lack of suitable carriers.A biomimetic carrier based on genetic engineering was constructed by Mangipudi et al.[55].And the adenoviral peptide was one of its components.The combination of genetic engineering and biomimetic technology has a good therapeutic potential.Molino et al.[56]demonstrated that a biomimetic strategy that mimicked viral properties could elicit an effective immune response in cancer therapy.
Biomimetic nano-systems combine nanotechnology with natural bio-derived materials to partially or completely replicate their process in vivo by simulating or directly using pathogen structures.The novel nano-systems can deliver drugs to target sites accurately and enhance the antitumor effects of drugs significantly,thus producing more efficient cancer therapy and enriching the tumor treatment strategies.
5.Other biomimetic materials
5.1.Apatite-based biomimetic nano-systems
Apatite is a natural structural component of the human body[57],which has structural and chemical similarities with the mineral components of mammalian bone and dentin.It has ideal biocompatibility [58]and is a commonly used material for bone repair [59].Nowadays,nanocrystalline apatite can be synthesized in the laboratory at a low cost with the advantages of good biocompatibility,high absorbability,easy surface functionalization and pH-dependent solubility[60].Recently,biomimetic nano-systems based on apatite nanocrystals have attracted the interest of researchers.What is exciting is that,as a new biomimetic material for tumor therapy,apatite nanocrystals perform well in the diagnosis and treatment of tumors.Recently,apatite nanocrystals functionalized with monoclonal antibodies (specifically targeting the tyrosine kinase receptor Met/hepatocyte growth factor receptor,which is overexpressed on tumor cells) were developed by Iafisco et al.[57].They selected DOX as a model drug and demonstrated the excellent targeting and anti-tumor activity in vitro of this novel nano-systems.Oltolina et al.[60]functionalized apatite nanocrystals with a monoclonal antibody that specifically targeted to the Met/hepatocyte growth factor receptor in a similar manner.The nanoparticles were labeled with Fluorescein-5-isothiocyanate.The physicochemical properties and behaviors in vitro and in vivo were characterized.The results showed that the nano-systems performed well in tumor targeting,and a strong fluorescent probe with more stable performance was obtained by the isothermal adsorption method.Al-Kattan et al.[61]prepared and studied Eu-doped apatite nanocrystals,which were able to be used for medical imaging and had potential as biocompatible luminescent nanoprobes.The low—methoxy amidated pectin (LMAP)microspheres were loaded into nanocrystalline apatite to obtain a drug delivery carrier with sustained release property.The research results obtained were unprecedented.Although the apatite-based biomimetic nano-systems are not mature enough and there are few literature reports on them,they perform well in the existing research and apatite is one of the promising candidate materials in the biomimetic nanofield.
5.2.Phospholipid and cholesterol-based biomimetic nano-systems
Phospholipids and cholesterol are both endogenous substances and important components of the cell membranes.Phospholipid-containing polymers can mimic the living tissues to some extent and have good compatibility with human body [62].Cholesterol plays a very critical role in regulating membrane fluidity [63].Hence,the design of phospholipid and cholesterol-mediated biomimetic nanosystems is a potential strategy to optimize therapeutic outcomes.Zuo et al.[64]designed a novel amphiphilic prodrug simulating a phospholipid structure and obtained a stable nanoacrrier by molecular self-assembly.The biomimetic nano-component has many advantages,such as excellent stability,tumor targeting,enhanced anti-cancer effect and enzyme triggered degradation.Wang et al.[65]constructed an eukaryotic cell-like nano-system using phospholipid membrane,mesoporous silica matrix and fullerene core,which was used for delivery of the chemotherapy drug DOX and in vivo imaging agent ICG.The nano-system was utilized for the combination of PTT,PDT and chemotherapeutic drugs,tumor imaging,etc.,which greatly improved the safety and efficiency of chemotherapy drugs.The biomimetic selfassembly micelle containing cholesterol,which responded to oxidation—reduction,was synthesized by Wang et al.[66].Studies showed that it had low polydispersity index (PDI) and good biocompatibility.In addition,it could achieve effective release of drugs owing to its good response to redox reagent.For these reasons,it is a promising anticancer drug carrier.A new type of biomimetic micelle was synthesized by Xu’s research team [67],they selected poly [2-(methacryloyloxy)ethyl phosphorylcholine](pMPC) as hydrophobicity chain segment and cholesterol as a hydrophobic segment.The micelle had weakened side effects on human body owing to the biocompatibility of phospholipids and cholesterol.The preparation process of biomimetic nano-systems based on phospholipids and cholesterol is relatively simple compared with cell membrane-based biomimetic nano-systems,and this endows phospholipid and cholesterol-based biomimetic nano-systems with more extensive applications.
5.3.Biomimetic nano-systems based on tumor microenvironment
Tumor microenvironment is closely related to tumor invasion,metastasis,the occurrence and development of related tumors [68—70].Liu et al.[71]simulated the environment of pancreatic dysplasia and prepared a biomimetic hydrogel,which provided a promising platform for studying the fate of pancreatic cancer cells.Park et al.[68]developed a threedimensional tumor/stem cell cluster biomimetic system that provided a good model for studying the migration and invasion of cancer cells in the extracellular matrix.The study of simulating tumor microenvironment biomimetic nanosystems is still in the initial stages,but simulating the tumor microenvironment may completely subvert the history of cancer diagnosis and treatment.
6.Conclusions and future perspectives
In this review,we highlighted the exciting research and application advances on biomimetic nano-systems.From chemotherapy,radiotherapy and surgery to the successfully application of various nano-systems,the therapeutic effect of the tumor has been significantly improved.But there are still many difficulties that prevent us from eradicating tumor.With the development of medical services,a variety of promising potential solutions have emerged.Biomimetic nano-systems have attracted a lot of interest from researchers because of their inherent advantages and some exciting research progress have been made,which make biomimetic nanosystems stand out in these new methods.First,the biomimetic principle gives us clear research ideas.The well-established knowledge of physiology,pathology and immunology has provided us with reliable theoretical support.When designing an experiment,the problems that may occur during the experiment and the experimental results are predictable to some extent.Second,the process of tumor growth is very complicated,and the intracellular environment of the tumor is quite different from that of normal cells,such as the increase in temperature,the exuberance of metabolism,the increase in the types and quantities of proteins and enzymes,the increase in the secretion of reducing substances,the changes in reactive oxygen species (ROS),pH,nucleic acids and H 2 O 2,which provide clear directions for the selection of biomimetic materials.In addition,the Cor111responding materials can be selected according to the differences of tumors owing to the rich variety of biomimetic materials,which can promote the personalized treatment of tumors.Finally,nano-systems based on the biomimetic principle have many obvious advantages compared with other antitumor strategies,such as better targeting effects,low toxicity,high bioavailability,easy metabolism,and excellent biocompatibility,which provide a huge opportunity for the application of biomimetic nanosystems to tumors,making biomimetic nanosystems one of the most promising antitumor tools.
However,things are two-sided.We cannot be addicted to the achievements,although the biomimetic nano-systems show great potential in the diagnosis and treatment of tumors,most of the research are still in the early stages.Before clinical practice,several challenges still need to be overcome.Firstly,biomimetic materials have both advantages and limitations,and these limitations hinder their further development.For example,in cell membrane-mediated biomimetic nano-systems,although erythrocyte membranes can prolong the circulation time of drugs,their targeting is relatively weak,further improved the accuracy of treatment are required; An immune response may occur when cancer cell membranes enter living tissues; Pathogen-mediated biomimetic nano-systems have potential biosafety hazards.In addition,how biomimetic systems target specific cells?How more biomimetic systems enter to specific cells? How biomimetic systems avoid certain host recognition? Its scientific principles are complicated and some even some mechanisms are still unclear.Hence,detailed in vivo research and further systematic research are needed,which would also be the inevitable development directions of future biomimetic nano-systems.
Despite the enormous challenges,biomimetic nanosystems possess great potential in the diagnosis and treatment of tumor,which are expected to play a critical role in this field although clinical applications may not emerge overnight.The applications of biomimetic nanosystems will not only be limited on tumor.We believe that biomimetic nano-systems will become a powerful tool for humans to overcome various diseases in the near future.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to financial support from National Natural Science Foundation of China (81202480,81302723 ),Natural Science Foundation of Liaoning Province (2015020749 ).
 Asian Journal of Pharmacentical Sciences2021年2期
Asian Journal of Pharmacentical Sciences2021年2期
- Asian Journal of Pharmacentical Sciences的其它文章
- A cleavable self-delivery nanoparticle for tumor photo-immunotherapy
- SARS-CoV-2 vaccine research and development:Conventional vaccines and biomimetic nanotechnology strategies
- Prospective of extracellular matrix and drug correlations in disease management
- Ocular prodrugs: Attributes and challenges
- Gas-blasting nanocapsules to accelerate carboplatin lysosome release and nucleus delivery for prostate cancer treatment
- A computer-aided chem-photodynamic drugs self-delivery system for synergistically enhanced cancer therapy
