Research on the Status Quo and Countermeasures of Patent Application in Liaoning Biopharmaceutical Industry Based on Patent Map
Ma Junhong,Zhang Wenfeng,Liu Yue,Yuan Hongmei ,Zhang Dawei
(School of Business Administration,Shenyang Pharmaceutical University,Shenyang 110016,China)
Abstract Objective To study the patent map and the relevant theories of the patent development of biopharmaceutical industry for conducting a comprehensive and in-depth analysis of the problems in patent application in Liaoning biopharmaceutical industry.Methods Literature review,information visualization method,empirical analysis method and comparative analysis method were used to research the problems in the patent application.Results and Conclusion While developing biopharmaceutical industry,Liaoning provincial government should strengthen the protection of pharmaceutical patents,creating a new R&D mode of“university+research institutes +enterprises+individuals”to broaden the scope of technical fields.Then,its biopharmaceutical industry will be developed in an all-round way.Besides,it should closely follow the national development direction so that the biopharmaceutical industry in Liaoning Province will have a bright future.
Keywords:patent map;biopharmaceutical industry;patent application
1 Introduction
In the 1990s,biopharmaceutical industry began to develop in China,but the state’s policy support for the biopharmaceutical industry originated from the official launch of the national science and technology special project“major new drug creation”.Subsequently,the National Development and Reform Commission officially promulgated“Bioindustry Development Plan”in 2012,which included the biopharmaceutical industry as one of the priority strategic industries.Compared with the traditional pharmaceutical industry,biopharmaceuticals,as an emerging industry,have the characteristics of high technology,high yield,high investment and high risk.Due to its high risk and long R&D cycle,the biopharmaceutical industry cannot achieve sound and fast development without government policy support.As an old industrial base in Northeast China,Liaoning Province has many biopharmaceutical enterprises.The Liaoning provincial government also regards the development of pharmaceutical technology and the revitalization of the pharmaceutical industry as the primary task.The“Guide of Made in China 2025 for Provinces and Cities (2017)”issued by the Ministry of Industry and Information Technology of the People’s Republic of China pointed out that seven major projects in the biomedical industry in Liaoning were selected as key projects,showing the great potential of Liaoning’s biopharmaceutical industry.However,facing the huge pressure of industrial upgrading,Liaoning Province has to solve the following problems in response to the call of the state,such as promoting the optimization of the industrial structure,and transforming the economic development model in recent years.
2 Overview of research
In this paper,the application of the visual analysis method of patent map and the related research on the development of China’s biopharmaceutical industry are systematically studied,aiming to understand the status of biopharmaceutical industry in all aspects and pave a way for further research.
There is a wide range of relevant research on using patented map analysis at home and abroad,mainly in the technical fields of energy,computers,batteries,and 3D printing.In terms of energy,some scholars,such as Cao Lijiang[1](2013),Liu Guifeng[2](2012),Simon C[3](2015),Zhang Huiming[4](2018) applied patent map analysis to study its development trend,R&D advantages and reasonable selection of development partners in solar energy,electrochemical,and wind energy industries.In terms of battery research,some scholars,such as Feng Lijie[5](2017),Li Xin[6](2018) and Hou Yuanyuan[7](2016) proposed to use patent map analysis to identify technological opportunities and predict future development trends in applications such as solar cells and electric vehicle batteries.Other scholars,such as Son Changhou[8](2012),Jiang Guihuang[9](2018),Wang Manrong[10](2017),Li Xiangyang[11](2016) used patent map analysis to discuss patent layout strategies and competitive situation in electrical appliances,automobile industry and big data.In the field of medicine,most scholars,such as Wu Jianlong[12](2009) and Liang Jinyu[13](2017),used patent maps to focus on the theoretical guidance of pharmaceutical enterprises in formulating patent strategies.
The development of the domestic biopharmaceutical industry started late compared to some developed countries.However,with the support of related policies,various universities,research institutions have paid more attention to this industry[14].Some domestic scholars,such as Su Yue[15](2009),Tao Ran[14](2012),Gao Zhenqian[16](2016) and Wu Dandan[17](2018) conducted research on the development direction and trend of the biomedical industry and found that it has the same global development trend as other emerging industries.Other scholars,such as Li Jingtan[18](2006) and Liang Rui[19](2007) conducted research from the perspective of the development model of the biopharmaceutical industry,concluding that its current development models in China mainly include independent growth,transformation and government-led.Some scholars,such as Liu Jianhua[20](2016) and Zhao Yan[21](2017) analyzed China’s biopharmaceutical industry based on patent information,and proposed model methods such as life cycle MATLAB evolution and dynamic input-output.Due to the late development of biopharmaceuticals and few scholars focusing on patent maps in the field of medicine,this article used the patent map analysis to study the status of patent applications in the biopharmaceutical industry in Liaoning Province.
3 Empirical analysis
3.1 Data source and processing
In this article,the IncoPat patent database is selected to search the keywords and international patent classification (IPC) numbers.The search time is July 4,2020.In order to focus on the research content,a secondary screening is performed based on the searched patent data.The patent data of invention and utility model from 2009 to 2018 in China are chosen as the data samples.After screening and removing irrelevant data,a total of 3 322 patents are retrieved.
3.2 Analysis of patent applications in Liaoning biopharmaceutical industry from the perspective of patent management map
3.2.1 Time series analysis
After analyzing the patent data of the biopharmaceutical industry in Liaoning Province from 2009 to 2018 in time series,this paper divides them into two major modules,namely,patent quantity trend graph and patent structure trend graph.
The patent quantity trend map is based on the number of patent applications and patent authorizations in the biopharmaceutical industry in Liaoning Province from 2009 to 2018.Then,Fig.1 is drawn to analyze the application status from the number of patent applications and the changing trend of the number of patent grants.
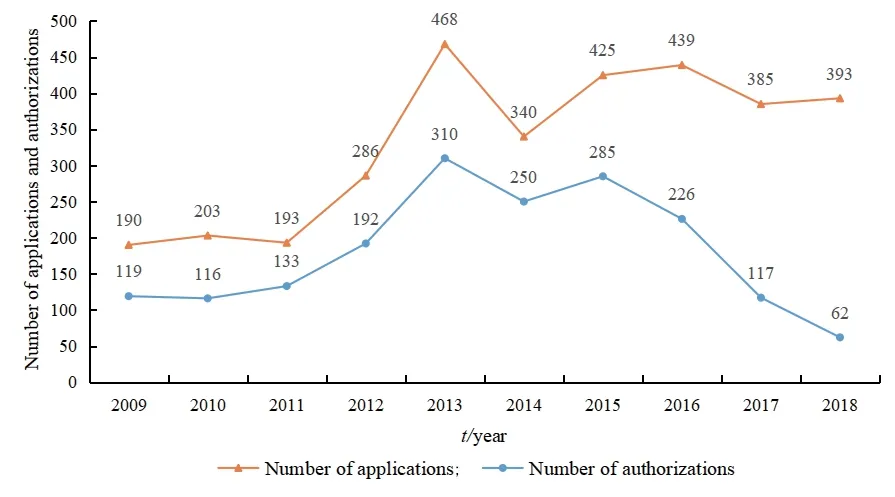
Fig.1 Trends in the number of patent applications in Liaoning’s biopharmaceutical industry
As shown in Fig.1,the number of patent applications in Liaoning biopharmaceutical industry shows an overall upward trend from 2009 to 2018.During 2009-2011,the number of patent applications in the biopharmaceutical industry is relatively small,and the number of patent applications declined in 2011.Since 2011,the number of patent applications increases continuously,and the rate of increase accelerates significantly,reaching a peak in 2013.The changes in the number of patent applications from 2009 to 2011 are due to the third revision of the Patent Law in 2008,which increases the protection of patents.The trend of changes in the number of patent applications in 2011 is closely related to the national macro-biopharmaceutical industry policy.During the“Twelfth Five-Year Plan”period,China has issued some policies to promote the development of China’s biopharmaceutical industry.Liaoning Province actively responded to the national policy and issued the“Liaoning Intellectual Property Strategy Implementation Promotion Plan (2012-2015)”and other documents.
Hulloa! said the shirt-collar, never before have I seen anything so slim and delicate, so elegant and pretty! May I be permitted to ask your name? I shan t tell you, said the garter
The patent structure trend chart is to count the number of patents according to patent types.There are no design patents in the search data.The statistical results are shown in Table 1.Since invention patents have the highest degree of innovation and technological content,the proportion of invention patents can be used to measure the innovation capability of a certain technology.It can be seen from Table 1 that invention patents occupy an absolute advantage in the number of patent applications in Liaoning’s biopharmaceutical industry with a proportion of 95.2%,which shows that Liaoning biopharmaceutical patents have high technical content and strong innovation capabilities.
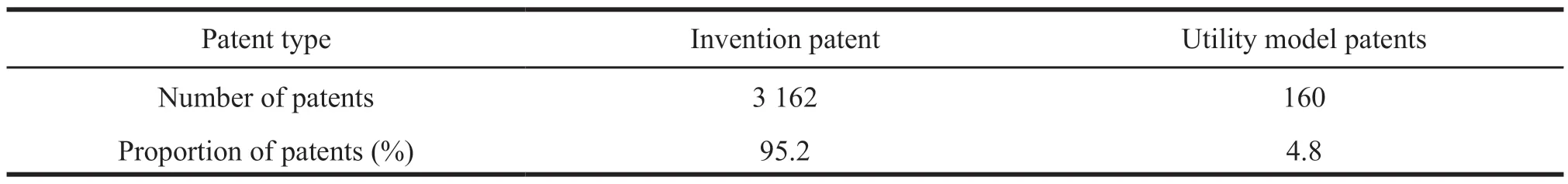
Table 1 Patent structure of Liaoning biopharmaceutical industry
3.2.2 Spatial sequence analysis
The spatial sequence analysis of the patent map in this article mainly refers to the regional distribution of patent applications.To a certain extent,regional analysis can reflect the technological development and technological R&D strength of a region.Based on the statistics of the regional distribution of patent applications in Liaoning’s biopharmaceutical industry from 2009 to 2018,the top 10 cities with the largest number of applications are sorted.Then,Fig.2 is drawn.
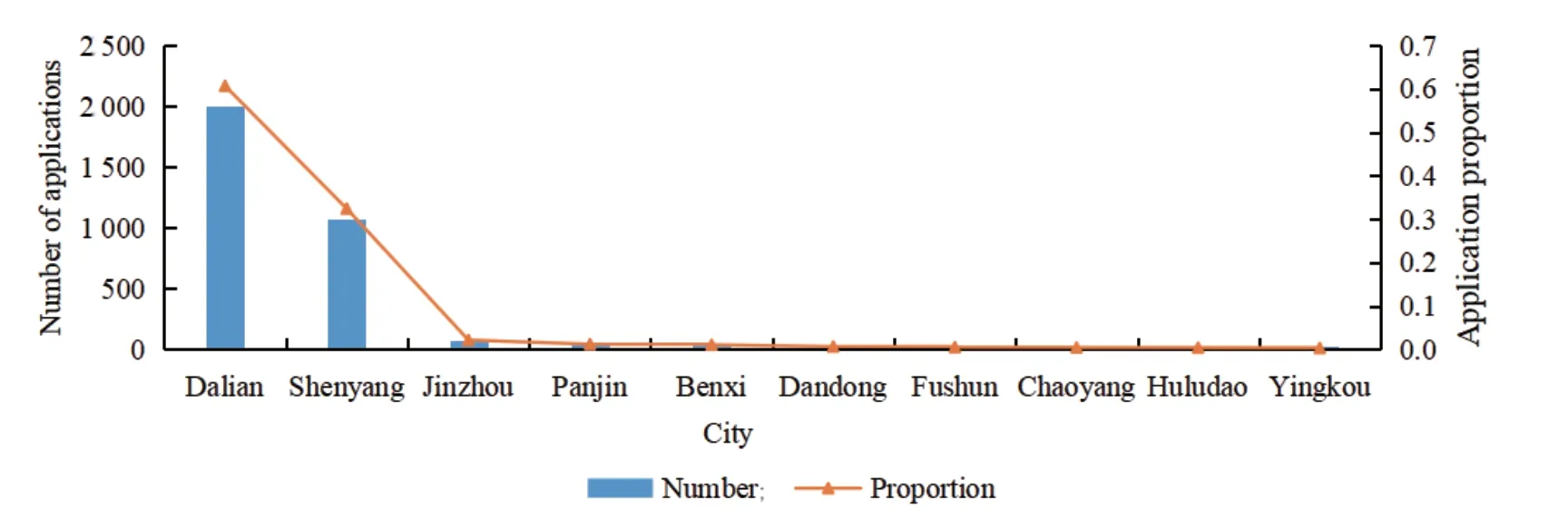
Fig.2 Regional layout of patent applications for the biopharmaceutical industry in Liaoning Province
It can be seen from Fig.2 that among the 10 cities in the statistics,Dalian and Shenyang have applied for 2 001 and 1 069 respectively,accounting for 60.69% and 32.42% of the patent applications.It means the patents of the biopharmaceutical industry in Liaoning are concentrated in Dalian and Shenyang,which is due to their early economic development and the strong industrial foundation.
3.2.3 Analysis of applicant composition
In this paper,the cooperation of patent applicants in the biopharmaceutical industry in Liaoning Province from 2009 to 2018 is statistically analyzed,as shown in Fig.3.
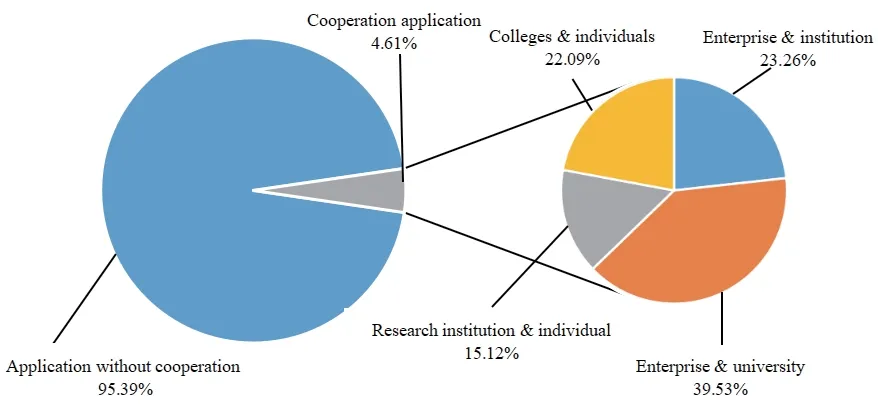
Fig.3 Diagram of patent applicants’ cooperation in Liaoning’s biopharmaceutical industry
From the perspective of applicants’ cooperation,the number of patents applications in the biopharmaceutical industry in Liaoning Province is not big.Among the applications,the proportion of cooperation between enterprises and universities ranks first.Although universities and scientific research institutions are the main body of biopharmaceutical innovation activities in Liaoning Province,patent applications mainly cooperate with enterprises.If the number and the quality of patent applications are to be further improved,attention should be paid to increasing the conversion rate of patent achievements among individuals,universities,scientific research institutions and enterprises.
3.3 Analysis of patent applications from the perspective of patent technology map in Liaoning biopharmaceutical industry
3.3.1 IPC analysis
Ten large groups of IPC are selected in this paper to conduct a statistical analysis on patent applications of the biopharmaceutical industry in Liaoning Province.Then,the number of patent applications of Liaoning’s biopharmaceutical industry is ranked in descending order,as shown in Table 2 and Fig.4.

Table 2 Top ten list of IPC group classification
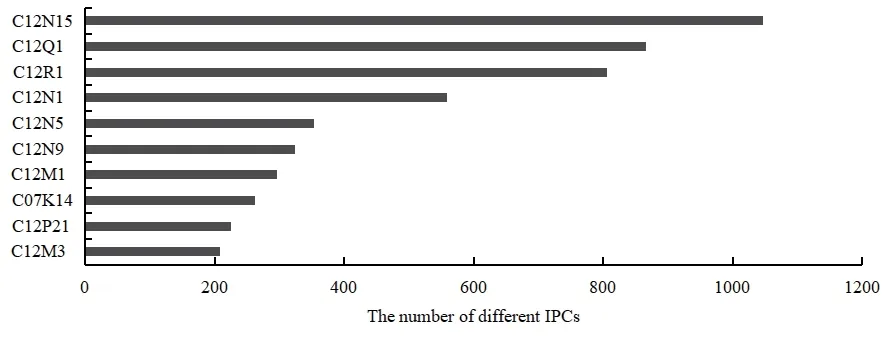
Fig.4 IPC classification of key patents in Liaoning biopharmaceutical industry
Table 2 and Fig.4 show that the composition of patented technologies in Liaoning biopharmaceutical industry is concentrated in such fields as C12N,C12Q,and C12R.The number of applications in these three fields exceeds 800,accounting for 81.79% of the total patent applications.Therefore,the patent applications of the biopharmaceutical industry in Liaoning Province in the past ten years mainly focused on microorganisms,enzymes,and nucleic acids.However,the technologies in the three fields of C12M (enzyme or microbiology device),C07K(peptide;gastrin;growth hormone release inhibitor;melatonin),and C12P (peptide or protein preparation)are relatively weak.The number of patent applications is balanced,none exceeding 300.The reason is the late start and slow development of the biopharmaceutical industry in Liaoning Province.
Table 3 Applicant information

Continued Table 3
3.3.2 Applicant-technical matrix analysis
In order to understand the key R&D direction and technical competitiveness of each applicant,this article uses the index of the number of patent applications in a certain technical f ield owned by each patent applicant.The patent applicants in Liaoning Province from 2009 to 2018 and their related technical fields are analyzed by applicant-technology matrix.This article selects the top 10 applicants for the analysis.The results are shown in Fig.5.
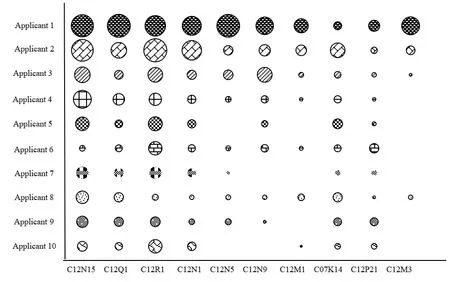
Fig.5 Technology matrix of major applicants in Liaoning’s biopharmaceutical industry
It can be seen from Fig.5 that different research institutions focus on different key technical fields.Among them,the top 10 applicants focus on four technical fields of C12N15,C12Q1,C12R1,and C12N1.The two main R&D organizations from the bubble chart are Dalian Institute of Chemical Physics,Chinese Academy of Sciences and Dalian University of Technology.The applicant’s technology matrix can clearly reflect the R&D focus and technical deficiencies of each applicant,which lays a foundation for them to f ind partners.
3.4 Comparative analysis of patented technology efficacy
Beijing,Jiangsu,and Guangdong are selected as the representative provinces of each industrial cluster to compare with the development of biopharmaceutical industry Liaoning Province.By comparing the efficacy of patented technologies,it can be seen whether the R&D focus of Liaoning Province is consistent with that of the selected key provinces.The top ten technologies in the IPC classification (large group) of patent applications in Liaoning,Beijing,Jiangsu,and Guangdong from 2009 to 2018 are chosen for statistical analysis,as shown in Fig.6.
From the analysis of the technical fields of Liaoning Province and other key provinces in Fig.6,it can be seen that the R&D focus of Liaoning biopharmaceutical industry is the same as that of the key provinces,which covers the two technical fields of C12N15 and C12Q1.Since the three key provinces selected in this article represent three industrial clusters,China’s current R&D focus on biopharmaceutical industry can be shown by three provinces.It also means that Liaoning biopharmaceutical industry should follow national trends.Liaoning Province should make full use of its own advantages,broaden the scope of technical f ields based on the existing technologies,and promote the development of the biopharmaceutical industry.
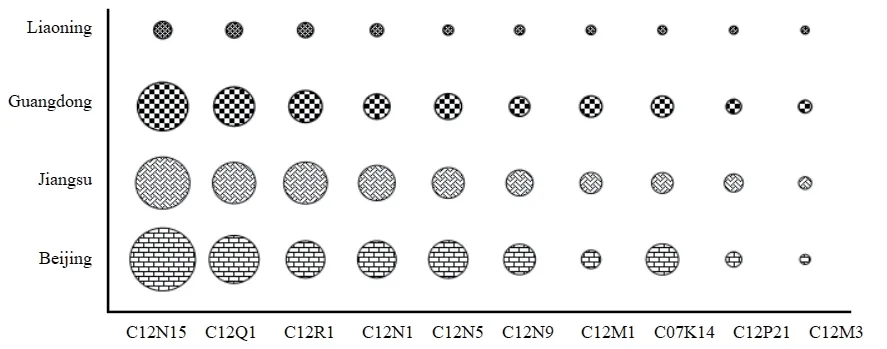
Fig.6 Comparative analysis of patent technology efficacy between Liaoning Province and key provinces
4 Analysis of Liaoning biopharmaceutical industry patent development strategies
4.1 Improving medical patent protection and building a good policy environment
From the perspective of pharmaceutical patent protection policy,since the pharmaceutical industry depends heavily on patent protection,the government should help enterprises strengthen their awareness of patent protection,and actively encourage biopharmaceutical enterprises and pharmaceutical universities to apply for patents.Besides,the government should provide corresponding policy support for enterprises to obtain patent protection.The government of Liaoning Province can issue patent policy documents suitable for the development of the biopharmaceutical industry,which is in accordance with the“National 13th Five-Year”strategic development plan.At the same time,it can issue a series of incentive policies for patentobtained enterprises,universities or individuals.While perfecting the patent protection of the pharmaceutical industry,we can build a good policy environment to promote the continuous innovation of biopharmaceutical industry in Liaoning Province.
4.2 Improving patent quality and promoting the transformation of scientific research results
From the perspective of patent quality and patent achievement transformation,the quality of patents in Liaoning’s biopharmaceutical industry needs to be improved.One feasible way to improve the quality of patents is to introduce more innovative talents,thus improving the quality of R&D personnel,who can raise the level of patents.Another way is to strengthen cooperation between various institutions.Based on the current cooperation,Liaoning Province should make full use of its own advantages to create a new R&D model of“university+research institution +enterprise+individual”.This will enable each part of the biopharmaceutical industry to be better linked.Eventually a clustering effect can be achieved to transform the scientific research results into enterprise production.The establishment of an integrated model of production,learning,and research can improve the economic benefits of biopharmaceutical industry.
4.3 Broadening the scope of technical fields and keeping up with the development direction
From the perspective of the technical fields,the current patent applications for biopharmaceutical industry in Liaoning are mainly concentrated in such technical fields as mutations and inspection methods.With the rapid development of world’s biopharmaceutical industry,Liaoning Province should utilize this opportunity to actively expand the technical fields while maintaining the original technical scope.A feasible plan is to cooperate with provinces with better development of the biopharmaceutical industry so that Liaoning Province can improve its technologies.In addition,the government must actively encourage universities,enterprises,and scientific research institutes to explore a wider range of technical fields,which will promote the technological development of Liaoning biopharmaceutical industry to be consistent with China’s overall biopharmaceutical industry.
4.4 Making full use of resources to reduce the gap in urban development
From the perspective of city,the current patent applications for the biopharmaceutical industry in Liaoning are mainly concentrated in Shenyang and Dalian.As an old industrial base in the Northeast,Liaoning has its unique advantages.It can make full use of the abundant resources of Benxi Pharmaceutical City to fully develop the biopharmaceutical industry,which will realize the development model of Shenyang driving Benxi,and universities driving enterprises.In addition,Jinzhou,Dandong and other cities all have certain potentials.The government can encourage universities and enterprises to conduct biopharmaceutical research and provide a certain degree of financial and technical support.In this way,the fast-developing cities such as Dalian and Shenyang will drive the slow-developing cities to reduce the gap in number of patents,and realize the all-round development of Liaoning’s biopharmaceutical industry.
- 亚洲社会药学杂志的其它文章
- Problems in China’s Licensed Pharmacists System and Suggestions for Improvement
- Investigation and Analysis of the Current Situation of Online Drug Sellers
- EU Real-World Evidence Supporting Drug Regulatory Decision and Its Enlightenment to China
- The Practice of Pharmacist-Driven Antimicrobial Stewardship Based on Value-Based Healthcare
- Current Situation and Development Strategy of Residents’Cognition and Use of TCM in Liaoning Province
- Empirical Analysis on Performance Evaluation of A Pharmaceutical Company Based on Economic Value Added

