Efficacy and safety of Huaiqihuang granule as adjuvant treatment for primary nephrotic syndrome in children: a meta-analysis and systematic review
Jiao Lin · Li-Min Hua ng · Jing-Jing Wang · Jian-Hua Mao
Abstract Background Huaiqihuang (HQH) granule is a traditional Chinese herbal complex that has been used as an adjuvant treatment in clinics for the primary nephrotic syndrome (PNS) for many years.However, the effectiveness and safety of HQH have not been systematically discussed.This review aimed to evaluate the effectiveness and safety of HQH in paediatric patients with PNS.Methods The following databases were searched from inception to Mar 2019: MEDLINE, Cochrane Library, EMBASE, CNKI, Wanfang Database, the Chinese Scientific Journal Database and the Chinese biomedical literature service system.All the randomized controlled trials (RCTs) eligible for inclusion were included.The primary outcomes were relapse, infection, remission and adverse events.The secondary outcomes included serum immunoglobulin levels (IgA, IgG or IgM), T-lymphocyte subtype (CD3+ , CD4+ , CD8+ , CD4+ /CD8+), IL-10, TNF- α, TNF- γ, total cholesterol and time of proteinuria turning negative.Results Fourteen RCTs (885 patients) were identified.Treatment with HQH reduced the chance of relapse [relative risk (RR): 0.47; 95% CI: 0.34, 0.66; P< 0.001] and infections (RR: 0.47; 95% CI: 0.35, 0.62; P< 0.001).No significant difference was found in adverse events.HQH also increased the serum levels of IgA [weighted mean difference (WMD): 0.40; 95% CI: 0.20, 0.60; P< 0.001] and IgG (WMD: 1.58; 95% CI: 1.38-1.78; P< 0.001), as well as CD4 + [standard mean difference (SMD): 0.90; 95% CI: 0.12-1.68; P= 0.02], CD3 + (WMD: 4.04; 95% CI: 3.27-4.82; P< 0.001), and the CD4 + /CD8 + ratio (WMD: 0.31; 95% CI: 0.21-0.41; P< 0.001), but decreased the level of CD8 + cells (WMD: -3.39; 95% CI: -5.73-1.05; P= 0.004).No statistically significant difference was found in IgM (WMD: 0.05; 95% CI: -0.13, 0.24; P= 0.57).Conclusions HQH could reduce the rate of relapse and the frequency of infection in children with PNS.No apparent adverse effects were found.Moreover, the beneficial infl uence of HQH may act through immunomodulation.Additional multi-center, large-sample, high-quality studies are needed to confirm the effectiveness and safety of HQH.
Keywords Children Huaiqihuang · Nephrotic syndrome · Meta-analysis
Introduction
Primary nephrotic syndrome (PNS), defined as massive proteinuria, hypoalbuminemia, hyperlipidaemia, and oedema, is one of the significant causes of chronic kidney disease in children worldwide [1].Since the wide application of steroids in 1950 [2], glucocortico steroids (GCs) have greatly improved the prognosis of patients with PNS due to their anti-infl ammatory and immunomodulatory effects [3].More than 90% of children with PNS response well to oral steroids, but 60% of patients frequently relapse with nephrotic syndrome (FRNS), even those with steroiddependent nephrotic syndrome (SDNS).The long-term use of steroids or immunosuppressants is necessary to control the symptoms [4]; however, exposure to the prolonged GC treatment may result in numerous metabolic side effects, such as growth retardation, hypertension, diabetes, central obesity and osteoporosis [5].Although the primary PNS treatment course is well established, the narrow balance between steroid side effects and potential nephrotoxicity remains challenging for paediatric nephrologists.
Huaiqihuang (HQH) granule is a traditional Chinese herbal medicine composed ofTrametesrobiniophila Murr, Lycium barbarumandPolygonatum.HQH has been used effectively in the treatment of respiratory and kidney diseases in China for years [6].Pharmacological studies indicate thatTrametesrobiniophila Murrcan adjust immunity and play an essential role as an anti-inflammatory and anti-allergic agent [7].In a prospective, randomized, controlled study, HQH improved the rate of complete remission in IgA nephropathy patients and reduced the levels of proteinuria and haematuria [8].Shao et al.indicated that HQH also improves proteinuria and hematuria and slows the progression of chronic kidney disease in primary glomerular diseases [9].In murine renal tubular cells, HQH extract inhibited the HMGB1/TLR4/NFκB/TNF-αpathway and reduced cisplatin-induced kidney damage [6].In addition, HQH protected against proteinuria by suppressing MPC5 podocyte damage and enhancing of nephrin expression [10, 11].These protective effects suggested that HQH might be a broad-spectrum nephroprotective agent.
The specific mechanism of PNS has not been fully elucidated and may be associated with immune disorders.Children taking GCs are more likely to become infected, which increases their chance of recurrence.In China, HQH is often considered to enhance immunity and decrease recurrence and infection.In this study, we evaluated the effectiveness and safety of HQH in children with PNS.A systematic review and meta-analysis on the effectiveness and safety of the adjuvant administration of HQH were performed for children with PNS in randomized controlled trials (RCTs).
Methods
Protocol and registration
A protocol of this meta-analysis and systematic review has been registered on PROSPERO with the registration number CRD42019116913.
Search strategy
The review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.We conducted a systematic search of the following three English databases and four Chinese databases: MEDLINE, Cochrane Library, EMBASE, CNKI, Wanfang Database, the Chinese Scientific Journal Database, and the Chinese biomedical literature service system.Studies were identified using the keyword search terms: “huaiqihuang” and “primary nephrotic syndrome”.The search was limited to Mar 2019 in each database.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) types of studies: RCTs; (2) study participants: children diagnosed with PNS; (3) types of interventions: HQH (< 3 years, 5 g dose, twice daily; ≥ 3 years, 10 g dose, twice daily, oral administration) together with the standard steroid therapy versus the standard steroid therapy only (prednisone or methyl prednisone); and (4) included studies must contain validity data and necessary outcome evaluation.Primary and secondary outcomes were measured.The primary outcomes were relapse, infection, remission and adverse events of PNS.The secondary outcomes included the levels of serum immunoglobulin (IgA, IgG or IgM) and T cell subtypes (CD3 + , CD4 + , CD8 + , CD4 + /CD8 + ), IL-10, TNF-α, TNF-γ, total cholesterol and time of proteinuria turning negative.
Studies were excluded if (1) the follow-up duration was less than 6 weeks; (2) patients had Lupus nephritis, Henoch-Schönlein purpura, IgA nephropathy, hepatitis B -associated glomerular nephritis, Alport's syndrome, etc.(3) other Chinese herbals were co-administered in the intervention group; and (4) the specific data of patients were not available from the articles or authors.All of the data included in this meta-analysis came from previously published articles; thus, ethics approval and patient consent were not required.
Study selection and data extraction
Two authors (LJ and HLM) reviewed the titles and abstracts of each study independently.The potentially eligible studies were assessed by reading the full text.All included studies were cross-checked, and any disagreement was resolved with the help of the third author (WJJ).
The data and study characteristics were extracted independently by two authors (LJ and HLM), and a predesigned standard form was used.The data extracted included the following: study ID, study characteristics (authors, title, publication year), patient characteristics (number of patients, age, sex), HQH intervention and control, treatment duration, follow-up duration, and outcomes.If necessary, the original authors of the studies were contacted for missing data or further information.
Quality assessment
The risk of bias of the included studies was evaluated by referencing the Cochrane Handbook, and seven domains were considered: random sequence generation method, allocation concealment, blinding of subjects and test personnel, blinding of outcome evaluator, incomplete outcome reporting, selective report study results, and other biases.The risk of bias was categorized as “high”, “low”, or “unclear” according to the actual criteria for each study.A trial would be judged to be at high risk if one domain was considered to be high.If each key domain was judged to be low risk, the study would be regarded as having a low risk of bias or an unclear risk of bias.
Outcome measures
RevMan5.3 software was used to conduct the data analysis.The weighted mean difference (WMD) or standardized mean difference (SMD) and 95% confidence intervals (CIs) were calculated for continuous data.Dichotomous data are reported as the relative risk (RR) and 95% CIs.According to the Cochrane Handbook for Systematic Reviews of Interventions [12], WMD can be used for studies using the same scale, and SMD can be used to measure the magnitude of the effect [13].The heterogeneity of the results among the trials was quantified using the inconsistency indexI2 [14].
I2represents the proportion of study variability with values ranging from 0-100%.Values of 25, 50, and 75% are considered low, moderate, and high, respectively [14].When statistical heterogeneity is present in a meta-analysis, the fixed-effects model is not appropriate [15].Therefore, a fixed-effect model was used if the heterogeneity of the data was not significant (I2 ≤ 50%); if heterogeneity existed (I2 > 50%), a random-effects model was applied.When the heterogeneity was high, a subgroup analysis was conducted, and sensitivity analysis was carried out to investigate possible sources of heterogeneity by eliminating individual trial in turn.If the studies were less than three, a qualitative description was provided [16].
Results
Description of the studies
A total of 1377 studies were initially identified after the search, but only 14 trials met the inclusion criteria and were enrolled in the analysis (Fig.1).All of the included trials were performed in China and were published in Chinese.A total of 885 patients were randomly assigned to Western medical treatment only or to HQH with Western medicine treatment.The sample sizes of the individual trials ranged from 42-112, with an average of 63 participants.The patient's ages varied from 1-14 years.The sex ratio of males was 65.5%.Nine studies reported interventions with HQH plus prednisone versus prednisone alone.Five studies administered HQH plus glucocorticoid versus glucocorticoid but did not mention the type of glucocorticoid used.The duration of treatment varied from 6 weeks-1 year.The study details are presented in supplemental Table 1.
Risk of bias of the included studies
A total of 14 RCTs were evaluated based on the Cochrane Collaboration, and assessment of the risk bias is shown in supplemental Fig.1.Although all trials were randomized, only six described the randomization procedures in detail.None of the trials mentioned blinding of patients or allocation concealment.All studies reported follow-up, and two studies described the dropout or withdrawal information.The baseline characteristics including age, sex, ethnicity, and disease course were similar in the different groups.
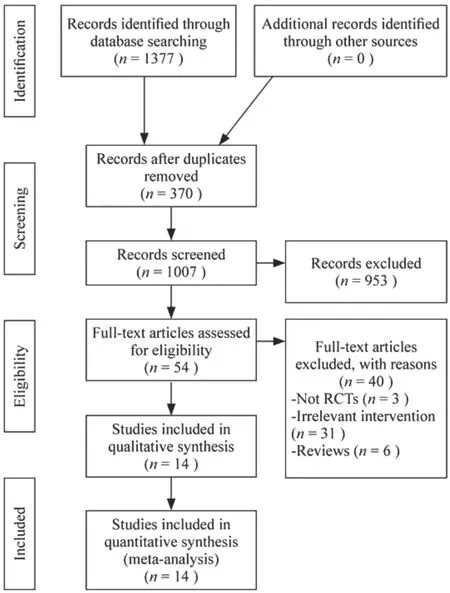
Fig.1 Flow diagram of study selection
Effect of the interventions
Main outcome measures
Six studies evaluated the rate of relapse [17- 22].Figure 2 a shows that co-treatment with HQH decreased the relapse rate compared with steroid alone (RR: 0.47; 95% CI: 0.34, 0.66;P< 0.001;I2 : 0.0%).Infection data were reported in five studies [18, 20- 23].The risk of infection was signifi-cantly reduced with HQH treatment compared with the conventional therapy (RR: 0.47; 95% CI: 0.35-0.62;P< 0.001;I2 : 0.0%) (Fig.2 b).Five studies reported adverse events as outcome measures [18, 20, 22- 24].There were no signifi-cant differences (RR: 2.80; 95% CI: 0.55-14.24;p= 0.22;I2 : 0.0%) between the two groups (Fig.2 c).Two studies reported remission as an outcome measure [20, 21].One of these studies reported a significant difference between the groups after 9 months; the other study reported no signifi-cant difference after 6 months; however, during the maintenance period in both studies, the dosage of GCs in the HQH group was reduced.
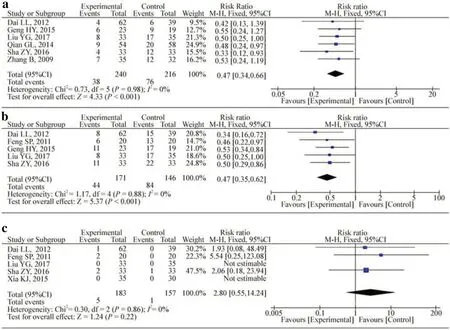
Fig.2 Effect of Huaiqihuang on the rate of relapse ( a), rate of infection ( b) and adverse events ( c) compared with the control group.M-Hmantel-Haenszel odds ratio, CIconfidence interval, dfdegrees of freedom, IVindependent variable, I2 inconsistency index, SDstandard deviation
Secondary outcome measures
The serum levels of IgG, IgA and IgM were analysed in eight studies [20- 27].The results indicated that treatment with HQH increased serum IgG (WMD: 1.58; 95% CI: 1.38, 1.78;P< 0.001;I2 = 46%) and IgA levels (WMD: 0.40; 95% CI: 0.20, 0.60;P< 0.001;I2 = 85%); however, IgM levels did not differ significantly compared with those in the control group (WMD: 0.05; 95% CI: -0.13, 0.24;P= 0.56;I2 : 65%) (Fig.3).
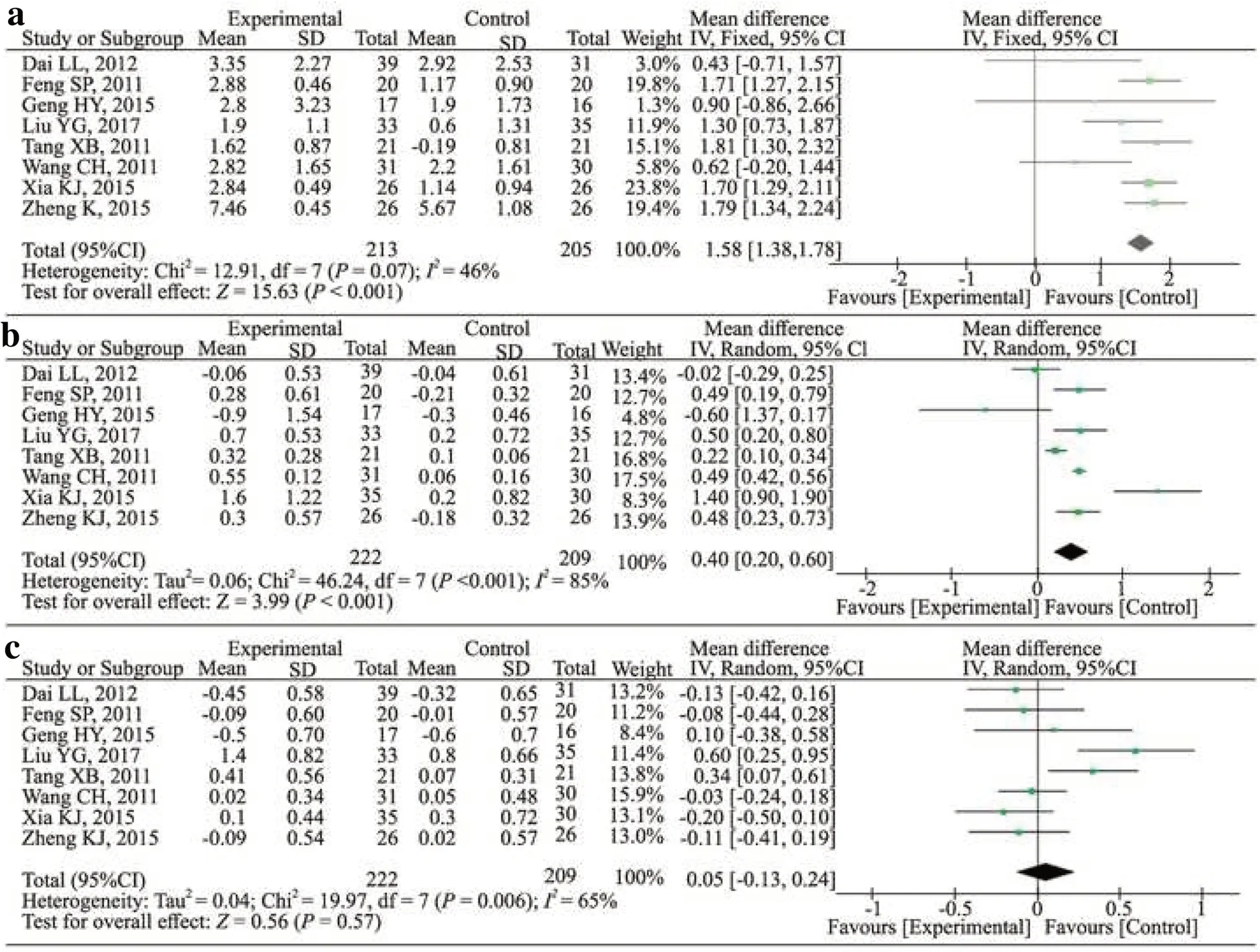
Fig.3 Effect of Huaiqihuang on the serum immunoglobulins levels compared with the control group ( a, IgG; b, IgA; c, IgM).CIconfidence interval, dfdegrees of freedom, IVindependent variable, I2 inconsistency index, SDstandard deviation
Eight studies measured the ratios of CD4+and CD8+counts [20- 27].Seven studies reported CD4+/CD8+counts [20- 26], and six reported CD3+counts [20- 23, 25, 27].Figure 4 shows that treatment with HQH increased CD4+(SMD: 0.90; 95% CI: 0.12, 1.68;P= 0.02;I2 : 92.0%) in a random effects model, but the treatment diminished the level of CD8+counts (WMD: -3.39; 95% CI: -5.73, -1.05;P= 0.004;I2 : 93%) compared with that in the control group.Treatment with HQH also increased the CD4+/CD8+counts (WMD: 0.31; 95% CI: 0.21, 0.41;P< 0.001;I2: 45%) (Fig.5).Furthermore, HQH significantly increased CD3+(WMD: 4.04; 95% CI: 3.27, 4.82;P< 0.001;I2 : 38%) (Fig.5).For CD3+counts, the pooled WMD was not stable through sensitivity analysis because of the study by Tang et al.The pool quality and small sample size in this study could account for the instability of the results.To add stability to the results, we removed this study from the analysis.
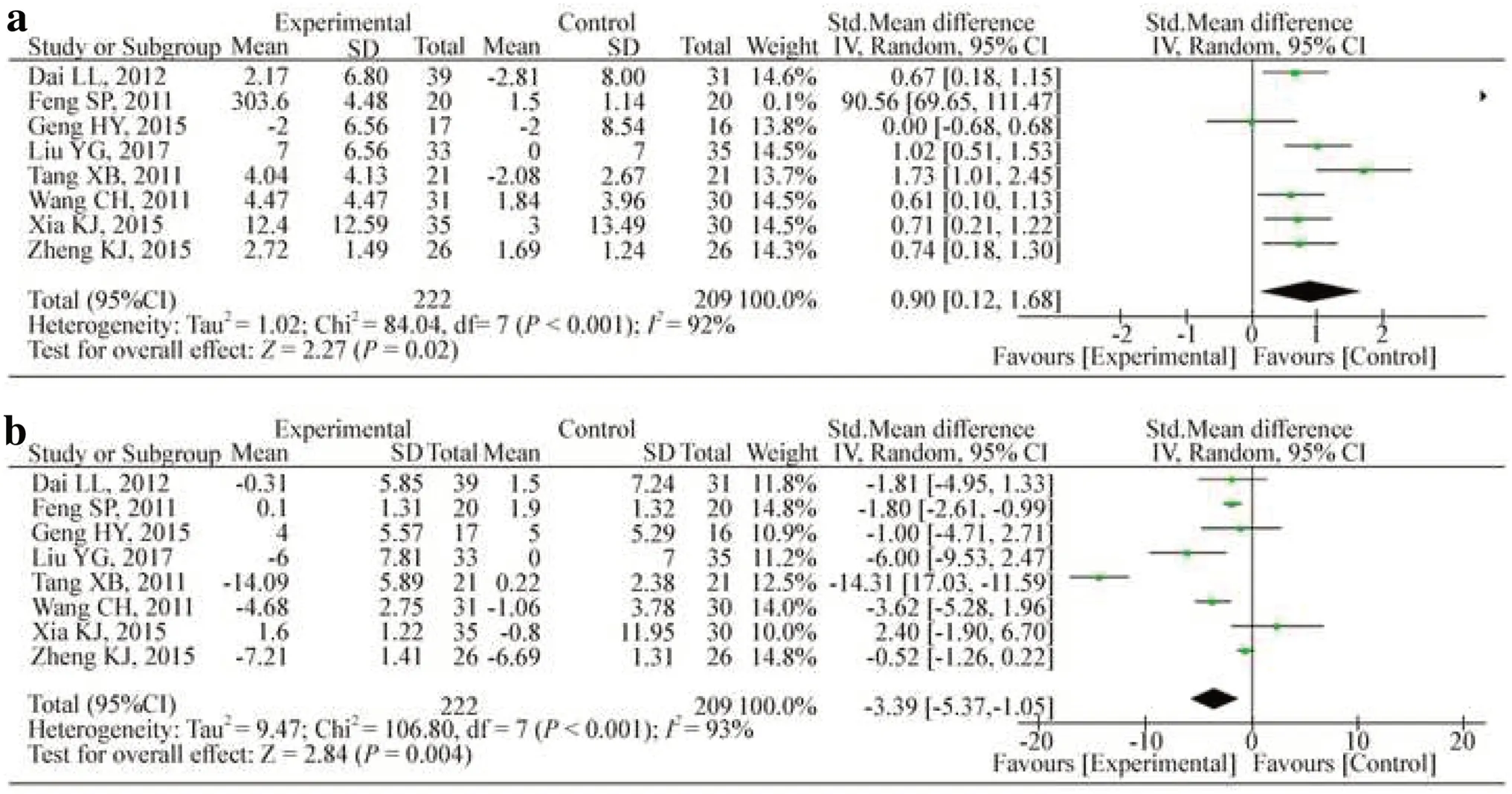
Fig.4 Changes in T-lymphocyte CD4 +( a) and CD8 +( b) counts.CIconfidence interval, dfdegrees of freedom, IVindependent variable, I2 inconsistency index, SDstandard deviation
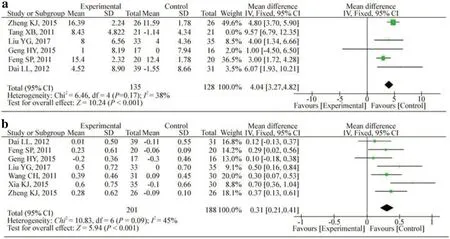
Fig.5 Changes in T-lymphocyte CD3 +( a) and CD4 +/8 +( b) counts.CIconfidence interval, dfdegrees of freedom, IVindependent variable, I2 inconsistency index, SDstandard deviation
Four studies evaluated the level of IL-10 [17- 19, 25].After removing the study by Qian et al.from the analysis [19], the heterogeneity was reduced to 0% and the treatment had a significant impact (WMD: 6.77; 95% CI: 4.80, 8.74;P< 0.001;I2 : 0.0%) (Fig.6).Regarding TNF-α, treatment with HQH significantly reduced the TNF-αlevel (WMD: -0.70; 95% CI: -1.27, -0.14;P= 0.01;I2 : 0.0%) in three studies [17, 18, 26].
INF-γwas measured in two studies [19, 27], and both showed a declining trend.Zhao et al.reported that INF-γwas much lower in the HQH group after treatment [27], but Qian et al.obtained the opposite results.
Two studies reported changes in serum cholesterol [20, 21].No significant heterogeneity was detected between the studies.Additionally, no significant difference in the time of proteinuria turning negative was found in the other two studies [21, 23].
Sensitivity and subgroup analyses on T-lymphocytes subtype and immunoglobulin levels
A sensitivity analysis was performed.Each study was removed once, and the remaining studies were used for meta-analysis.The appearance of no additional changes indicated the robustness of the data analysis.For CD3+, the source of study heterogeneity was reduced once the study by Tang et al.was removed (WMD: 4.04; 95% CI: 3.27, 4.82;P< 0.001;I2 : 38%) [28].For IL-10, removing the study by Qian et al.reduced study heterogeneity to 0% (RR: 6.77; 95% CI:4.80, 8.74;P< 0.001;I2 : 0.0%) [19].Subgroup analysis was conducted based on the treatment period of HQH (≥ 6 months vs.< 6 months) and follow-up time (≥ 6 months vs.< 6 months) in IgA, IgG, IgM, CD4+, CD8+, and CD4+/CD8+.The results suggested that the treatment duration and follow-up period did not identify the source(s) of heterogeneity (Tables 1, 2).
Discussion
A total of 14 studies involving 885 patients were included in this review.The results indicated that treatment with HQH promoted positive therapeutic effects by reducing relapse rates and infection rates without adverse effects.HQH also increased the serum levels of IgA, IgG, CD3+, CD4+, CD4+/CD8+, and IL-10, while the levels of CD8+and TNF-α decreased compared with the standard steroid therapy alone.No significant difference was found in the IgM levels.Two studies that referred to remission had different conclusions.Another two studies mentioned the time of proteinuria turning negative and cholesterol; however, no significant difference was found between the two groups.Subgroup analysis was performed, but we did not find any source(s) of heterogeneity.
PNS is an immune-mediated renal disease related to T cell disorder and secondary to B cell derangement [29].Regarding T cells, many studies have shown an imbalance of the CD4+/CD8+ratio, lower CD4+activity and activated CD8+T-lymphocytes and natural killer cells in PNS [30, 31].These studies provide evidence that T cells participate in the pathogenesis of PNS.This finding is also supported by the absence of immunoglobulins and complement components in glomeruli, the positive response to treatment with T cell inhibition, such as steroids, cyclosporine, and mycophenolate, and the association of remission following measles infection (which is known to depress T cell immunity) [32].In an animal model of PNS, decreased ratios of CD4+induced an increased proportion of CD8+and an aggravation of the disease, suggesting that CD4+cells play a protective role [33].The role of B-lymphocytes and the therapeutic effect of rituximab, a monoclonal antibody against CD20, have received increased attention in recent years.Previous studies showed that B-lymphocytes may regulate the pathology of PNS; however, the mechanism of rituximab treatment for PNS remains unclear.One possibility is that its effect is to induce regulatory T cells rather than deplete simple B cells.This article mainly describes the effect of HQH on T-lymphocytes, such as increasing the amounts of CD3+and CD4+lymphocytes, and balancing the CD4+/CD8+ratio, which may play an important part in the immunomodulatory effects in PNS; however, no data were reported for the B lymphocyte subset, so whether HQH affects PNS through B lymphocytes is unclear.
Most patients with PNS often present low IgG levels and high serum IgM levels.Xia et al.found that PNS relapse is often induced by infections due to low serum IgG levels.Decrease IgG levels can act as a predictive index for an unfavorable PNS prognosis [34].In our study, HQH increased IgG serum levels and reduced the rate of relapse.This result was in agreement with that of prior studies.Remission was the most important prognostic indicator of PNS and was mentioned in two articles.Although the results of the two articles were inconsistent, both showed that the dosage of GCs decreased significantly, which could eliminate the longterm and high-dose side effects of GCs.PNS also carries a high risk of infection.The incidence of infection in PNS varies from 8 to 84% in different reports [35, 36].Infection may cause frequent relapses and treatment failure and ultimately may result in an increased rate of morbidity in PNS patients [37].The pathogenesis of infection frequency is mostly associated with immunosuppressive therapy, malnutrition, and the urinary loss of immunoglobulin complement factors [38].HQH extracts are often used as adjuvant medicine in kidney disease and respiratory infection in children.HQH granules can reduce the incidence of re-infection in children with severe Mycoplasma pneumoniae, can regulate immune functions, and can increase the levels of serum IgA, IgG, and IgM, as well as CD4+and CD4+/CD8+[39].Through this meta-analysis, we also found a similar conclusion, suggesting that HQH could promote the proliferation of CD4+, CD3+, and immunoglobulin.
The key component of HQH,Trametesrobiniophila Murr, has been considered an effective chemotherapeutic agent with a safe and broad anticancer spectrum [6].More attention should be given toTrametesrobiniophila Murrand on the development of drugs that are more effective and have fewer side effects.
Five studies mentioned adverse events as an outcome measure, and no difference was discovered.Three of the included RCTs described mild adverse events, such as diarrhoea, nausea, and emesis, indicating that HQH was likely to be safe and well tolerated in children with PNS.However, although HQH is a natural product, that does not imply that it is absolutely safe.First, the data on complications were scarce and only included in five studies.Second, articles mentioning complications were based on clinical observation and did not give exact data, such as SCR and ALT.In addition, the combined pharmacological activities of medicinal plants may exert adverse effects [40].Although mild adverse reactions were reported, the absolute safety of HQH should be clarified based on more accurate and adequate studies.
This study has several limitations.First, the risk of bias of the included RCTs was generally high.Less than half of the studies provided detailed random sequence generation, none of the trials described allocation concealment and there was a lack of blinding, which may cause selection bias and detection bias.Second, all included studies were from China and, therefore, racial differences may lead to selection bias.No negative studies were generated, and publication biases were inevitable.Although each article described the diagnostic criteria for PNS, most of the patients were first diagnosed and had not used immunosuppressants; thus, data for patients with FRNS or SDNS were lacking.In addition, none of the included studies performed a kidney biopsy, the relationship between pathological type and therapeutic effect cannot be analyzed.Further, the sample size was relatively small, and the follow-up time was too short.Most articles lacked details, such as the methods of hormone application, and thereby prohibit firm conclusions.Overall, we should pay more attention to a few points in future articles.The diagnostic criteria of PNS can be clarified with biopsy techniques, and patients with FRNS or SDNS should be included.Second, the usage and type of GCs can be described more accurately.Third, the drug concentration may be used to determine the reasonable and effective dosage of HQH.Further large-scale, multicentre studies are still needed, or a meta-analysis based on individual patient data can be considered to promote credibility.Finally, considering the suboptimal quality of the studies and lack of detailed methodology, it is difficult to draw a firm general conclusion.
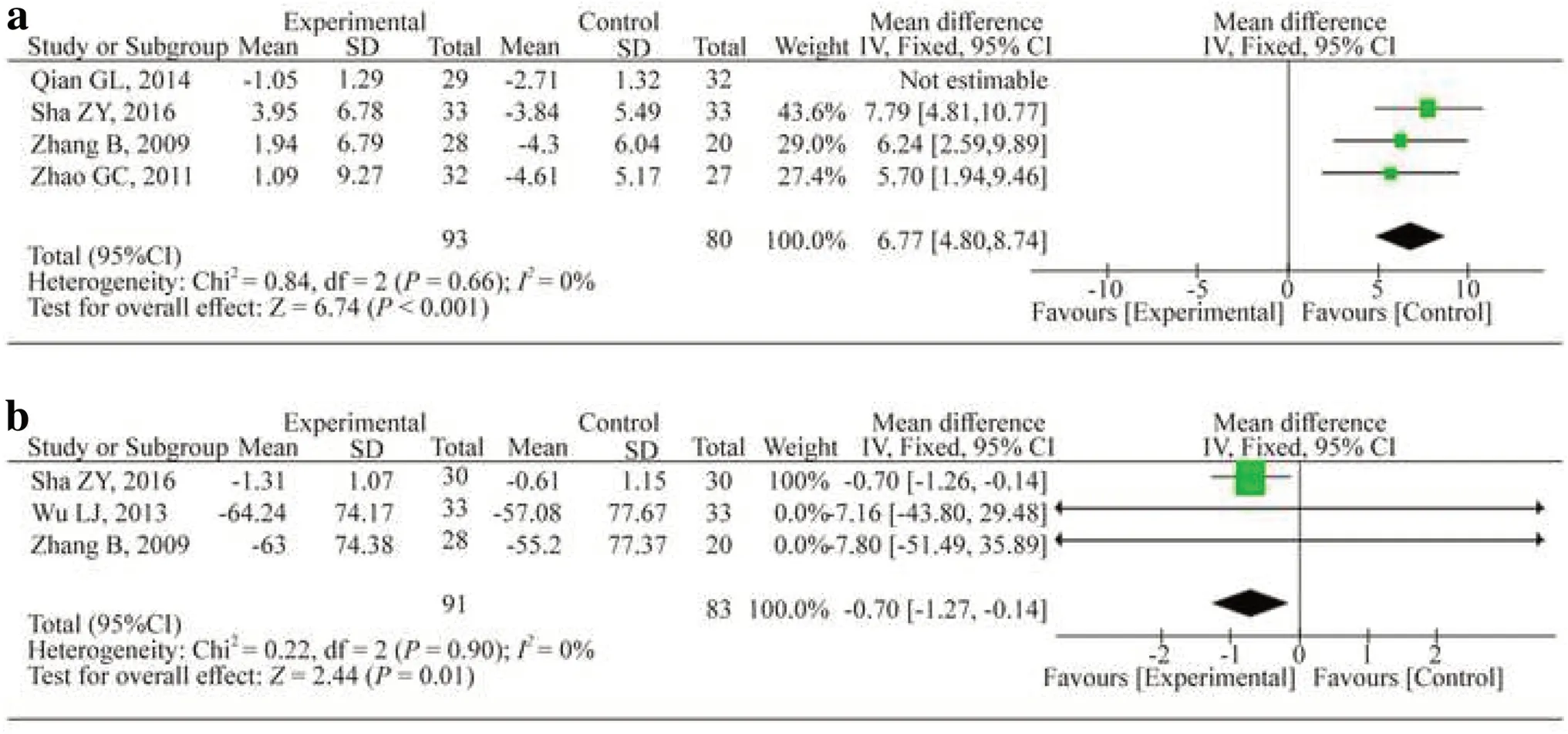
Fig.6 Changes in IL-10 ( a) and TNF- α( b).CIconfidence interval, dfdegrees of freedom, IVindependent variable, I2 inconsistency index, SDstandard deviation
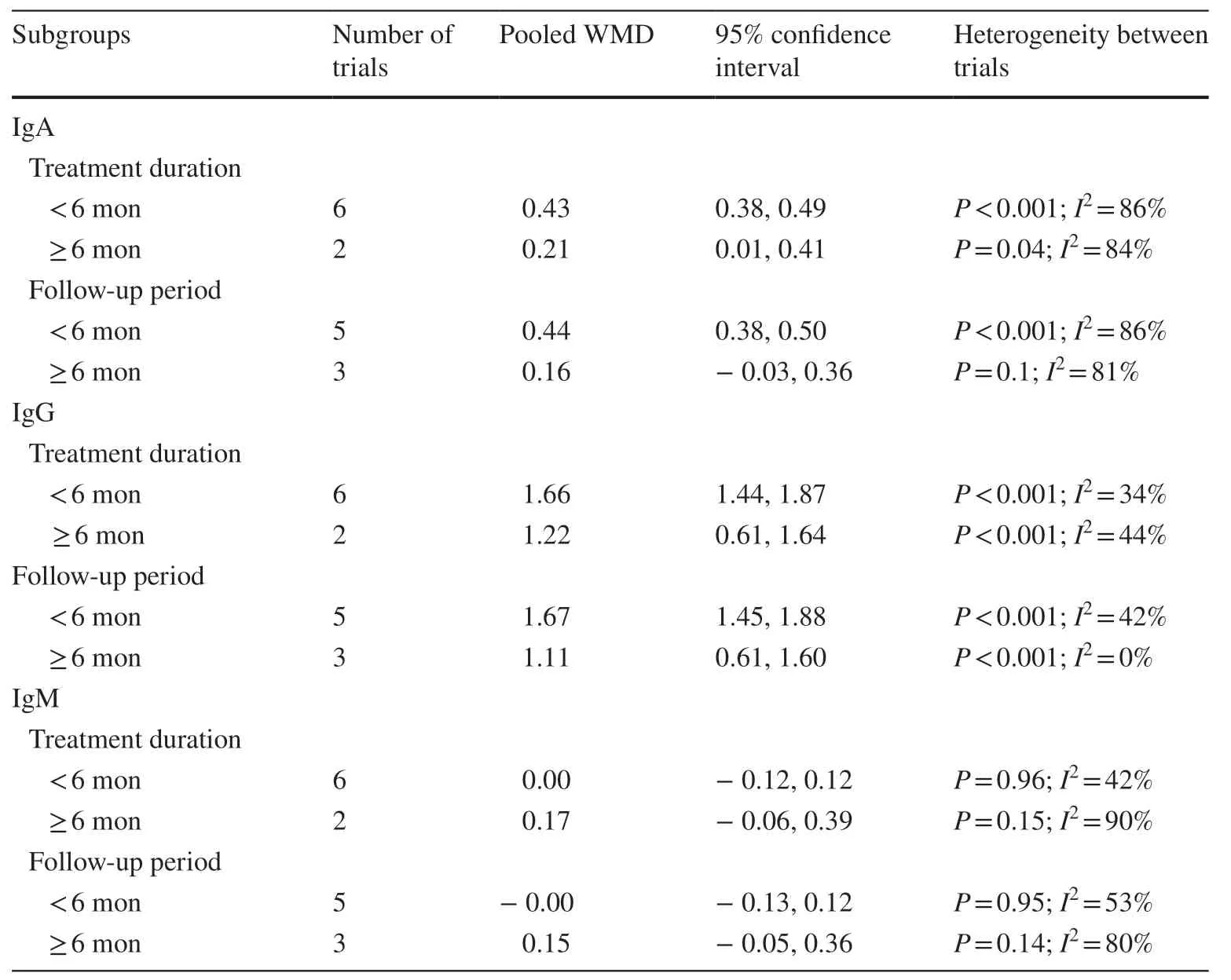
Table 1 Subgroup analysis on the changes of immunoglobulin level
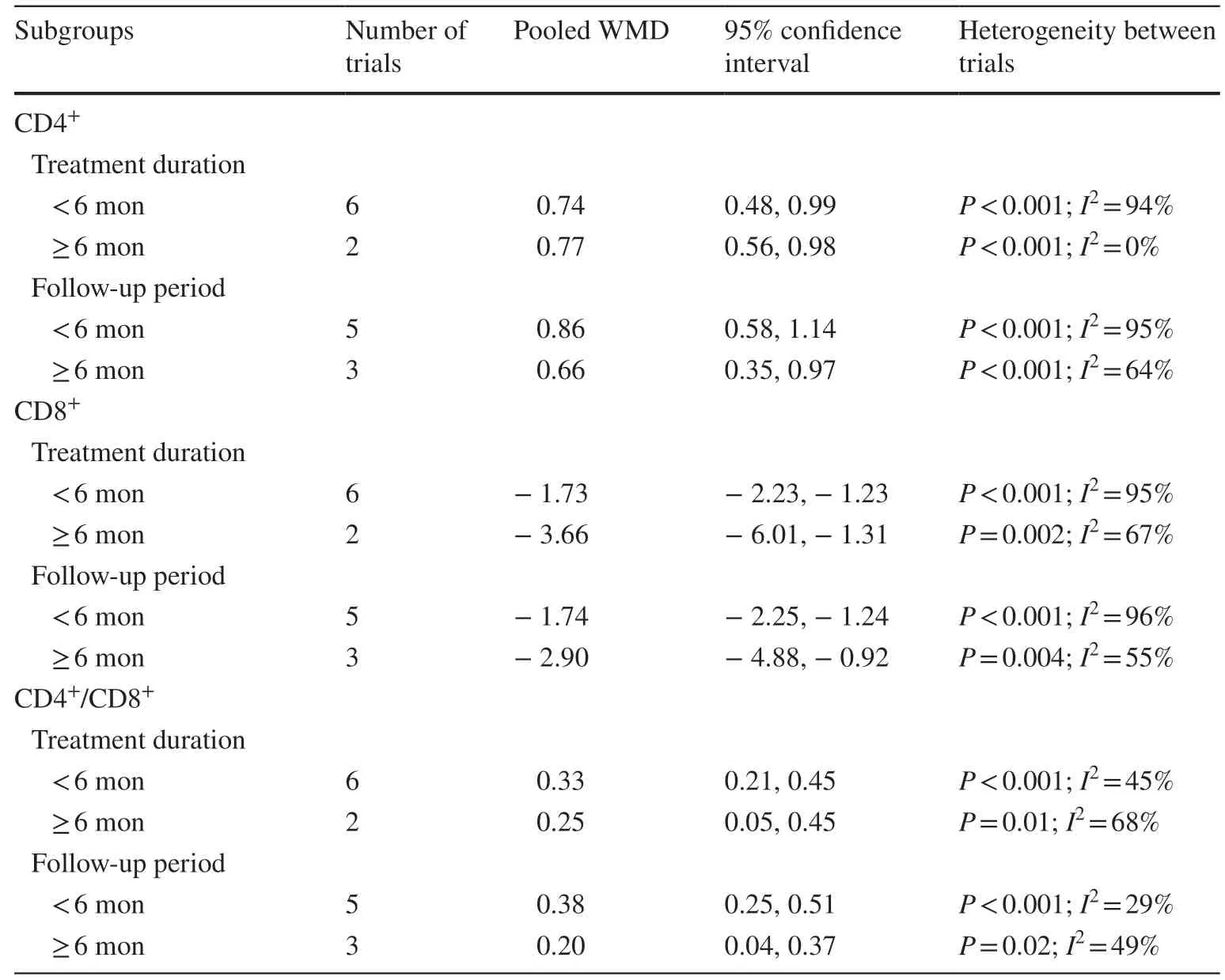
Table 2 Subgroup analysis on the changes of CD4 +, CD8 +and CD4 +/CD8 +
In conclusion, HQH could reduce the frequency of relapse and infection rates.No obvious side effects were found.HQH may play an immunomodulatory role.Considering the quality of the original trials, further multi-centre and high-quality RCTs are needed to provide high-quality evidence.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/ s12519- 020- 00405-w.
Author contributionsMJH was responsible for the conception, reviewed and revised the manuscript.LJ managed the literature searches, data extraction and wrote the first draft of the manuscript.HLM was responsible for the literature searches, data extraction and inclusion and exclusion of studies.WJJ was responsible for data extraction, paper writing and revision.All authors read and approved the final manuscript.
AcknowledgementsAuthor Jian-Hua Mao is a member of the Editorial Board for World Journal of Pediatrics.The paper was handled by the other Editor and has undergone rigrous peer review process.Author Jian-Hua Mao was not involved in the journal's review of, or decisions related to, this manuscript.
FundingThis study was supported by the National Natural Foundation of China (81470939 and 81770710), the Natural Science Foundation of Zhejiang Province (LH14H050002, LY15H050001), and the Zhejiang Medical and Health Science and Technology Project (2016KYB177).
Compliance with ethical standards
Ethical approvalNot required for this meta-analysis.
Conflict of interestThe authors declare that they have no competing interests.
 World Journal of Pediatrics2021年3期
World Journal of Pediatrics2021年3期
- World Journal of Pediatrics的其它文章
- COVID-19 ocular findings in children: a case series
- Associations between measures of pediatric human resources and the under-five mortality rate: a nationwide study in China in 2014
- Genetic etiologies associated with infantile hydrocephalus in a Chinese infantile cohort
- Hedgehog signaling pathway gene variant influences bronchopulmonary dysplasia in extremely low birth weight infants
- Causes of severe neonatal hyperbilirubinemia: a multicenter study of three regions in China
- Maternal mental health and well-being during the COVID-19 pandemic in Beijing, China
