SDF5 Encoding P450 Protein Is Required for Internode Elongation in Rice
Yang Yachun, Li Juan, Li Hao, Xu Zuntao, Qin Ruiying, Wu Wenge, Wei Pengcheng, Ding Yong, Yang Jianbo
Letter
Encoding P450 Protein Is Required for Internode Elongation in Rice
Yang Yachun1, 2, Li Juan2, Li Hao2, Xu Zuntao3, Qin Ruiying2, Wu Wenge2, Wei Pengcheng2, Ding Yong3, Yang Jianbo2
(Agricultural College, Anhui Agricultural University, Hefei 230036, China; Rice Research Institute, Anhui Academy of Agricultural Sciences, Hefei 230031, China; School of Life Sciences, University of Science and Technology of China, Hefei 230026, China)
Plant height is a critical trait for yield in rice (), and gibberellic acid (GA) is involved in modulating rice height. Here, we identified the() mutant by screening a rice T-DNA insertion mutant library using genome- resequencing.contains a T-DNA insertion that causes the ectopic expression of, whichencodes a 14-α-demethylase cytochrome P450 (CYP51) protein. Levels of the active GA species GA1and GA4were reduced in themutant, and the semi-dwarf phenotype of themutant was rescued by exogenous GA4. In addition to its semi-dwarf height, themutant exhibited compact plant architecture, dark green leaves and low 1000-grain weight. Together, these results suggest thatis involved in GA biosynthesis pathway and modulates rice height and plant development.
Short plant stature is a primary goal in rice breeding to enhance grain yield, and commonly involves the() mutation (Monna et al, 2002; Sasaki et al, 2002; Spielmeyer et al, 2002). Using semi-dwarfvarieties, rice yield is increased by 100% in southern China, which has been a breakthrough for food security in China. More than 60 dwarf genes have been characterized in rice.encodes GA20-oxidase andencodes endo-kaurene oxidase, and both of them are responsible for the synthesis of gibberellins. The() mutation is associated with GA signal transduction andencodes a heterotrimeric G protein (GTP-binding protein) α-subunit (Ashikari et al, 1999; Fujisawa et al, 1999; Ueguchi-Tanaka et al, 2000).andare involved in the brassinosteroid (BR) biosynthesis pathway, whereas,andare involved in biosynthesis of strigolactones, which are responsible for tiller number and stem elongation (Yan et al, 2007; Arite et al, 2009; Lin et al, 2009; Zhang et al, 2010; Jiang et al, 2013; Sun et al, 2013). In addition, the epigenetic regulation is important for rice height.is a dominant dwarf mutant. The level of H3K9me2 at the 5-end ofdecreases, while the level of H3K4me3 increases, which proves thathas gained function due to apparent modification. Yeast two-hybrid results show that FIE1 interacts with iEZ1 and CLF, which indicates that FIE1 is involved in PRC2-mediated transcriptional repression (Zhang et al, 2012)., which is identified as a dominant half-dwarf gene, acts as a repressor of strigolactone signaling.encodes a 14-α-demethylase cytochrome P450 (CYP51) protein. Decreasedexpression reduces phytosterol and BR concentrations (Xia et al, 2015). After oxidized squalene cyclase mediated cyclization, CYP450 and acyltransferase modify the skeletal structure of triterpene and sterol (Osbourn et al, 2011). The characterization of dwarf genes has greatly improved our understanding of the factors regulating plant morphology. Many recessive dwarf genes have been cloned, and some of their regulatory mechanisms have been revealed. However, the dominant dwarf genes are not well known.
mutant was derived from a T-DNA insertion mutation of therice variety Nipponbare (wild type, WT). It was approximately 23.6 cm in height, which exhibited a semi-dwarf phenotype (Fig. 1-A). Moreover, the internodes and panicle lengths were shorter than those of the wild type (Fig. 1-B and Fig. S1). Tiller number, total grain number, filled grain number, 1000-grain weight and grain length were remarkably reduced inmutant (Fig. 1-C and Fig. S1). We then investigated the cell length in the fifth internode and showed that the cell length in themutant was reduced (Fig. 1-D).
Genetic analysis ofwas performed in the T1population following self-fertilization of the T-DNA insertion line. In the progeny, there were 14 semi-dwarf mutants and 7 normal plants. The seeds of 21 plants in the T2generation were planted with 20 seedlings per line. Among the 21 lines, 5 lines were semi-dwarf (100 seedlings), whereas 7 lines were normal, and 9 lines segregated in the next generation (142 semi-dwarf and 38 normal). These results suggested thatis a dominant dwarf mutant based on aχ2test (Table S1).
To isolate the gene responsible for the dominant dwarf mutation of, we determined the initial position of the inserted fragment by pool re-sequencing and population verification of the candidate gene. DNA was isolated from 10 plants with the semi-dwarf phenotype and 10 plants with normal height from the progeny of the self-fertilized T2lines. Re-sequencing showed that one T-DNA each was inserted into chromosomes 3 and 5. For chromosome 3, a T-DNA was inserted in the region of, which encodes a Cu/Zn superoxide dismutase-like protein. However, this locus was not linked to the dominant dwarf phenotype based on molecular verification. For chromosome 5, a T-DNA was inserted into the intergenic region, 1 743 bp downstream of(encoding a β-hexosaminidase precursor) and 3 488 bp upstream of(encoding a cytochrome P450) (Fig. 1-E).

Fig. 1. Characterization of
A, Phenotypes of the wild type (WT) and themutant. B, Internodes of WT and themutant. C, Grain size of WT and themutant. D, Cell length of the fifth internode in WT and themutant. E, Diagram of T-DNA insertion in themutant. FP1, FP2, RP1, and RP2 indicate the primers used for genotyping. F, Transcripts ofand. G, Phenotypes of wild type,mutant andoverexpression lines. T11, T12 and T13 indicate different overexpression plants. H, Transcripts ofin wild type,, T11, T12 and T13. Data are Mean ±SD (= 3). * and ** indicate significant differences at the 0.05 and 0.01 levels by the Student’s-test, respectively.
These results were further confirmed by genotyping (Fig. S2 and Table S2). T-DNA insertion on chromosome 5 was linked tousing the T2population. The transcription ofwas remarkably induced in themutant but the expression level ofwas not changed (Fig. 1-F). The expression level ofin the mutantwas 715 times higher than that in the wild type. These results suggested that the semi-dwarf phenotype of themutant might be caused by ectopic expression of.
To test this hypothesis, we generated vectors harboringandcoding sequences driven by the 35S promoter and transformed them into Nipponbare (primers are shown in Table S2). The transgenic plants containingexhibited a shorter height, similar to themutant (Fig. 1-G), but those containingexhibited a similar phenotype to the wild type. The severity of the dwarf phenotype increased with increasingtranscript levels (Fig. 1-G and -H). These results suggested that the dwarf phenotype of themutants was caused by the ectopic expression of, which was named as.
encodes a member of the P450 protein family, which includes proteins involved in fundamental processes such as synthesis of sterols, and modification of sterols and cyclic terpenes in the BR, abscisic acid and GA pathways. Phylogenetic analysis showed that SDF5belongs to the CYP51 sub-family, which includes six members in rice. CYP51 proteins are conserved in yeast, mammals and plants, which are responsible for sterol biosynthetic pathway in all biological kingdoms (Fig. S3).expressed in stem, leaf and spikelet, but not in root (Fig. S4).
We treated themutant with active gibberellins GA3and GA4(Fig. S5). The internodes of the wild type and themutant increased in the presences of GA3and GA4. However, the height ofmutant was close to that of the wild type with GA4(Fig. 2-A and -B), but not GA3(Fig. S5-A and -C). GA3slowly promoted the growth of,but the semi-dwarf was not rescued by GA3(Fig. S5)These results suggested thatwas involved in GA biosynthesis. We then treated themutant with paclobutrazol (PAC), an inhibitor of GA biosynthesis, and showed that PAC shortened the internodes of the wild type and themutant. However, themutant died in the presence of high concentrations of PAC (Fig. S5).
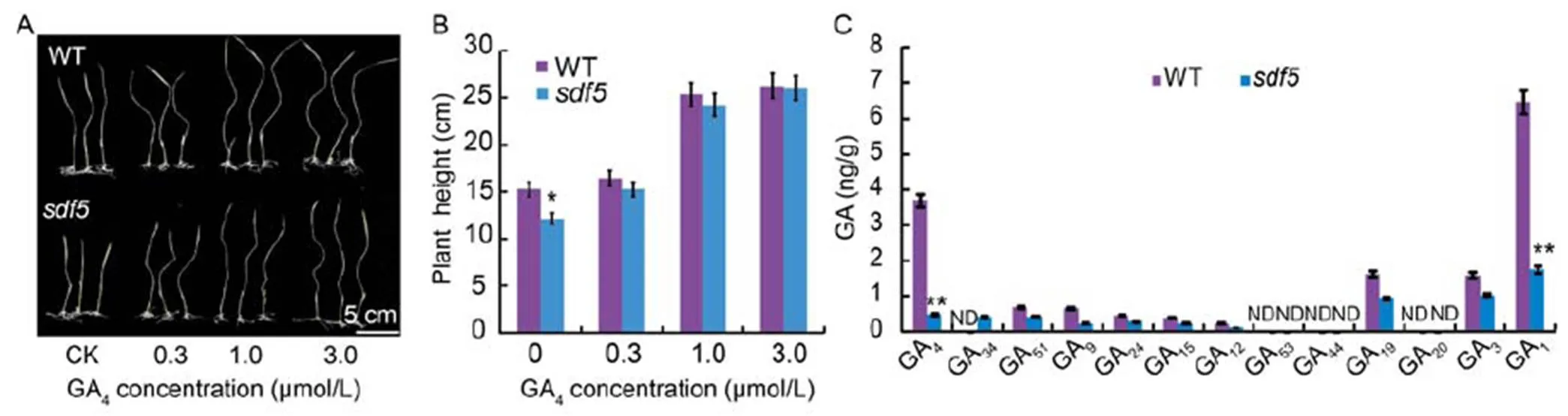
Fig. 2. Gibberellin (GA) compounds were tested in wild type (WT) andmutant.
A, Phenotypes of WT andmutant treated with different concentrations of GA4. Fifteen-day-old seedlings were subjected to 10 d of homone culture and their plant heights were measured. CK, Control. B, Height of WT andseedlings in different concentrations of GA4. C, Endogenous levels of GAs were measured in WT andmutant. ND indicates not detectable. Data are Mean ± SD (= 3). * and ** indicate significant differences at the 0.05 and 0.01 levels by the Student’s-test, respectively.
We further tested whether GA levels were affected in themutant. Indeed, GA biosynthesis was reduced in themutant. In particular, the levels of GA1and GA4were much lower in themutant compared with the wild type (Fig. 2-C). We analyzed the transcript levels involving GA biosynthetic pathway in the wild type andmutant, including the genes ent(),, ent(),(),,,and. We also analyzed(),and(), which are involved in GA signaling pathways. The expression levels of,,andwere reduced in themutant compared with the wild type, but,andwere not affected (Fig. S6). These results suggested that the ectopic expression ofrepressed GA biosynthesis.
We then investigated whether the induced expression ofmodulates the BR or auxin. Plants were treated with different concentrations of BR and benzoyl-CoA ligase (BZL). We detected no differences in the rate of stem elongation between the wild type and themutant, which suggested thatwas not involved in BR or indole-acetic acid biosynthesis (Fig. S5-E to -H, Fig. S7).
Plant height is an important agronomic trait in rice and is associated with crop yield, lodging and photosynthetic efficiency. In this study, we characterized the dominant semi-dwarf mutant, which is a T-DNA insertion mutant and induced the expression of. GA content was reduced in themutant, including GA biosynthetic components and the active GA species GA1and GA4. These results were confirmed by treatment with exogenous GAs. The semi-dwarf phenotype of themutant was rescued by active GA4, but not by GA3. GA3promoted the internode elongation in the wild type andmutant, but failed to recuse thephenotype. These results are consistent with the results of low GA4content inmutant. Our study showed that theis a new factor involved in GA biosynthesis. GA precursor GA12was reduced in themutant, suggesting thatmight be involved in the upstream of GA biosynthesis. Whyfunctions in GA4content but not in GA3remained to be studied.
Bothandencode the P450 proteins, which play roles in the growth of rice by reducing the biological activity of GA and are involved in the homeostasis of GA (Magome et al, 2013)encodes a P450 protein and belongs to the CYP51 sub-family, which was placed from sterol 14-α-demethylation. The CYP51 family was first characterized from yeast, followed by determination of the primary structure of several family members.is involved in the BR pathway andis targeted by the stress-responsive microRNA osa-miR1848 (Xia et al, 2015).
Several dominant dwarf materials have been reported, such as(Xia et al, 2015),(t)(Liu et al, 2009),(Wang et al, 2008),(Asano et al, 2009),(Sunohara et al, 2009),(Zhang et al, 2012) and(Liang et al, 2011). Most of these materials were obtained by mutagenesis or regeneration. Compared with recessive dwarf materials, dominant dwarf materials can reduce plant height in F1generation.has important applications in recurrent selection breeding. Whenis aggregated on the reincarnation parent, it can quickly reduce the height of the reincarnation parent. After the plant height is reduced, the reincarnation parents can better accept foreign pollen to increase the outcrossing rate of seeds.
ACKNOWLEDGeMENTS
This work was supported by the Natural Science Foundation of Anhui Province in China (Grant No. 1808085MC66), and the Program of Rice Genetic Breeding Key Laboratory of Anhui Province (Grant No. SDKF-2020-02) and University Synergy Innovation Program of Anhui Province (Grant No. GXXT- 2019-033) in China.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
File S1. Methods.
Fig. S1. Agronomic traits of wild type andmutant.
Fig. S2. Segregation ofin F2population with self-pollilation.
Fig. S3. Phylogenetic tree of CYP51 proteins.
Fig. S4. Expression pattern of.
Fig. S5. Wild type andmutant treated with gibberellin (GA3), paclobutrazol (PAC) and brassinosteriod (BR).
Fig. S6. Transcriptional analysis of gibberellic acid (GA) genes in wild type (WT) andmutant.
Fig. S7. Indoleacetic acid (IAA) in wild type andmutant.
药后30 d,30g/L甲基二磺隆4个剂量处理及对照药剂(CK)对野燕麦的鲜重防效分别为82.32%,88.65%,92.88%,97.36%,88.65%;对雀麦的鲜重防效分别为85.74%,90.13%,94.67%,97.81%,91.22%(表3)。
Table S1. Genetic analysis of.
Table S2. Primers used for gene over-expression, PCR and RT-qPCR analysis.
Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J. 2009., a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers., 50(8): 1416‒1424.
Asano K, Hirano K, Ueguchi-Tanaka M, Angeles-Shim R B, Komura T, Satoh H, Kitano H, Matsuoka M, Ashikari M. 2009. Isolation and characterization of dominant dwarf mutants,, in rice., 281: 223‒231.
Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A. 1999. Rice gibberellin-insensitive dwarf mutant geneencodes the α-subunit of GTP-binding protein.,96(18): 10284‒10289.
Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, Asahi T, Iwasaki Y. 1999. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice., 96: 7575‒7580.
Jiang L, Liu X, Xiong G S, Liu H H, Chen F L, Wang L, Meng X B, Liu G F, Yu H, Yuan Y D, Yi W, Zhao L H, Ma H L, He Y Z, Wu Z S, Melcher K, Qian Q, Xu H E, Wang Y H, Li J Y. 2013.acts as a repressor of strigolactone signalling in rice., 504: 401‒405.
Lin H, Wang R X, Qian Q, Yan M X, Meng X B, Fu Z M, Yan C Y, Jiang B, Su Z, Li J Y, Wang Y H. 2009., an iron- containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth., 21(5): 1512‒1525.
Liu B M, Wu Y J, Fu X D, Qian Q. 2009. Characterizations and molecular mapping of a novel dominant semi-dwarf gene(t) in rice ()., 127(2): 125‒130.
Magome H, Nomura T, Hanada A, Takeda-Kamiya N, Ohnishi T, Shinma Y, Katsumata T, Kawaide H, Kamiya Y, Yamaguchi S. 2013. CYP714B1 and CYP714B2 encode gibberellin 13-oxidases that reduce gibberellin activity in rice., 110(5): 1947‒1952.
Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M, Sato M, Nasu S, Minobe Y. 2002. Positional cloning of rice semidwarfing gene,: Rice ‘green revolution gene’ encodes a mutant enzyme involved in gibberellin synthesis., 9(1): 11‒17.
Osbourn A, Goss R J M, Field R A. 2011. The saponins: Polar isoprenoids with important and diverse biological activities., 28: 1261‒1268.
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush G S, Kitano H, Matsuoka M. 2002. A mutant gibberellin-synthesis gene in rice., 416(2): 701‒702.
Spielmeyer W, Ellis M H, Chandler P M. 2002. Semidwarf (), ‘green revolution’ rice, contains a defective gibberellin 20-oxidase gene., 99(13): 9043‒9048.
Sun L J, Li X J, Fu Y C, Zhu Z F, Tan L B, Liu F X, Sun X Y, Sun X W, Sun C Q. 2013., a member of the GRAS gene family, negatively regulates grain size in rice., 55(5): 938‒949.
Sunohara H, Kawai T, Shimizu-Sato S, Sato Y, Sato K, Kitano H. 2009. A dominant mutation ofencoding an α-tubulin protein causes severe dwarfism and right helical growth in rice., 84(3): 209‒218.
Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, Ashikari M, Iwasaki Y, Kitano H, Matsuoka M. 2000. Rice dwarf mutant, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction., 97(21): 11638‒11643.
Wang X, Yu H X, Tang D, Huang J, Gong Z Y, Cheng Z K. 2008. Genetic analysis of a dominant dwarf mutant in rice (L.)., 41: 3959‒3966. (in Chinese with English abstract)
Xia K F, Ou X J, Tang H D, Wang R, Wu P, Jia Y X, Wei X Y, Xu X L, Kang S H, Kim S K, Zhang M Y. 2015. Rice microRNA osa-miR1848 targets the obtusifoliol 14α-demethylase geneand mediates the biosynthesis of phytosterols and brassinosteroids during development and in response to stress., 208(3): 790‒802.
Yan H F, Saika H, Maekawa M, Takamure I, Tsutsumi N, Kyozuka J, Nakazono M. 2007. Rice tillering dwarf mutanthas increased leaf longevity during darkness-induced senescence or hydrogen peroxide-induced cell death., 82(4): 361‒366.
Zhang S Y, Li G, Fang J, Chen W Q, Jiang H P, Zou J H, Liu X, Zhao X F, Li X B, Chu C C, Xie Q, Jiang X N, Zhu L H. 2010. The interactions among, auxin and cytokinin underlie lateral bud outgrowth in rice., 52(7): 626‒638.
Zhang L G, Cheng Z J, Qin R Z, Qiu Y, Wang J L, Cui X K, Gu L F, Zhang X, Guo X P, Wang D, Jiang L, Wu C Y, Wang H Y, Cao X F, Wan J M. 2012. Identification and characterization of an Epi-allele ofreveals a regulatory linkage between two epigenetic marks in rice., 24(11): 4407‒4421.
Ding Yong (dingyong@ustc.edu.cn); Yang Jianbo (yjianbo@263.net)
31 July 2020;
18 November 2020
Copyright © 2021, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2021.05.001
——黔南扁穗雀麦
- Rice Science的其它文章
- Genome-Wide Association Study of Nitrogen Use Efficiency and Agronomic Traits in Upland Rice
- Development of Chromosome Segment Substitution Lines and Genetic Dissection of Grain Size Related Locus in Rice
- Water Management for Improvement of Rice Yield, Appearance Quality and Palatability with High Temperature During Ripening Period
- Effect of Milling and Parboiling Processes on Arsenic Species Distribution in Rice Grains
- iTRAQ-Based Proteomics Investigation of Critical Response Proteins in Embryo and Coleoptile During Rice Anaerobic Germination
- A New Approach to Select Doubled Haploid Rice Lines under Salinity Stress Using Indirect Selection Index

