Fasudil prevents liver fibrosis via activating natural killer cells and suppressing hepatic stellate cells
Qiu-Ju Han, Yong-Liang Mu, Hua-Jun Zhao, Rong-Rong Zhao, Quan-Juan Guo, Yu-Hang Su, Jia n Zhang
Abstract
Key Words: Liver fibrosis; Natural killer cells; Fasudil; Hepatic stellate cells
INTRODUCTION
Liver fibrosis is caused by inflammation and characterized by the accumulation of extracellular matrix (ECM), which are major characteristics of chronic liver diseases such as cirrhosis and primary hepatic carcinoma[1 ]. The traditional view is that liver fibrosis is irreversible, but recent studies have shown that liver fibrosis can be partially reversed in experimental models of liver fibrosis[2 ]. Unfortunately, to date, there is no clinically safe and efficient drug to treat liver fibrosis in humans.
The mechanisms underlying hepatic fibrosis are inflammatory reaction, hepatic stellate cell (HSC) activation, and the biological function of various cytokines[3 ]. HSCs,as hepatic lipid-storing cells, are sensitive to hepatic injury and participate in the pathogenesis of liver fibrosis[4 ]. During chronic diseases, agents from damaged hepatocytes or other cells can promote the activation of HSCs. Then these activated HSCs become highly proliferative myofibroblast-like cells, exhibiting a migratory phenotype and producing large amounts of extracellular matrix proteins such as collagens and fibronectin, thereby leading to the development of hepatic fibrosis[5 ,6 ].Therefore, HSC-specific targeted strategies are required for the effective treatment of liver fibrosis.
Immune cells in the liver exert different roles during the pathogenesis of liver fibrosis. Hepatic macrophages promote fibrosis by perpetuating an inflammatory phase, which leads to the release of pro-inflammatory cytokines and chemokines, and then the activation of HSCs[7 ]. T helper type 2 (Th2 ) cells can produce cytokines,including interleukin (IL)-13 , IL-4 , and IL-5 , which are shown to promote fibrosis[8 ].Natural killer (NK) cells suppress the pathogenesis of liver fibrosis by killing HSCs and producing interferon-γ (IFN-γ). Also, NK cells can induce apoptosis of HSCs and help clear senescent-activated HSCs, thereby facilitating the resolution of fibrosis,while the exact mechanisms remain unknown. NK cells are suppressed during the advanced stage of liver fibrosis in mice and patients[9 ,10 ]. Thus, properly regulating the activation of intrahepatic immune cells is essential for treating liver fibrosis.Practically, the restoration and promotion of NK cell activity might be an attractive strategy for the regression of liver fibrosis.
Ras homology family member A (RhoA) and its major downstream effector Rhoassociated protein kinase (ROCK) play important roles in several downstream effects of the small GTP-binding protein Rho. Several cellular events including adhesion,motility, and contractility are regulated by Rho kinase[11 ]. Furthermore, increasing evidence has demonstrated that Rho kinase inhibition can improve liver fibrosis. The Rho kinase, Y27632 , decreases fibrotic parameters in models of liver fibrosis, and inhibits the activation status of the primary HSCs[12 ]. Additionally, RhoA-ROCK signaling pathway also plays a key role in regulating the function of immune cells. For example, the RhoA-ROCK pathway promotes the activation of downstream chemokine receptors. Therefore, T-cell activation, polarization, and migration are promoted by chemokines[13 ]. Fasudil, as a first-generation selective Rho/ROCK inhibitor in the clinic, is clinically used to improve brain microcirculation and promote nerve regeneration[14 ]. More importantly, Fasudil can prevent lung and skin fibroses in hypochlorous acid-injected mice[15 ]. Recently, Fasudil was shown to exert antiinflammatory effects and markedly reduce the accumulation of ECM in type 1 diabetic rats[16 ]. However, the viability of Fasudil for liver fibrosis therapy and the associated mechanisms are still unclear. The regulatory effects of Fasudil on NK cells in liver fibrosis have not been fully explained.
In this study, we determined the effects of Fasudil on the progression of liver fibrosis and clarified the related mechanisms. We found that Fasudil performed antiproliferative effects in a mouse model of thioacetamide (TAA)-induced liver fibrosis,providing a feasible solution for the clinical treatment of liver fibrosis.
MATERIALS AND METHODS
Animal model
C57 BL/6 mice (male, 4 -6 wk old) were provided by HuaFuKang Biological Technology Co., Ltd. (Beijing, China). The animals were caged under specific pathogen-free conditions, housed under a controlled temperature 23 ± 1 °C and relative humidity 45 %. The animal model of hepatic fibrosis was induced by administration of TAA at 200 mg/kg (T104039 ; Aladdin, Shanghai, China) once every 3 d for 12 times[17 ]. At 1 wk after induction with TAA, Fasudil (10 mg/kg) was intraperitoneally injected once a day for 3 wk. The procedures were approved by the Research Ethics Committee of Shandong University (Jinan, China).
Cell line and reagents
LX-2 , an immortalized human hepatic stellate cell line preserved in our laboratory,was grown in Dulbecco’s modified Eagle medium (Thermo Fisher Scientific, Inc.,Waltham, MA, United States). This cell line carries a typical phenotype and biochemical properties of activated HSCs, and was checked by referring to the International Cell Line Authentication Committee and National Center for Biotechnology Information databases[18 ]. Fasudil was purchased from Tianjin Chase Sun Pharmaceutical Co. Ltd. (Tianjin Shi, China).
Hematoxylin and eosin staining
In a TAA-induced hepatic fibrosis model, the mice were sacrificed under mild ether anesthesia after Fasudil treatment. Then mouse liver specimens were fixed overnight in 4 % paraformaldehyde/phosphate-buffered saline (PBS) and embedded in paraffin.Then liver sections were examined and photographed under a light microscope after hematoxylin and eosin (Beyotime, Shanghai, China) staining. The criteria used for scoring fibrosis and inflammation were as follows: Score 0 , normal (no visible fibrosis and inflammation); score 1 , fibrosis and inflammation present (5 %-30 %); score 2 , mild fibrosis and inflammation (31 %-50 %); and score 3 , severe fibrosis and inflammation(51 %-75 %).
Sirius red staining
Liver specimens were stained with 0 .4 % sirius red in saturated picric acid for 0 .5 h.The positively stained area was selected by threshold adjustment on a gray scale picture using Image J software (NIH, Bethesda, MD, United States) and the ratio of positively stained area/total area was then calculated. At least five different fields on each slide were measured. The stained area were determined using Image-pro Plus 6 .0 software.
RNA extraction and real-time reverse transcription-polymerase chain reaction
The treated cells were added to TRIzol (Invitrogen, Thermo Fisher Scientific, Inc.), and the total RNA was extracted from cells following the manufacturer’s instructions. Then cDNA was obtained and synthesized using M-MLV Reverse Transcriptase (Invitrogen;Thermo Fisher scientific, Inc.). The mRNA levels of matrix metalloproteinase 2 (MMP-2 ), MMP-9 , transforming growth factor beta (TGF-β), B-cell lymphoma 2 (Bcl-2 ), Bcl-2 -associated X protein (Bax), and Ki67 were detected according to the SYBR Green Master Mix kit instructions (Roche Diagnostics, Indianapolis, IN, United States). The primer sequences for quantitative polymerase chain reaction (qPCR) amplification are listed in Table 1 . PCR was conducted using the iCycleriQ real-time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, United States).
Cell cycle analysis and apoptosis assay
After treatment, the cell cycle distribution of HSCs seeded was assayed by flow cytometry. Briefly, HSCs (1 .5 × 105 ) were plated in 12 -well plates and treated with Fasudil at the indicated concentrations (5 and 10 mM). After 24 h, cells were harvested and washed with ice-cold PBS. Then they were fixed in cold 70 % ethanol and stored at 4 °C overnight, and the fixed cells were washed and re-suspended in 1 mL staining solution containing 50 mg/mL propidium iodide (PI) (Beijing Solarbio Science &Technology, Co., Ltd.) and 100 mg/mL RNase for 30 min at 37 °C. Then cell cycle analysis was conducted using a flow cytometer (BD Calibur).
11.燕京地毯厂——为男童提供工作(Hou Ku Lo Yuan和Ho kung Hsiao Tsai Yuan)
FITC-Annexin V/PI (eBioscience; Thermo Fisher Scientific, Inc.) was utilized to determine rates of apoptosis. LX2 cells were plated and treated with Fasudil (5 mM, 10 mM) for 24 h. Cells were harvested and stained following the manufacturer’s instructions. Finally, the flow cytometry data were analyzed using WinMDI.
Western blotting
Protein was extracted from LX-2 cells, NK-92 cells, or liver tissues with RIPA protein lysis buffer (1 % Triton X-100 , 1 % deoxycholate, 0 .1 % sodium dodecyl sulfate [SDS])(Beyotime, Shanghai, China). The protein concentration of each sample was determined by the BCA assay. Proteins (30 μg/lane) were separated by SDS-polyacrylamide gel electrophoresis using a 10 % polyacrylamide gel, and then transferred to PVDF membranes (Millipore, Billerica, MA, United States). The following primary antibodies were used: Anti-phosphorylated extracellular signal-related kinase (p-ERK)(#14227 S; Cell Signaling Technology, Danvers, MA, USA), anti-ERK (#4348 S, Cell signaling Technology), alpha smooth muscle actin (α-SMA) (#4668 S; Cell signaling Technology), Collagen I (WL008 ; Wanleibio Co., Ltd., Shenyang, China), and β-actin(#3700 ; Cell signaling Technology). For ECL detection, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (#A0208 ;Beyotime, Shanghai, China) at a dilution of 1 :10000 for 1 h at 37 °C. ECL detection was performed according to the manufacturer’s protocol (Millipore). Analysis of blots was performed using Image J.
Isolation of liver mononuclear cells and flow cytometry
Mice were euthanized and the liver tissues were harvested, and then intrahepatic liver mononuclear cells were isolated as indicated previously[19 ]. Antibodies used for fluorescence-activated cell sorting (FACS) analysis were PerCP-Cy5 .5 anti-cluster of differentiation 3 (CD3 ) (#35 -0031 -82 ), FITC anti-NK1 .1 (#11 -5941 -82 ), PE anti-CD69 (#12 -0691 -82 ), PE anti-NKG2 A (#12 -5897 -81 ), PE anti-NKG2 D (#12 -5882 -82 ), or isotype-matched controls (eBioscience, San Diego, CA, United States). Cells were analyzed on a FACSCalibur flow cytometer, and then analyzed using WinMDI analysis software.
Cytolysis assay
The cytolytic activity of NK-92 cells against LX2 was determined using the MTT assay.Briefly, 1 × 104 target cells (LX2 cells) were seeded into 96 -well plates. NK-92 cells,pretreated with Fasudil (10 mM) overnight, were added to target cells at effector/target ratios of 1 :20 , 1 :10 , or 1 :5 . Then the effector and target cell were coincubated for 12 h, and then 20 μL MTT (5 mg/mL) were added. After 4 h, the absorbance (A) at 490 nm was determined using a scanning multi-well spectrophotometer (Bio-Rad). The percent specific lysis was calculated using the following formula: % specific lysis =1 - (ODE+T- ODE) / ODT × 100 %.
Statistical analyses
Data are shown as mean ± SD of three independent experiments. Significant differences were analyzed using the Student’st-test or one-way analysis of variance.Statistical analyses were conducted using GraphPad Prism (version 5 .0 a). aP < 0 .05 ,bP< 0 .01 , cP < 0 .001 .
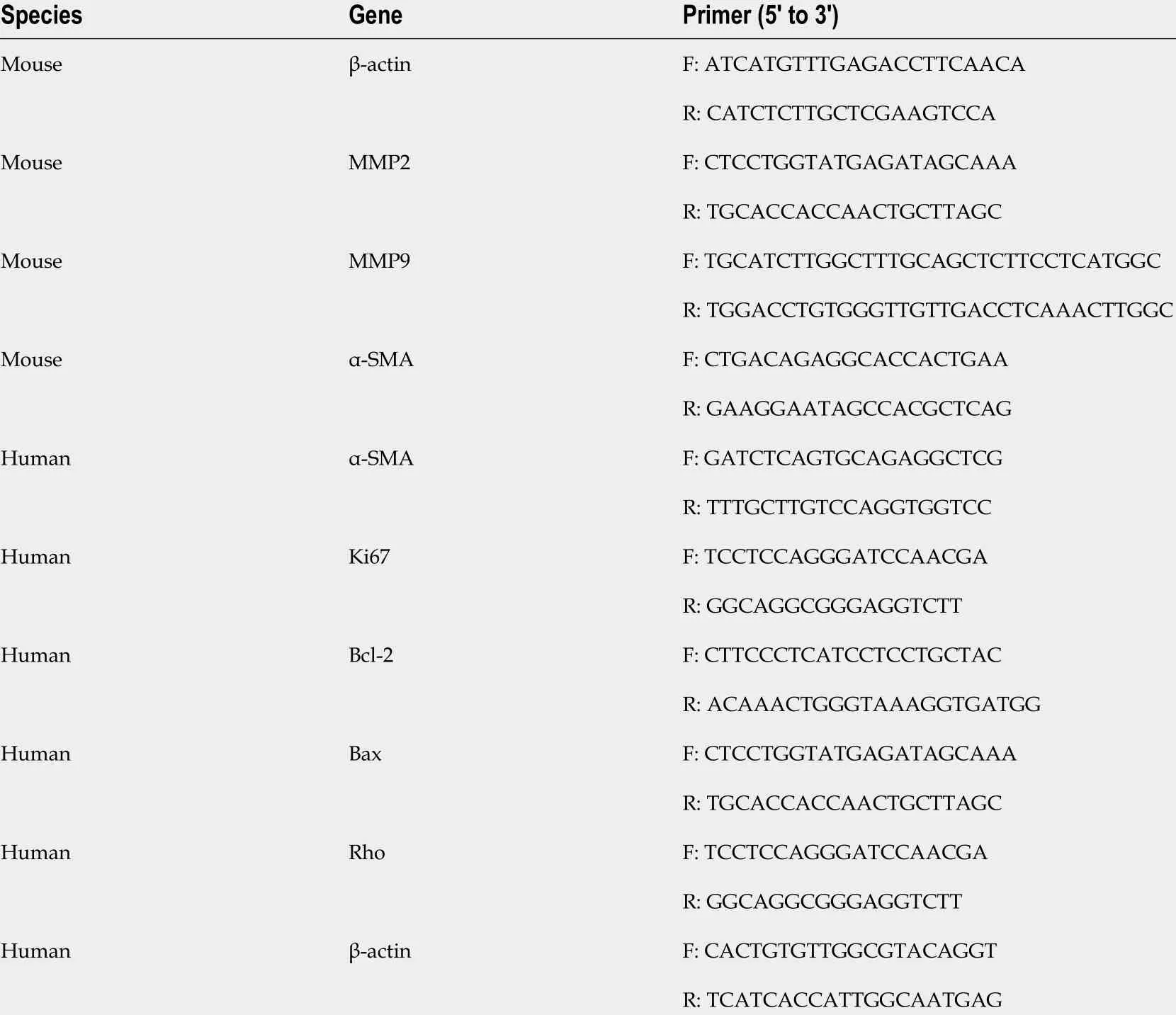
Table 1 Primers used in this study
RESULTS
Fasudil protects TAA-induced liver injury
To clarify whether Fasudil influences hepatic fibrosis, a fibrotic mouse model was induced by intraperitoneal injection of TAA (200 mg/mL) once time every 3 d[11 ]. At 7 d after induction with TAA, Fasudil (10 mg/kg) was injected once a day for 3 wk,and then mice were sacrificed (Figure 1 A). As shown in Figure 1 B, compared with the normal gross features of the livers observed in the control group, the liver surface from the TAA-treated group showed several spots of nodules, while the livers from the TAA + Fasudil group had much fewer nodular spots. There was no significant difference in liver/body ratio among these three groups. Meanwhile, the liver of the TAA-treated group exhibited a lobular structure destruction and inflammatory cell infiltration. Also, livers from TAA-treated mice gained loss of structural integrity,while TAA + Fasudil-treated mice revealed structural integrity of liver with no significant inflammatory cell infiltration (Figure 1 C). Fasudil significantly lowered the indices of liver (Table 2 ). Furthermore, we observed a significant reduction of alanine aminotransferase and aspartate aminotransferase in serum after Fasudil treatment compared with TAA-treated mice (P< 0 .05 ; Figure 1 D). These data suggest that Fasudil might blunt TAA-induced liver fibrosis by preventing liver injury.
Fasudil prevents TAA-induced liver fibrosis in vivo
Hepatic collagen I deposition is an important characteristic of liver fibrosis level in mice[20 ]. As shown in Figure 2 A, sirius red staining exhibited a significant increase in perisinusoidal collagen fibers in the TAA-treated group, which was significantly decreased by Fasudil treatment (P< 0 .05 ). Furthermore, fibrosis-related genes such as α-SMA, MMP-2 /9 , and TGF-β1 [21 ] were analyzed to evaluate the impact of Fasudil on hepatic fibrosis. As shown in Figure 2 B, the expression of α-SMA and collagen I inTAA + Fasudil-treated mice was significantly lower than that in TAA-treated group.Meanwhile, mRNA levels of MMP-2 and MMP-9 were also downregulated by Fasudil treatment (Figure 2 C). TGF-β1 induced by TAA was significantly decreased at both the mRNA and protein levels after Fasudil treatment (P< 0 .05 ; Figure 2 D). These data clearly demonstrate that TAA-induced liver fibrogenesis is markedly inhibited by Fasudil treatment.
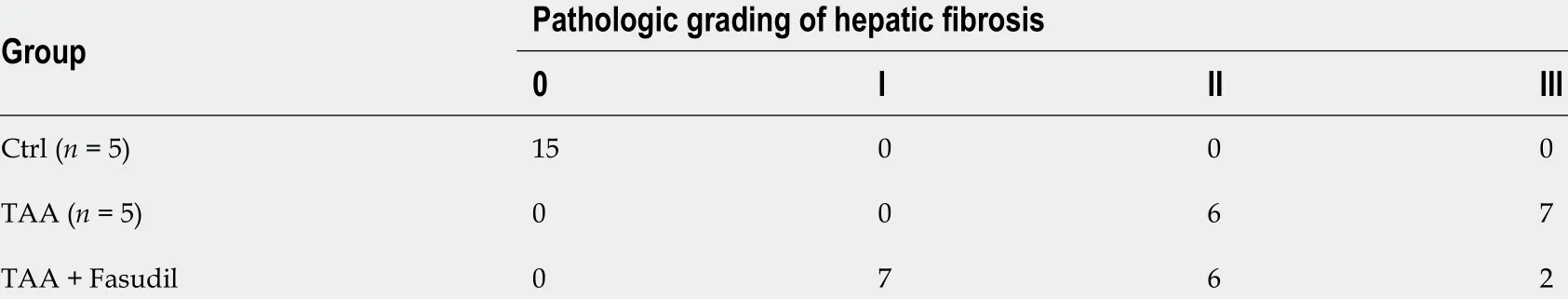
Table 2 Effect of Fasudil on the pathologic grading of hepatic fibrosis mice induce by thioacetamide
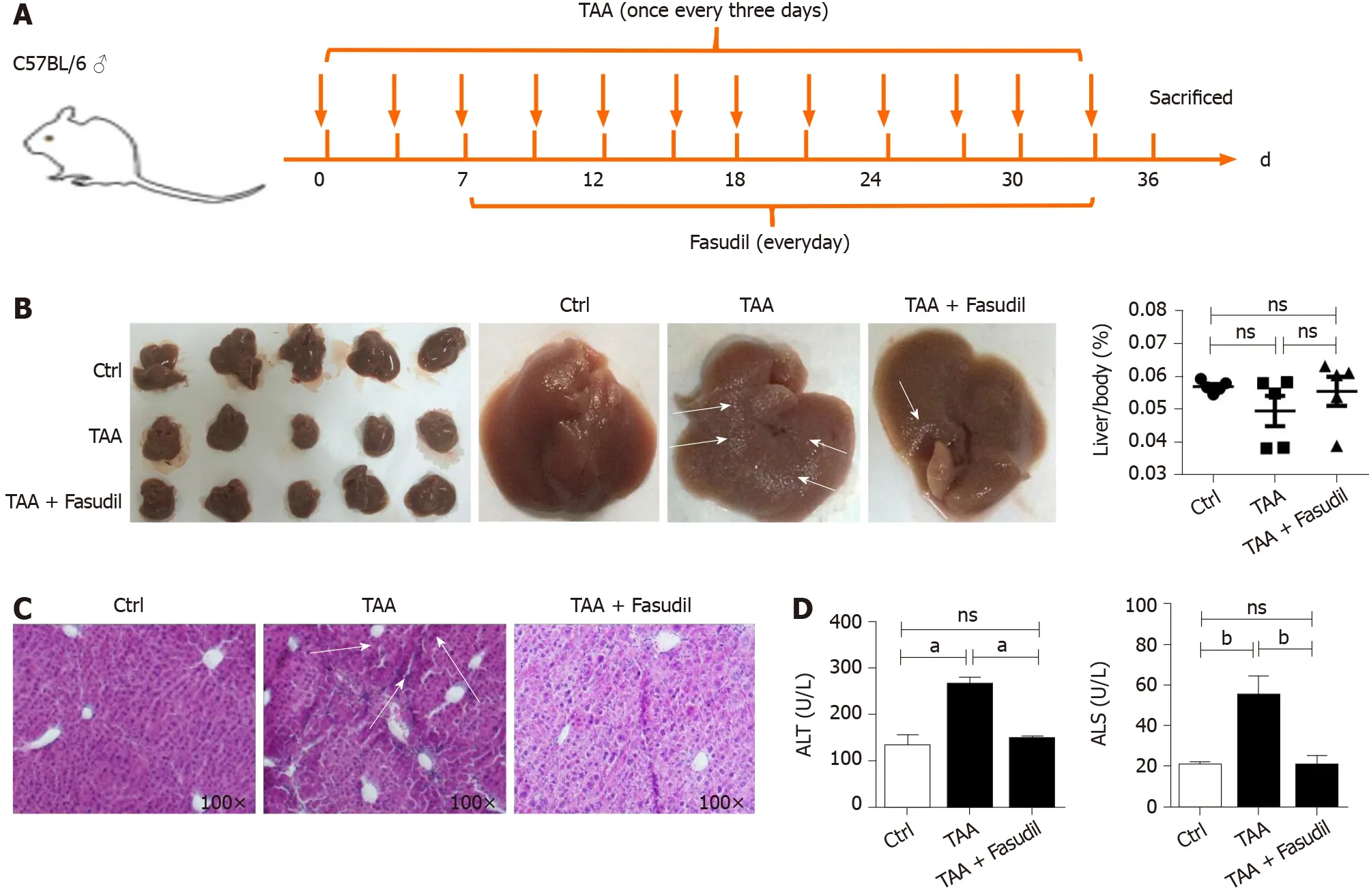
Figure 1 Fasudil protects thioacetamide-induced liver injury. A: Establishment of a liver fibrosis mouse model; B: Representative detection of liver images is shown. Gross pathological examination of liver taken from normal control, thioacetamide (TAA)-treated, and TAA + Fasudil-treated groups, and the liver/body ratio was assayed among these three groups; C: Histological observation of liver fibrosis by hematoxylin and eosin staining under light-field microscope with × 100 magnification; D: Enzyme-linked immunoassay was used to assess the level of serum alanine aminotransferase and aspartate aminotransferase. Data represent the mean ± SD from at least three independent experiments (n = 5 /group). aP < 0 .05 , bP < 0 .01 .
NK cells are activated by Fasudil in a TAA-induced fibrotic mouse model
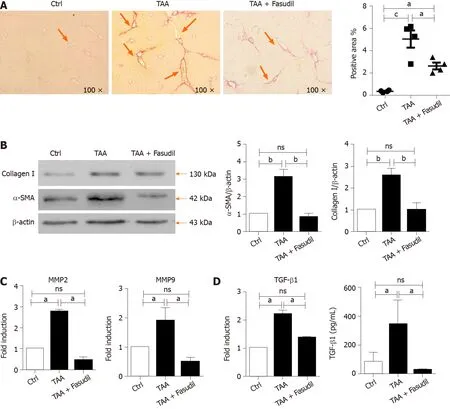
Figure 2 Fasudil prevents thioacetamide-induced liver fibrosis. A: Histological observation of collagen deposition by sirius red staining under a light-field microscope with × 100 magnification (left). Quantitative analysis of sirius red staining of each group (right); B: Expression of alpha smooth muscle actin (a-SMA) and collagen I in liver tissues was assayed by western blotting. Right panel shows the quantification of α-SMA and collagen I protein levels; C: Levels of matrix metalloproteinase 2 (MMP2 ) and MMP9 mRNA in liver tissues were detected by real time-reverse transcription polymerase chain reaction (RT-PCR); D: Hepatic expression of transforming growth factor beta 1 (TGF-β1 ) mRNA was detected by real time-RT-PCR, the secretion level of TGF-β1 in serum was assayed by enzymelinked immunoassay. Data represent the mean ± SD from at least three independent experiments. aP < 0 .05 , bP < 0 .01 .
Immune cells in the liver play critical roles during the development of liver fibrosis[22 ]. As shown in Figure 3 A, TAA treatment increased the total number of mononuclear cells in the liver compared to the normal control group, while no significant differences were observed between TAA-treated and TAA + Fasudiltreated groups. NK cells suppress liver fibrosisviastrong cytolysis against HSCs[23 ,24 ], and impaired NK cell may contribute to accelerated liver fibrosis progression in a TAA-induced fibrotic mouse model. Here, the proportion of NK cells was not significantly affected after Fasudil treatment (Figure 3 B), while the level of the activation marker CD69 was markedly increased in NK cells from TAA + Fasudiltreated mice than that from TAA-treated mice (P< 0 .05 ; Figure 3 C), accompanied by the elevation of activation receptor NKG2 D (Figure 3 D). Meanwhile, the proportion of CD69 +CD8 +T cells (Figure 3 E) and CD69 +CD4 +T cells (Figure 3 F) in the liver was not significantly affected by Fasudil treatment. These data suggest that NK cells might be activated by Fasudil and exhibit anti-liver fibrosis effects in a TAA-induced fibrotic mouse model.
Fasudil promotes NK cell activation via ERK and nuclear factor kappa B signaling pathways
To investigate the mechanism by which Fasudil treatment suppresses the progression of liver fibrosis by activating NK cells, human NK-92 cells were treated with different concentrations of Fasudil (5 mM, 10 mM) for 24 h in vitro, and the concentration of 10 mM was equivalent toin vivoexperiments. Then the levels of ERK, nuclear factor kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3 ) were examined by western blotting. The levels of p-ERK and p-NF-kB were significantly increased in Fasudil-treated NK-92 cells, while activation of the STAT3 signaling pathway was not affected by Fasudil (Figure 4 A). To determine the effects of NK cells activated by Fasudil on HSCs, we assayed the lysis of LX2 cells (human HSC cell line)by NK cells at different effector: target ratios. First, we treated NK-92 cells with Fasudil(10 mM) for 24 h, and assayed the cytotoxicity against cultured LX2 cells. We found that Fasudil treatment increased the lysis activity of NK cells against cultured LX2 cells(Figure 4 B). Then, liver mononuclear cells from normal mice were isolated and treated with different concentrations of Fasudil for 24 hin vitro. FACS analysis showed the frequency of CD69 + NK cells increased after Fasudil treatment (Figure 4 C).Furthermore, the activation receptor NKG2 D was enhanced in NK cells, while the inhibitory receptor NKG2 A was not significantly influenced by Fasudil treatment(Figure 4 D). In addition, the proportion of IFN-γ+NK cells was increased after Fasudil treatment (Figure 4 E).
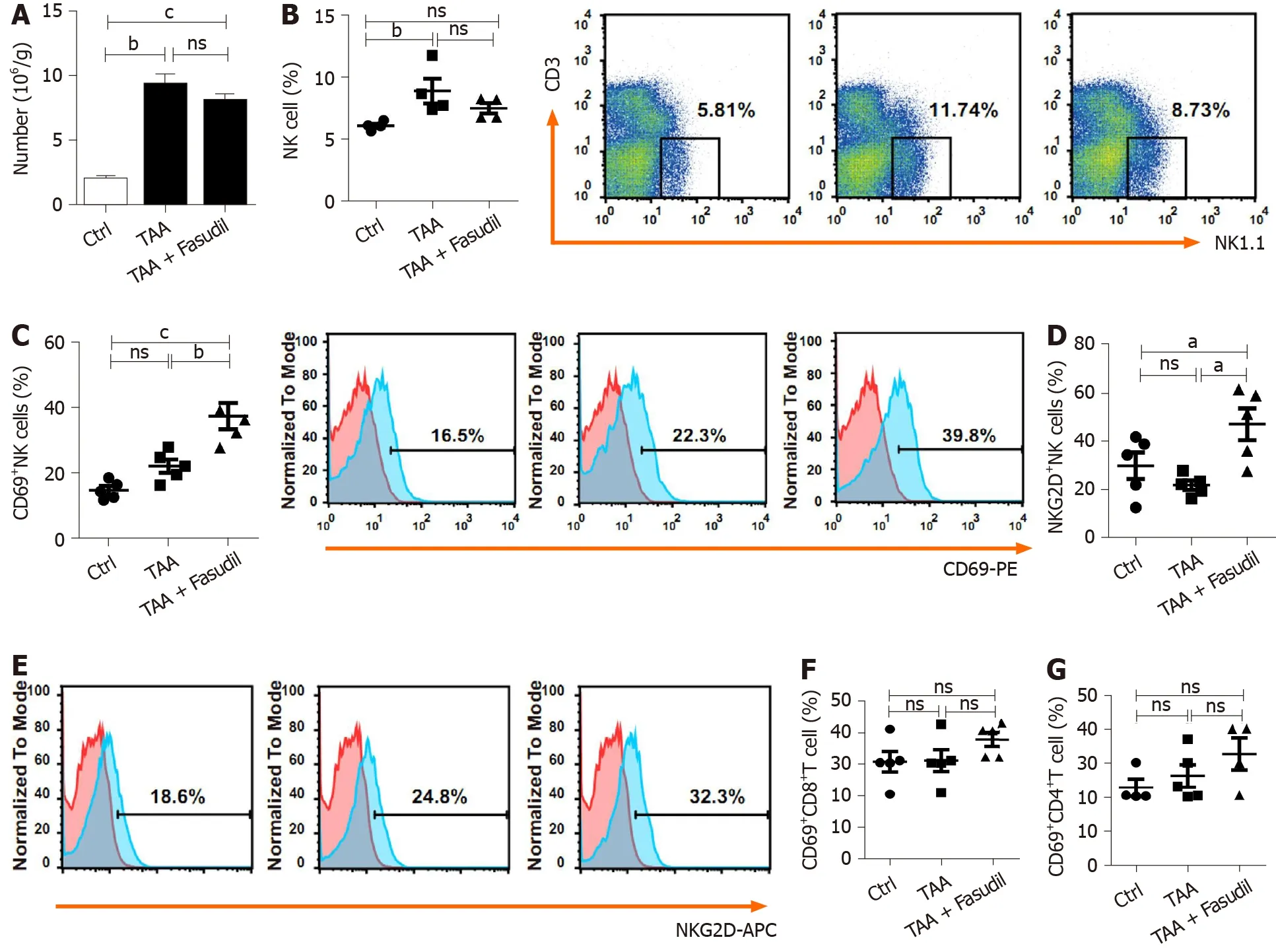
Figure 3 Natural killer cells are activated in a Fasudil-treated liver fibrotic mouse model. After the last treatment, the mice were sacrificed and the intrahepatic lymphocytes were isolated. A: Absolute number of mononuclear cells in the liver from the normal control, thioacetamide (TAA)-induced, and TAA +Fasudil groups was assessed; B-G: Then the percentages and representative fluorescence-activated cell sorting (FACS) plots of natural killer (NK) cells among lymphocytes (B), CD69 + NK cells and representative FACS plots (C), quantification of NKG2 D+ NK cells (D), representative FACS plots of NKG2 D on NK cells (E),CD69 +CD8 + T cells (F) and CD69 +CD4 + T cells (G) in liver from the above three groups were analyzed by FACS. Data represent the mean ± SD from at least three independent experiments (n = 5 /group). aP < 0 .05 , bP < 0 .01 , cP < 0 .001 .
Taken together, these results demonstrate that Fasudil can promote NK cell activation and cytotoxicity by activating the ERK and NF-κB signaling pathways.
Fasudil inhibits proliferation but promotes the apoptosis of HSCs
The proliferation and activation of HSCs are one of the main triggers of liver fibrosis[25 ]. To observe the direct effects of Fasudil on HSCs, LX2 cells were treated with different concentrations of Fasudil (5 mM, 10 mM) for 24 hin vitro, and then the survival of these HSCs was observed. As shown in Figure 5 A, the percentage of apoptotic HSCs was promoted by Fasudil treatment. Meanwhile, the level of the antiapoptotic gene Bcl-2 was decreased, while the level of the pro-apoptotic gene Bax was induced in LX2 cells treated with Fasudil (Figure 5 B), accompanied by an increase in p-ERK level (Figure 5 C). Furthermore, Fasudil arrested the HSC cell cycle in the G1 phase (Figure 5 D). Accordingly, the proliferation-related gene Ki67 in LX2 cells was downregulated (Figure 5 E). Previous studies have reported that RhoA is involved in expression of the fibrosis-associated protein α-SMA[26 ]. Fasudil is a RhoA kinase inhibitor; we found that Fasudil decreased the expression of α-SMA (Figure 5 E and F)accompanied by the downregulation of RhoA (Figure 5 G). These results indicate that Fasudil has a direct inhibitory effect on HSCs.
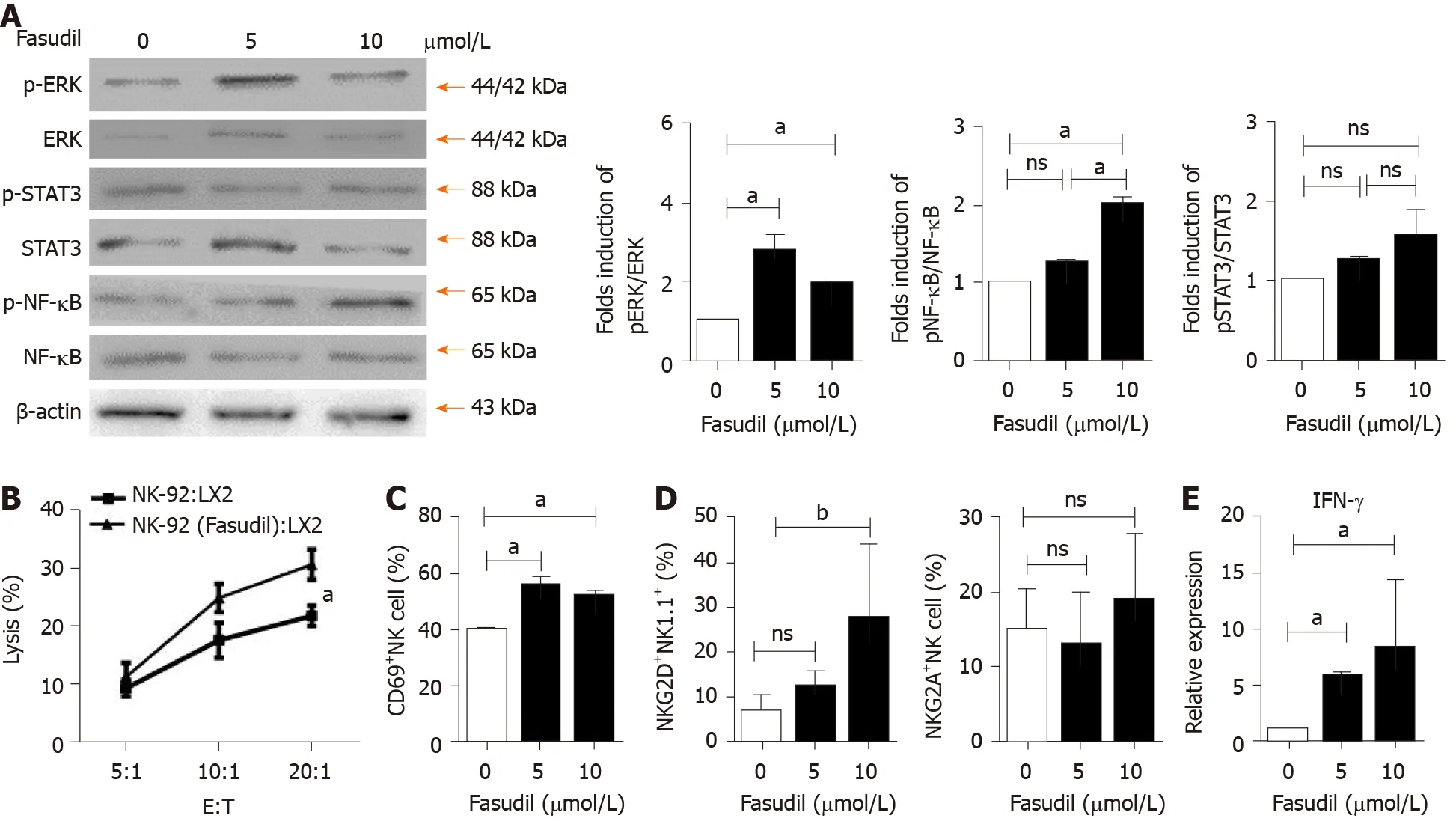
Figure 4 Fasudil can promote natural killer cell activation via extracellular signal-related kinase and nuclear factor kappa B signaling pathways. A: Natural killer (NK)-92 cells were treated with different concentrations of Fasudil (5 mM, 10 mM) for 24 h in vitro, and extracellular signal-related kinase(ERK), nuclear factor kappa B (NF-kB), and signal transducer and activator of transcription 3 (STAT3 ) levels were detected by western blotting. Then mononuclear cells from the livers of normal mice were isolated and then treated with Fasudil (5 mM, 10 mM) for 24 h in vitro; B: 1 × 104 LX2 cells were seeded into 96 -well plates.NK-92 cells pretreated with Fasudil (10 mM) were added at different ratios (1 :20 , 1 :10 , or 1 :5 ) for 12 h. Then the cytotoxicity was analyzed; C-E: Frequencies of CD69 + NK cells (C), NKG2 D NK cells, and NKG2 A NK cells (D), as well as the proportion of IFN-γ-producing NK cells (D) were analyzed by FACS (E). Data represent the mean ± SD from at least three independent experiments. aP < 0 .05 , bP < 0 .01 , cP < 0 .001 .
DISCUSSION
The current therapeutic interventions for liver fibrosis include removing the injurious stimuli, decreasing hepatic inflammation, suppressing HSC activation, and accelerating matrix degradation[27 ]. Repurposing old drugs for new clinical applications is a superior strategy for drug development. Recently, Rilpivirine, a widely used anti-HIV drug, was found to ameliorate liver fibrosis through selective STAT1 -dependent induction of HSC apoptosis[28 ]. So, repositioning existing drugs might be an effective strategy for obtaining therapeutics against liver fibrosis.
Fasudil, a RhoA kinase inhibitor, is clinically applied for improving brain microcirculation and promoting nerve regeneration. Xieet al[16 ] found that RhoA/ROCK signaling pathway activation plays an important role in the development of diabetic hepatic fibrosis. Interestingly, the RhoA/ROCK-2 signaling pathway is necessary for activated HSC; the administration of RhoA/ROCK inhibitor can exert anti-inflammatory effects, markedly decrease collagen synthesis, and delay hepatic fibrosis[16 ,29 ]. In accordance with this, we found that liver injury and liver fibrosis induced by repeated injection of TAA were prevented by Fasudil treatment (Figures 1 and 2 ). In addition, several studies have proved that RhoA/ROCK pathway activation promotes TGF-β1 secretion in several models[30 ]. As a RhoA/ROCK inhibitor, Fasudil inhibited the induction of TGF-β1 secretion in TAA-induced liver fibrosis, consistent with previous studies.
The immune response plays important roles in the development and treatment of liver fibrosis[31 ]. NK cells can promote the secretion of IFN-γ through the Janus kinase-STAT signaling pathway, thereby promoting the killing of HSCs[32 ]. The inhibition of NK cells cytotoxicity promotes the accumulation of LX2 cells[33 ]. In this study, with the elevation of CD69 and NKG2 D, hepatic NK cell activation was promoted by Fasudil treatment (Figure 3 ). Additionally, NK cells were activated in spleen from mice with Fasudil treatment (data not shown). ERK and NF-κB signals are critical for NK cell-mediated cytolysis of target cells[34 ]. Here, we found that Fasudil drove ERK and NF-κB activation, and augmented the cytolysis activity of NK cells against HSCs. In addition, Fasudil promoted the secretion of IFN-γ in NK cells(Figure 4 ). Although STAT3 activation is essential for NK cells to exert anti-tumor effects[35 ], it was not significantly influenced by Fasudil treatment (Figure 4 ). These results demonstrate the impact of Fasudil on NK cell function in fibrotic mouse model.
In terms of T cells, different T-cell subpopulations play different roles in the process of liver fibrosis. Previous studies have reported that T-cell impairment generally does not alter fibrogenesis, while Th2 and Th17 CD4+T cells are involved in the inflammatory process, promoting cytokine production and HSC activation[36 ]. Here, the ratio of CD4 + T cells and CD8+T cells was upregulated in the liver and spleen (data not shown), but the activation of these T cells was not significantly affected by Fasudil treatment (Figure 3 ). The exact roles of T cell subtypes in Fasudil-treated liver fibrosis need to be further investigated.
HSCs play a vital role in the pathogenesis of liver fibrosis. They can secret fibrogenic factors and then promote several kinds of cell types including portal fibrocytes,fibroblasts, and bone marrow-derived myofibroblasts to produce collagen and thereby propagate fibrosis[4 ,37 ]. Interestingly, we found that Fasudil treatment significantly increased apoptosis and inhibited the proliferation of LX2 cells, indicating the direct effect of Fasudil on HSCs (Figure 5 ), consistent with previous studies[38 ]. Actually, the effect of ROCK signal pathway on apoptosis is complicated. For example, inhibition of the RhoA/ROCK signaling pathway promotes the apoptosis of human urethral scar fibroblasts[38 ], cardiac fibroblasts[39 ], and neutrophils[40 ]. However, the decrease of apoptosis by RhoA/ROCK inhibition is also found in several cell types, such as nitrergic neurons[41 ] and progenitor cells[42 ]. RhoA is positively correlated with the expression of α-SMA, a gene involved in liver fibrosis[43 ]. Indeed, Fasudil, as an inhibitor targeting RhoA kinases, significantly inhibited both the expression of RhoA and α-SMA in HSCs. In addition, ERK is an important mediator of signal transduction in HSCs, which is involved in the growth, differentiation, survival, and apoptosis of HSCs[44 ]. In line with previous studies, we observed an increase in the p-ERK level by Fasudil treatment, which would promote HSC apoptosis. Thus, Fasudil directly disrupts HSC activation and survival in TAA-induced liver fibrosis.
CONCLUSION
In conclusion, our findings demonstrated that Fasudil prevented TAA-induced liver fibrosis by directly suppressing the cell cycle and activation of HSCs, and activating NK cells. Because Fasudil is a drug that has been used clinically, it has high safety and reliability and can provide a feasible solution for clinical treatment of liver fibrosis.
ARTICLE HIGHLIGHTS
 World Journal of Gastroenterology2021年24期
World Journal of Gastroenterology2021年24期
- World Journal of Gastroenterology的其它文章
- COVID-19 and its effects on the digestive system
- Weight loss interventions in living donor liver transplantation as a tool in expanding the donor pool: A systematic review and meta-analysis
- Disorders of the brain-gut interaction and eating disorders
- Stem cell injection for complex anal fistula in Crohn’s disease: A single-center experience
- Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis
- Chronic intestinal failure and short bowel syndrome in Crohn’s disease
