鲤病毒性疾病发生与防控的研究进展
蒋昕彧 王啸宇 裴超 周勇 李鹏飞 孔祥会

摘要:鯉(Cyprinus carpio L.)是我国重要的水产养殖品种之一,但随着养殖密度和养殖规模的不断扩大,其养殖生态系统受到不同程度破坏,各类疾病频繁暴发,其中又以病毒性疾病的影响范围最广、死亡率最高,已成为制约鲤养殖产业可持续健康发展的瓶颈问题。文章通过综述鲤春病毒血症(Spring viraemia of carp,SVC)、锦鲤疱疹病毒病(Koi herpesvirus disease,KHVD)、鲤病毒性浮肿病(Viral edema of carp disease,VEC)和鲤痘疮病毒病(Carp pox disease,CPD)等病毒性疾病的病原生物学、发生机理、流行特点及其防控策略,发现鲤病毒性疾病仍存在诸多问题亟待深入研究,包括KHV可感染多种鲤科近缘鱼类,但为何只发展成为病毒携带者而不发病;养殖水温是否是决定病毒感染的关键因素;病毒可通过哪些途径逃避免疫监视而进入鱼体;在不同感染阶段病毒的传播方式是否存在差异等。此外,由于目前尚缺乏高效的防控方法,加强检疫和免疫防控仍是防控鲤病毒性疾病的主要手段。因此,今后要继续加强对病毒致病机理和传染途径的研究,研发新型口服疫苗载体及传送系统,并基于RNAi技术研制能有效防治病毒性疾病的药物,以确保我国鲤养殖产业的健康可持续发展。
关键词: 鲤;病毒性疾病;流行特点;防控策略;SVC;KHVD;VEC;CPD
中图分类号: S941.41 文献标志码: A 文章编号:2095-1191(2021)02-0509-09
Abstract:Common carp(Cyprinus carpio L.) is an important economic fish species in China. With the gradual increase of breeding range and density in aquaculture, the aquaculture ecosystems are being damaged to varying degrees, the diseases frequently break out in common carp. Among them, the influence range caused by viral diseases is the most extensive, and the mortality is the highest. It has become a bottleneck problem restricting the sustainable and healthy development of carp breeding industry. In this paper, the pathogen biology, occurrence, epidemic characters and prevention and control strategies of viral diseases in common carp such as spring viraemia of carp(SVC), koi herpesvirus disease(KHVD), viral edema of carp disease(VEC) and carp pox disease(CPD) were summarized, and the future research trends of viral diseases were prospected. There are still many problems to be further studied, such as why KHV could infect a variety of Cyprinidae related fishes, but the fish only carried virus not becoming a disease-causing? Whether the water temperature is a key factor for virus infection? How can viruses escape immune surveillance and infect fish? Whether there are differences in the transmission of viruses at different stages of infection? Since lack of effective methods to control the carp viral diseases, strengthening quarantine and immune control remains the main means of preventing and controlling viral diseases of carp. Therefore, in the future, the researchers should continue to strengthen the research on the pathogenic mechanism and transmission pathway of the virus, develop a new oral vaccine carrier and transmission system, and develop drugs that can effectively treat viral diseases based on RNAi technology, in order to ensure the healthy and sustainable development of carp breeding industry in China.
Key words: common carp; virus disease; epidemic characteristics; control and prevention strategies; SVC; KHVD;VEC; CPD
Foundation item: National Key Research and Development Program of China(2019YFD0900105); Scientific and Technological Project in Henan(202102110260); Construction Project of Innovative Scientific and Technological Team of Aquatic Animal Immunity and Disease Prevention and Control in Henan(201706081)
0 引言
鯉(Cyprinus carpio L.)是我国重要水产的养殖品种之一,近年来我国的鲤年均产量维持在296万t左右,其养殖产业形成了巨大的社会效益和经济效益。我国鲤养殖历史悠久,据河南省贾湖遗址考古数据显示,早在8000年前的新石器时代就开始驯养鲤(Nakajima et al.,2019)。但随着鲤养殖密度和养殖规模的不断扩大,其养殖生态系统受到不同程度破坏,导致疫病频繁暴发而造成严重的经济损失。至今,已发现多种病原微生物能侵染鲤,并造成暴发流行,其中又以病毒性疾病的影响范围最广、死亡率最高(朱霞等,2011),已成为制约鲤养殖产业可持续健康发展的瓶颈问题。鲤易感的病毒性疾病有鲤春病毒血症(Spring viraemia of carp,SVC)、锦鲤疱疹病毒病(Koi herpesvirus disease,KHVD)及鲤病毒性浮肿病(Viral edema of carp disease,VEC)等,且这些病毒性疾病的死亡率均在50%以上,一旦大规模暴发流行,其病情很难控制,给鲤养殖业带来重大经济损失(高隆英等,2002;朱霞等,2011;陈昌福,2017)。为了更好地防控鲤病毒性疾病的暴发流行,本文通过综述鲤病毒性疾病的病原生物学、发生机理、流行特点及其防控策略,以期为实际生产中鲤病毒性疾病的科学防控提供参考依据。
1 鲤春病毒血症
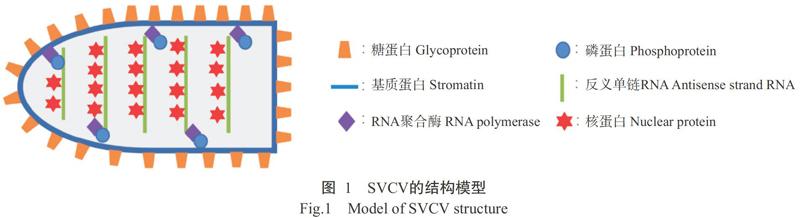
SVC是由鲤春病毒血症病毒(Spring viraemia of carp virus,SVCV)引起的一种急性高致死率传染病,已被世界动物卫生组织(World Organization for Animal Health,OIE)列为必须申报的疫病之一,也是我国农业农村部规定的二类动物疫病。SVCV是一类高致病性病毒,属于弹状病毒科(Rhabdoviridae),其病毒粒子长80~180 nm,直径60~90 nm,形态与其他弹状病毒相似,呈一面凸出而另一面扁平的子弹状(Ahne et al.,2002)。SVCV遗传物质为11 kb的反义单链RNA,包含5个开放阅读框(ORF),分别编码核蛋白(Nucleoprotein,N)、磷蛋白(Phosphoprotein,P)、基质蛋白(Matrix protein,M)、糖蛋白(Glycoprotein,G)及RNA依赖的RNA聚合酶(RNA-dependent RNA polymerase,L)(图1)。上述5个病毒基因的排列顺序为3'-N-P-M-G-L-5'(Teng et al.,2007;Stone et al.,2013;Ashraf et al.,2015;Zhang and Gui,2015),是弹状病毒的典型特征。
1. 1 鲤春病毒血症的发生及流行特点
SVCV于1971年在南斯拉夫首次被发现,随后在美洲、亚洲和一些欧洲国家(英国、法国、德国、西班牙、丹麦、荷兰和俄罗斯等)相继报道有鱼体感染SVCV(Hoffmann et al.,2005;Miller et al.,2007;Warg et al.,2007;Basic et al.,2009;Stone et al.,2013)。SVCV能感染多种鲤科鱼类,其中鲤是主要易感宿主,不同年龄的鲤均可感染。仔鱼和幼鱼感染SVCV后,其死亡率相对于其他年龄阶段较高,1龄仔鱼的感染死亡率达70%,而成鱼的感染死亡率相对较低。SVC主要在春季流行,该病的暴发与水温密切相关,当水温处于10~17 ℃时,SVCV最易感染鲤并造成高死亡率,进而导致SVC的暴发流行。已有研究表明,当水温高于17 ℃时,SVCV可感染成年鲤;水温处于22~23 ℃时,SVCV虽然可感染仔鱼,但不会造成大规模发病(Ahne,1986)。这可能是由于水温较高时,机体代谢旺盛,免疫系统处于活跃阶段,由体液免疫产生的抗病毒类物质如γ干扰素(IFNγ),能有效抑制SVCV在鱼体内复制;以及由细胞免疫产生的特异性抗体,促使被感染而不致死的鲤可抵御SVCV的二次感染。SVCV可通过鱼类的排泄物和体表黏液进行传播,同时可利用某些寄生虫作为媒介进行传播,排出体外的病毒颗粒仍具有较高感染活性。在4~10 ℃的自然水体中,SVCV感染活性保持周期长达4周,而在底泥中可维持6周。因此,在养殖过程中暴发SVC时需对养殖环境进行彻底消杀,才能有效消灭病原体(Ahne et al.,2002)。
1. 2 鲤春病毒血症的典型症状及组织病理学变化
鲤感染SVCV后其体色变暗发黑,腹部肿大,鳃丝无血色,眼球突出,体表出现血色斑点,肌肉由于出血而呈鲜红色;体内渗透压遭到破坏,造成脏器出血、腹腔积水,进而引发腹膜炎症和肠道炎症,鳔出现血色斑点,部分鱼体脾脏肿大(Ghasemi et al.,2014;Misk et al.,2016)。SVCV可在鲤的体内大量增殖,破坏鱼体稳态,致使免疫力下降,临床上常伴随其他细菌或寄生虫的继发感染,且呈大规模暴发流行。SVC的组织病理学变化主要表现为:肝脏血管壁水肿,部分血管壁结构消失,肝实质充血,多灶性坏死及脂肪变性。脾脏水肿,网状内皮细胞大量增生(Ghasemi et al.,2014);高铁红细胞内脂褐质储存量增加,淋巴管明显扩张,巨噬细胞和淋巴细胞肿大(图2),部分细胞破碎(Ahne et al.,2002;Misk et al.,2016)。肝胰腺出现多灶性坏死和非化脓性炎症,心脏呈心包炎及间断性肌变性。在肠道中可见血管周炎和绒毛萎缩。体肾出现空泡,且有玻璃样病变,肾小管堵塞(Misk et al.,2016)。鳔上皮细胞层形成不连续的多层结构,黏膜下层有出血现象(Ahne et al.,2002)。
1. 3 鯉春病毒血症的诊断与防控
SVC可对鲤养殖业造成重大影响,因此快速检测鉴定SVCV是控制SVC暴发的关键。我国针对SVC的诊断标准为:将病鱼组织匀浆10倍梯度稀释后,接种至生长约24 h的鲤上皮瘤细胞(EPC)、草鱼性腺细胞(CO)或胖头鱥肌肉细胞(FHM)中,接种的细胞板置于(20±2)℃培养箱中培养7 d。若接种匀浆稀释液的细胞在培养过程中出现细胞病变效应(CPE),则立即采用实时荧光定量PCR、酶联免疫吸附测定(ELISA)或间接免疫荧光法(IFAT)进行SVCV鉴定。但该方法操作繁琐,试验周期较长,难以实现快速检测;且采用ELISA和IFAT时,SVCV易与其他弹状病毒发生交叉反应,而导致假阳性结果(Way,1991;Rodák et al.,1993)。单克隆抗体是检测SVCV的重要工具,但传统的单克隆抗体制备方法繁杂,且对专业技术水平要求较高(Chen et al.,2008;Luo et al.,2014;Li et al.,2015)。因此,亟待研发新的检测方法,替代传统单克隆抗体检测,实现简便、快捷检测鉴定SVCV。Liu等(2013)研发出一种可快速检测SVCV的新型抗体,其原理是将SVCV特异性抗体可变区的单链片段,通过噬菌体表面展示的方法表达于噬菌体表面,而实现对SVCV的快速检测。
鉴于PCR检测方法的高灵敏性,已广泛应用于SVCV检测。高隆英等(2002)首次报道利用RT-PCR和半嵌套PCR扩增SVCV的糖蛋白基因序列,结果分别扩增获得长度为714和606 bp的特异核酸序列片段,说明2种方法均可用于SVCV检测。Koutná等(2003)研究表明,利用RT-PCR与巢式PCR相结合的方法能快速从细胞培养物和鱼组织中检测出SVCV,其灵敏度达10-1 TCID50/mL。传统的PCR检测需配备专门的仪器设备及具备熟练的RNA提取操作技术,不利于现场快速诊断。为此,Shimahara等(2016)依据编码G蛋白基因序列设计一对特异性反转录引物,成功建立从鱼体组织中精确检出SVCV的方法,且适用于非实验室条件下的常规诊断。
目前,SVCV防控的主要策略是严格控制疾病暴发期水体环境的稳定性,并及时清除和处理已被感染的病鱼。由于病毒寄生于细胞内,至今尚无针对SVC的特效治疗药物,SVCV疫苗研发也还处于实验室试验阶段。SVCV的G基因能编码其衣壳糖蛋白,诱导宿主产生免疫应答反应,因此包含G基因的DNA疫苗得到广泛关注(Kanellos et al.,2006;Emmenegger and Kurath,2008)。Kanellos等(2006)通过检测10种表达G基因的DNA疫苗,结果发现这些DNA疫苗的免疫保护效果较弱,多数DNA疫苗的相对免疫保护率只有11%~48%。Emmenegger和Kurath(2008)研究证实,基于SVCV南美株G基因设计的DNA疫苗对锦鲤(Cyprinus carpio haematopterus)和金鱼(Carassius auratus Linnaeus)具有较好的免疫保护力,其相对免疫保护率均在50%以上。可见,利用G基因设计的DNA疫苗可作为SVCV预防性治疗的候选疫苗。
2 锦鲤疱疹病毒病
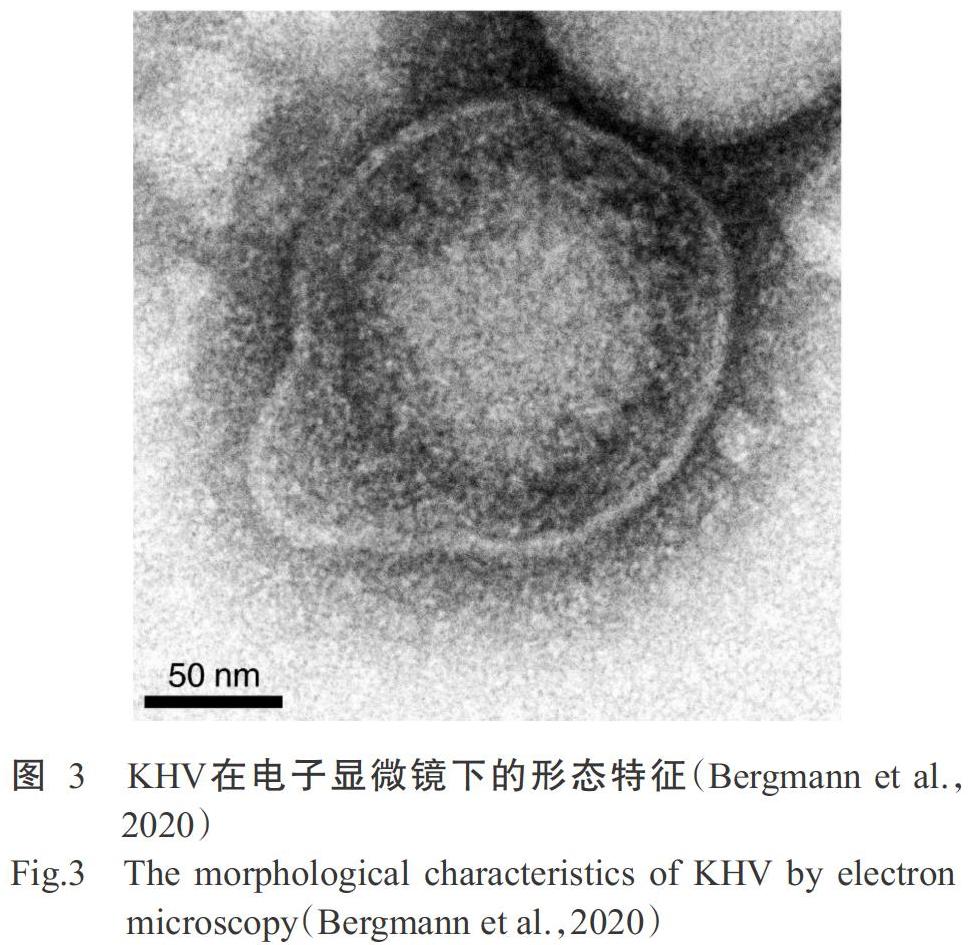
锦鲤疱疹病毒(Koi herpesvirus,KHV)又称3型鲤疱疹病毒(Cyprinid herpesvirus 3,CyHV-3)(图3),属于异疱疹病毒科(Alloherpesviridae)鲤疱疹病毒属(Cyprinivirus)(Waltzek et al.,2005;Hedrick et al.,2006)。目前,已分离鉴定出4种鲤疱疹病毒,分别为CyHV-1、CyHV-2、CyHV-3(KHV)及感染淡水鳗鲡(Anguilla japonica)的鳗鲡疱疹病毒(Anguillid herpesvirus 1,AngHV-1)。其中,CyHV-1常被称为鲤痘疮病毒,CyHV-2是引起疱疹病毒性造血器官坏死病的病原体。KHV是一种高致病性和高传染性的病原微生物,通常存在7~15 d的感染潜伏期,鲤感染KHV后7 d内的累计死亡率可达80%以上(刘宗晓等,2006;罗璋等,2018;周瑶佳等,2018)。因其传播特性和高致病性,OIE已将KHV引发的KHVD列为必须申报的动物疫病之一,也是全球进口检验检疫必检的疫病;在我国KHV是农业农村部规定的二类动物疫病病原之一。KHV对常见消毒试剂及紫外线的抵抗力较差,经35 ℃作用48 h或60 ℃作用30 min均可使其丧失感染能力。KHV在23 ℃的自然水体中存活不超过21 h,但在鱼体分泌物和池塘底泥中存活时间较长。
2. 1 锦鲤疱疹病毒病的发生及流行特点
1998年,美国科学家分别从鲤和锦鲤中首次分离出KHV,之后在欧洲、美洲、非洲和亚洲相继报道KHVD大规模暴发流行(Haenen et al.,2004;Hedrick et al.,2006)。KHV传染性强、致死率高,但其宿主范围单一,仅感染包括鲤在内的少数鲤科鱼类,而不感染其他鱼类,包括与鲤亲缘关系较近的金鱼、鲢(Hypophthalmichthys molitrix)、鲫(Carassius caras-sius)、草鱼(Ctenopharyngodon idella)及罗非鱼(Oreo-chroms mossambcus)等(Bergmann et al.,2010;Michel et al.,2010;Fabian et al.,2013)。KHV可在上述鱼体内复制增殖,并将具有感染活力的病毒颗粒释放到水体环境中,但不会表现出KHV感染的临床症状。鲤对KHV尤为敏感,不同年龄段的鲤均可感染,且成鱼较幼鱼更易感(Bergmann et al.,2010;Michel et al.,2010)。KHV主要流行于秋季,当养殖水温维持在18~28 ℃时易发病,在23~28 ℃时极易暴发流行;养殖水温低于18 ℃时,KHV虽然也感染鲤,但无临床症状,一旦温度适宜,病毒暴发,带毒鱼体即表现出KHVD的典型临床症状,可造成80%以上的死亡率。
KHV主要依賴于养殖水体进行传染,水体中的病毒主要通过鳃组织进入鱼体,也可通过后肠进入鱼体(Gilad et al.,2004;Haenen et al.,2004;Pikarsky et al.,2004)。Costes等(2009)研究认为,KHV主要依赖皮肤进入鱼体。但KHV感染48 h内,在无损伤且黏膜覆盖完好的皮肤中检测不到KHV;而通过浸泡感染鲤2~5 h,在被感染鱼体的鳃组织和肠道中均能检测到KHV,感染4~6 h后在鳃组织的巨噬细胞和肠道组织的淋巴细胞中也能检测到KHV(Monaghan et al.,2015)。此外,KHV通过血液循环系统在感染后5~7 d可将病毒粒子运送到机体各组织和器官(Haenen et al.,2004)。
2. 2 锦鲤疱疹病毒病的典型症状及组织病理学变化
鲤感染KHV后的典型症状为:反应迟钝,无法正常进食,在水体表面离群散游,或头朝下悬垂于水面;体表黏液分泌异常,局部发白,部分表皮不分泌黏液,且表现出砂纸样纹理结构;眼球凹陷,鱼鳍出血甚至腐烂;病鱼鳃丝末端溃烂(图4),丧失生理功能,导致呼吸困难;剖开腹腔可观察到体肾肿大,且伴有腹水(Oh et al.,2001;Gray et al.,2002)。组织病理学研究显示,鲤感染KHV后其鳃上皮细胞发生退行性病变,在感染细胞中发现核内包涵体。肝脏、脾脏、肾脏和肠道薄壁细胞坏死,且在单核细胞/巨噬细胞内部发现被吞噬的细胞碎片。Hedrick等(2000)研究显示,鲤感染KHV后其神经元细胞中出现核内包涵体,但在KHV感染过程中神经系统并未参与免疫应答反应。
2. 3 锦鲤疱疹病毒病的诊断与防控
感染KHV后患病鲤临床症状明显,首先可通过临床病症和流行病学进行初步诊断;其次可通过细胞培养技术进行诊断,感染KHV的鲤脑组织细胞系(CCB)经20 ℃培养5 d后可产生特异性细胞病变,具体表现为细胞体积变大,部分细胞发生融合及出现明显的细胞质空泡化;最后可通过分子生物学技术进行确诊,利用巢式PCR或环介导等温扩增法(Loop-mediated isothernal amplification,LAMP)等分子检测方法确定鲤是否感染KHV(Gunimaladevi et al.,2004)。我国防控KHV主要采取以预防为主的策略。KHV大规模暴发时应保持水质稳定,养殖水体的溶解氧含量需保持在5 mg/L以上,同时避免拉网及其他作业对鱼体产生应激反应。目前,市场仅有一种获批销售的KHV商业化疫苗,即以色列生产的弱毒疫苗(KoVax Ltd./Phibro Animal Health Corp.)(Ronen et al.,2003;Perelberg et al.,2005)。此外,一些传统疫苗和DNA疫苗也正在研发之中,并证实对KHVD有一定预防效果(Rosenkranz et al.,2008;Zhou et al.,2014;Boutier et al.,2015;Klafack et al.,2019;Schroder et al.,2019)。Matras等(2017)研究表明,螺旋藻胞外多糖可有效治疗由KHV感染鲤引发的病症,持续给药4~6周能显著降低KHV的感染发病强度。以色列烈日大学Vanderplasschen教授认为防治KHVD最有效的方法是接种疫苗,但KHV疫苗需满足以下3个方面:(1)研发的KHV疫苗必须适用于大规模接种;(2)疫苗的成本效益比率应尽可能低,生产和管理成本过高是制约渔用疫苗推广应用的重要因素;(3)疫苗的安全性需得到保证,且能产生较好的免疫效果,即相对免疫保护率接近100%(Boutier et al.,2019)。
3 其他病毒性疾病
3. 1 鲤病毒性浮肿病
鲤病毒性浮肿病(VEC)又称锦鲤嗜睡病(Koi sleepy disease,KSD),其病原体为鲤浮肿病毒(Carp edema virus,CEV)。CEV是一种双链DNA病毒,属于痘病毒科(Poxviridae)。1976年CEV在日本首次被发现,随后在世界各地迅速传播(Haenen et al.,2014;Jung-Schroers et al.,2015;Swaminathan et al.,2016;Matras et al.,2017)。CEV主要感染鲤和锦鲤,是一种急性感染源,可造成87.5%的感染率及80.0%~100.0%的死亡率。感染CEV的鱼体行动缓慢,呼吸功能减弱,常浮头于水面,或侧卧于池塘底部,呈昏睡状,最终因缺氧而死。病鱼体表溃烂,皮下组织水肿,鳃丝无血色、末端溃烂(Miyazaki et al.,2005)。
VEC常暴发于春秋两季,暴发水温一般维持在15~25 ℃。但也有研究显示,当水温在6~22 ℃时,CEV可感染鲤或锦鲤,并造成流行传播(Oyamatsu et al.,1997;Amita et al.,2002);而水温高于28 ℃时,CEV难以感染锦鲤,不会造成VEC大规模暴发流行。目前,尚未发现对CEV敏感的细胞系,故难以获得大量纯化的病毒粒子,而制约CEV基因组测序及其疫苗的研发(Swaminathan et al.,2016)。
3. 2 鲤痘疮病毒病
鲤痘疮病毒病(Carp pox disease,CPD)是由CyHV-1感染致病,因此CyHV-1又被称为鲤痘疮病毒(Carp pox virus)或乳头瘤病毒(Papilloma virus)。CyHV-1是一种DNA病毒,与KHV同属于疱疹病毒科(Alloherpesviridae)鲤疱疹病毒属(Cyprinivirus)。CyHV-1的病毒核心为二十面体,外面有囊膜包被,病毒粒子核心和囊膜的直径分别为113和190 nm (Sano et al.,2004)。
CyHV-1于1985年从日本患病锦鲤表皮组织中分离获得,能感染EPC和FHM,造成这2种细胞系出现CPE,被感染的细胞发生空泡化,且在核内形成包涵体(Sano et al.,1985)。CyHV-1感染鲤的临床症状表现为:感染初期鱼体表出现白色斑点,并分泌大量白色黏液,随着病情发展,体表的白色斑点范围逐渐扩大,且逐渐凸起,形成增生物。增生物为上皮细胞与结缔组织增生形成的乳头状凸起,其表面光滑呈石蜡样或玻璃样。增生物的主要成分为胶原纤维,能自然脱落,但在脱落部位又会重新出现增生物。CyHV-1常于流行春冬季,暴发流行时的水温一般在10~16 ℃。当水温高于22 ℃时,患病鱼体能自然痊愈。CyHV-1对成鱼的危害较小,其死亡率低于10%,但感染2周龄仔鱼的死亡率可高达60%~90%。相对于其他病毒,CyHV-1的致病率和致死率均较低,且危害较小,因此针对CyHV-1的研究较少,通常认为该病毒是通过接触传染,但也有研究证实水生寄生虫是其传播媒介(Sano et al.,1985)。
4 展望
当前,针对鲤病毒性疾病的防控主要采取消灭传染源、切断传播途径及保护易感鱼群。消灭传染源是通过对池塘和塘泥进行彻底消毒,发病池塘水体需全面消杀;切断传播途径主要通过加强苗种检疫,对疫区亲鱼、苗种和成鱼严格管控;保护易感鱼群主要通过对敏感鱼类注射疫苗或提高鱼体免疫力。由于缺乏高效的防控方法,因此加强检疫和免疫防控仍是防控鲤病毒性疾病的主要手段。疫苗的导入方式直接决定其免疫效果。疫苗通过腹腔注射方式导入鱼体,可诱导鱼体产生抗病毒免疫反应,但这种导入方式对鱼体应激较大,且成本较高,难以实现大规模推广应用(Adelmann et al.,2008)。鱼类的黏膜组织在抵御病毒侵染过程中发挥重要作用(Costes et al.,2009;Gomez et al.,2013),但通过腹腔注射方式接种疫苗难以激活黏膜免疫系统。口服疫苗可成功激活鱼体黏膜免疫系统,且这种疫苗导入策略已在多个鱼种上成功诱导抗病毒免疫应答反应(Chen,2000;Liu et al.,2012),但口服疫苗需抵抗鱼体消化系统的侵蚀才能到达后肠而能被黏膜免疫系统识别和吸收,因此,研发新型口服疫苗载体及传送系统是今后鱼类病毒疫苗的重点研究内容之一。
随着集约化、工厂化养殖模式的推广普及,尾水排放等环境污染问题日益加剧,同时为病毒性疾病的大暴发流行创造了条件。环境友好型生态养殖模式是水产养殖发展的必然趋势,生态养殖需具备优良的养殖品种及优质的养殖环境和饲料,同时要构建完善的养殖生态系统。近年来,中药等植物源药物在水产养殖中的应用研究逐渐深入。赵倩等(2013)以嗜水气单胞菌对鲤进行攻毒,结果发现中药喂食组鲤的死亡率显著低于基础饲料组,且鱼体溶菌酶活力显著提高。谢炎福等(2015)研究表明,在黄河鲤基础饲料中添加中药制剂能显著上调鱼体超氧化物歧化酶(SOD)活性,同时降低丙二醛(MDA)含量。中药制剂还具有净化水质及抑制水体中病原微生物增殖的作用(汤菊芬等,2016;孟彬等,2018)。目前,利用中药对鲤病毒性疾病进行防治的研究较少,中药在鲤抵御病毒入侵过程中发挥的作用机理尚有待进一步探究。
KHV可感染多种鲤科近缘鱼类,但为何只发展成为病毒携带者而不发病?养殖水温是否是决定病毒感染的关键因素?病毒可通过哪些途径逃避免疫监视而进入鱼体?在不同感染阶段病毒的传播方式是否存在差异?可见,鲤病毒性疾病仍存在诸多问题亟待深入研究,以揭示病毒与鱼体间的相互作用机理及病毒免疫逃逸的作用机制。近年来,RNA干扰(RNAi)技术在抗病毒药物研发领域已取得长足进展,并获准进行下一步临床试验(Gotesman et al.,2015)。因此,今后要加强对病毒致病机理和传染途径的研究,并基于RNAi技術研制能有效防治病毒性疾病的药物,以确保我国鲤养殖产业的健康可持续发展。
参考文献:
陈昌福. 2017. 日本鱼病学者福田颖穗关于锦鲤疱疹病毒病和鲤病毒性浮肿病的症状描述与防控措施[J]. 当代水产,42(11):86-88. doi:10.3969/j.issn.1674-9049.2017.11. 029. [Chen C F. 2017. Description of symptoms and prevention and control measures of koi herpesvirus disease and koi viral edema disease by Yoshiho Fukuda,a Japanese fish disease scholar[J]. Current Fisheries,42(11):86-88.]
高隆英,史秀杰,刘荭,江育林. 2002. 用RT-PCR法快速检测鲤春病毒血症病毒基因[J]. 水生生物学报,26(5):452-456. doi:10.3321/j.issn:1000-3207.2002.05.005. [Gao L Y,Shi X J,Liu H,Jiang Y L. 2002. Detection of spring viremia of carp virus(SVCV) gene using reverse-polymerase chain-reaction(RT-PCR)[J]. Acta Hydrobiologica Sinica,26(5):452-456.]
刘宗晓,刘荭,江育林. 2006. 锦鲤疱疹病毒病的研究进展[J]. 检验检疫科学,16(4):77-80. [Liu Z X,Liu H,Jiang Y L. 2006. Research progress of koi herpesvirus disease[J]. Quality Safety Inspection and Testing,16(4):77-80.]
罗璋,郝爽,张振国,孟一耕,史谢尧,冯守明. 2018. 锦鲤疱疹病毒天津株的分离与鉴定[J]. 水产学报,42(4):575-585. doi:10.11964/jfc.20170410788. [Luo Z,Hao S,Zhang Z G,Meng Y G,Shi X Y,Feng S M. 2018. Isolation and characterization of a strain of koi herpesvirus from diseased koi in Tianjin[J]. Journal of Fisheries of China,42(4):575-585.]
孟彬,孙敬锋,吕爱军. 2018. 发酵中草药在水产养殖中的应用[J]. 水产科学,37(3):421-426. doi:10.16378/j.cnki. 1003-1111.2018.03.023. [Meng B,Sun J F,Lü A J. 2018. Application of fermented chinese herbal medicine in aquaculture[J]. Fisheries Science,37(3):421-426.]
汤菊芬,黄瑜,蔡佳,丘金珠,孙建华,徐中文,简纪常. 2016. 中草药复合微生态制剂对吉富罗非鱼(Oreochromis niloticus)生长、肠道菌群及抗病力的影响[J]. 渔业科学进展,37(4):104-109. doi:10.11758/yykxjz.20150810002. [Tang J F,Huang Y,Cai J,Qiu J Z,Sun J H,Xu Z W,Jian J C. 2016. Effects of a compound probiotics combined with chinese herbal medicine on growth performance,intestinal flora and resistance to diseases of GIFT strain of Nile tilapia(Oreochromis niloticus)[J]. Progress in Fishery Sciences,37(4):104-109.]
謝炎福,代春梅,杜亚. 2015. 益生菌发酵复方中药对黄河鲤生长性能和免疫性能的影响[J]. 黑龙江畜牧兽医,(8):198-200. [Xie Y F,Dai C M,Du Y. 2015. Effects of probiotics fermentation compound Chinese herbs on growth performance and immune performance of yellow river carp[J]. Heilongjiang Animal Science and Veterinary Me-dicine,(8):198-200.]
赵倩,陈玉春,谷巍,李丕武. 2013. 益生菌发酵中草药对鲤鱼生长指标、生化指标及抗病力的影响[J]. 饲料与畜牧,(5):31-34. [Zhao Q,Chen Y C,Gu W,Li P W. 2013. Effects of Chinese herbs fermentation with probiotics on growth indexes,biochemical indexes and disease resistance of Cyprinus carpio[J]. Animal Agriculture,(5):31-34.]
周瑶佳,涂尊方,税斐,阳瑞雪,汪开毓,耿毅,黄小丽,欧阳萍. 2018. 温度对锦鲤疱疹病毒体外培养和致病性的影响[J]. 华南农业大学学报,39(4):20-24. doi:10.7671/j.issn.1001-411X.2018.04.004. [Zhou Y J,Tu Z F,Shui F,Yang R X,Wang K Y,Geng Y,Huang X L,Ouyang P. 2018. Effects of temperature on culture in vitro and pathogenicity of Cyprinid herpesvirus 3[J]. Journal of South China Agricultural University,39(4):20-24.]
朱霞,王好,李新伟,周井祥. 2011. 锦鲤疱疹病毒病的研究进展[J]. 中国兽医科学,41(1):106-110. doi:10.16656/j.issn.1673-4696.2011.01.020. [Zhu X,Wang H,Li X W,Zhou J X. 2011. Advances in koi herpesvirus disease[J]. Chinese Veterinary Science,41(1):106-110.]
Adelmann M,K?llner B,Bergmann S M,Fischer U,Lange B,Weitschies W,Enzmann P J,Fichtner D. 2008. Development of an oral vaccine for immunisation of rainbow trout(Oncorhynchus mykiss) against viral haemorrhagic septicaemia[J]. Vaccine 26(6):837-844. doi:10.1016/j.vaccine.2007.11.065.
Ahne W. 1986. The influence of environmental temperature and infection route on the immune response of carp (Cyprinus carpio) to spring viremia of carp virus(SVCV)[J]. Veterinary Immunology and Immunopathology,12(1-4):383-386. doi:10.1016/0165-2427(86)90144-3.
Ahne W,Bjorklund H V,Essbauer S,Fijan N,Kurath G,Winton J R. 2002. Spring viremia of carp(SVC)[J]. Diseases of Aquatic Organisms,52(3):261-272. doi:10.3354/dao 052261.
Amita K,Oe M,Matoyama H,Yamaguchi N,Fukuda H. 2002. A survey of koi herpesvirus and carp edema virus in colorcarp cultured in Niigata Prefecture,Japan[J]. Fish Pathology,37(4):197-198. doi:10.3147/jsfp.37.197.
Ashraf U,Ye J,Ruan X D,Wan S F,Zhu B B,Cao S B. 2015. Usutu virus:An emerging flavivirus in Europe[J]. Viruses,7(1):219-238. doi:10.3390/v7010219.
Basic A,Schachner O,Bilic I,Hess M. 2009. Phylogenetic analysis of spring viraemia of carp virus isolates from Austria indicates the existence of at least two subgroups within genogroup Id[J]. Diseases of Aquatic Organisms,85(1):31-40. doi:10.3354/dao02069.
Bergmann S M,Jin Y,Franzke K,Grunow B,Wang Q,Klafack S. 2020. Koi herpesvirus(KHV) and KHV disease (KHVD)-a recently updated overview[J]. Journal of App-lied Microbiology,129(1):98-103. doi:10.1111/jam. 14616.
Bergmann S M,Sadowski J,Kie?piński M,Bart?omiejczyk M,Fichtner D,Riebe R,Lenk M,Kempter J. 2010. Susceptibility of koi×crucian carp and koi×goldfish hybrids to koi herpesvirus(KHV) and the development of KHV di-sease(KHVD)[J]. Journal of Fish Diseases,33(3):267-272. doi:10.1111/j.1365-2761.2009.01127.x.
Boutier M,Gao Y,Donohoe O,Vanderplasschen A. 2019. Current knowledge and future prospects of vaccines against cyprinid herpesvirus 3(CyHV-3)[J]. Fish and Shellfish Immunology,93:531-541. doi:10.1016/j.fsi.2019.07.079.
Boutier M,Ronsmans M,Ouyang P,Fournier G,Reschner A,Rakus K,Wilkie G S,Farnir F,Bayrou C,Lieffrig F,Li H,Desmecht D,Davison A J,Vanderplasschen A. 2015. Rational development of an attenuated recombinant cyprinid herpesvirus 3 vaccine using prokaryotic mutagenesis and in vivo bioluminescent imaging[J]. PLoS Pathogens,11(2):e1004690. doi:10.1371/journal.ppat.1004690.
Chen H. 2000. Recent advances in mucosal vaccine development[J]. Journal of Controlled Release,67(2-3):117-128. doi:10.1016/s0168-3659(00)00199-1.
Chen Z Y,Liu H,Li Z Q,Zhang Q Y. 2008. Development and characterization of monoclonal antibodies to spring viraemia of carp virus[J]. Veterinary Immunology and Immunopathology,123(3-4):266-276. doi:10.1016/j.ve-timm.2008.02.011.
Costes B,Raj V S,Michel B,Fournier G,Thirion M,Gillet L,Mast J,Lieffrig F,Bremont M,Vanderplasschen A. 2009. The major portal of entry of koi herpesvirus in Cyprinus carpio is the skin[J]. Journal of Virology,83(7):2819-2830. doi:10.1128/JVI.02305-08.
Emmenegger E,Kurath G. 2008. DNA vaccine protects ornamental koi(Cyprinus carpio koi) against North American spring viremia of carp virus[J]. Vaccine,26(50):6415-6421. doi:10.1016/j.vaccine.2008.08.071.
Fabian M,Baumer A,Steinhagen D. 2013. Do wild fish species contribute to the transmission of koi herpesvirus to carp in hatchery ponds?[J]. Journal of Fish Diseases,36(5):505-514. doi:10.1111/jfd.12016.
Ghasemi M,Zamani H,Hosseini S M,Karsidani S H,Bergmann S M. 2014. Caspian white fish(Rutilus frisii kutum) as a host for spring viraemia of carp virus[J]. Veterinary Microbiology,170(3-4):408-413. doi:10.1016/j.vetmic. 2014.02.032.
Gilad O,Yun S S,Zagmutt-Vergara F J,Leutenegger C M,Bercovier H,Hedrick R P. 2004. Concentrations of a koi herpesvirus(KHV) in tissues of experimentally infected Cyprinus carpio koi as assessed by real-time TaqMan PCR[J]. Diseases of Aquatic Organisms,60(3):179-187. doi:10.3354/dao060179.
Gomez D,Sunyer J O,Salinas I. 2013. The mucosal immune system of fish:The evolution of tolerating commensals while fighting pathogens[J]. Fish and Shellfish Immuno-logy,35(6):1729-1739. doi:10.1016/j.fsi.2013.09.032.
Gotesman M,Soliman H,Besch R,Elmatbouli M. 2015. Inhibition of spring viraemia of carp virus replication in an Epithelioma papulosum cyprini cell line by RNAi[J]. Journal of Fish Diseases,38(2):197-207. doi:10.1111/jfd. 12227.
Gray W L,Mullis L,LaPatra S E,Groff J M,Goodwin A. 2002. Detection of koi herpesvirus DNA in tissues of infected fish[J]. Journal of Fish Diseases,25(3):171-178. doi:10.1046/j.1365-2761.2002.00355.x.
Gunimaladevi I,Kono T,Venugopal M N,Sakai M. 2004. Detection of koi herpesvirus in common carp,Cyprinus carpio L.,by loop-mediated isothermal amplification[J]. Journal of Fish Diseases,27(10):583-589. doi:10.1111/j.1365- 2761.2004.00578.x.
Haenen O,Way K,Bergmann S M,Ariel E. 2004. The emergence of koi herpesvirus and its significance to European aquaculture[J]. Bulletin of the European Association of Fish Pathologists,24(6):293-307.
Haenen O,Way K,Stone D,Engelsma M. 2014. Koi sleepy disease(KSD) door ‘carp edema virus:Eerste detectie in Nederlandse koi[J]. Koi Wijzer,113:65-67.
Hedrick R P,Gilad O,Yun S,Spangenberg J V,Marty G D,Nordhausen R W,Kebus M J,Bercovier H,Eldar A. 2000. A herpesvirus associated with mass mortality of juvenile and adult koi,a strain of common carp[J]. Journal of Aquatic Animal Health,12(1):44-57. doi:10.1577/1548-8667(2000)012<0044:AHAWMM>2.0.CO;2.
Hedrick R P,Waltzek T B,McDowell T S. 2006. Susceptibility of koi carp,common carp,goldfish,and goldfish×common carp hybrids to cyprinid herpesvirus-2 and herpesvirus-3[J]. Journal of Aquatic Animal Health,18(1):26-34. doi:10.1577/H05-028.1.
Hoffmann B,Beer M,Schütze H,Mettenleiter T C. 2005. Fish rhabdoviruses:Molecular epidemiology and evolution[J]. Current Topics in Microbiology and Immunology,292(1):81-117. doi:10.1007/3-540-27485-5_5.
Jung-Schroers V,Adamek M,Teitge F,Hellmann J,Bergmann S M,Schütze H,Kleingeld D W,Way K,Stone D M,Runge M,Keller B,Hesami S,Waltzek T,Steinhagen D. 2015. Another potential carp killer?:Carp edema virus di-sease in Germany[J]. BMC Veterinary Research,11(1):114. doi:10.1186/s12917-015-0424-7.
Kanellos T,Sylvester I D,DMello F,Howard C R,Mackie A,Dixon P F,Chang K C,Ramstad A,Midtlyng P J,Russell P H. 2006. DNA vaccination can protect Cyprinus carpio against spring viraemia of carp virus[J]. Vaccine,24(23):4927-4933. doi:10.1016/j.vaccine.2006.03.062.
Klafack S,Fiston-Lavier A S,Bergmann S M,Hammoumi S,Schr?der L,Fuchs W,Lusiastuti A M,Lee P Y,Heredia S V,Consortium M S,Gosselin-Grenet A S,Avarre J C. 2019. Cyprinid herpesvirus 3 evolves in vitro through an assemblage of haplotypes that alternatively become dominant or under-represented[J]. Viruses,11(8):754. doi:10.3390/v11080754.
Koutná M,Vesel? T,Psikal I,H?lová J. 2003. Identification of spring viraemia of carp virus(SVCV) by combined RT-PCR and nested PCR[J]. Diseases of Aquatic Orga-nisms,55(3):229-235. doi:10.3354/dao055229.
Li Z M,Zhang Q,Luo P X,Liu G X,Wang M,Liu X Q. 2015. Monoclonal antibody against M protein of spring viremia of carp virus[J]. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy,34(2):122-125. doi:10.1089/mab.2014.0056.
Liu H,Zheng X C,Zhang F,Li Y,Zhang X H,Dai H P,Hua Q Y,Shi X J,Lan W S,Jia P,Yuan L. 2013. Selection and characterization of single-chain recombinant antibo-dies against spring viraemia of carp virus from mouse phage display library[J]. Journal of Virological Methods,194(1-2):178-184. doi:10.1016/j.jviromet.2013.08.017.
Liu M,Zhao L L,Ge J W,Qiao X Y,Li Y J,Liu D Q. 2012. Immunogenicity of Lactobacillus-expressing VP2 and VP3 of the infectious pancreatic necrosis virus(IPNV) in rainbow trout[J]. Fish and Shellfish Immunology,32(1):196-203. doi:10.1016/j.fsi.2011.11.015.
Luo P X,Ruan X D,Zhang Q,Li Z M,Wang M,Liu X Q. 2014. Monoclonal antibodies against G protein of spring viremia of carp virus[J]. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy,33(5):340-343. doi:10.1089/mab.2014.0025.
Matras M,Borzym E,Stone D,Way K,Stachnik M,Maj-Paluch J,Palusińska M,Reichert M. 2017. Carp edema virus in polish aquaculture-evidence of significant sequence divergence and a new lineage in common carp Cyprinus carpio(L.)[J]. Journal of Fish Diseases,40(3):319-325. doi:10.1111/jfd.12518.
Michel B,Leroy B,Raj V S,Lieffrig F,Mast J,Wattiez R,Vanderplasschen A F,Costes B. 2010. The genome of cyprinid herpesvirus 3 encodes 40 proteins incorporated in mature virions[J]. The Journal of General Virology,91(2):452-462. doi:10.1099/vir.0.015198-0.
Miller O,Fuller F J,Gebreyes W A,Lewbart G A,Shchelkunov I S,Shivappa R B,Joiner C,Woolford G,Stone D M,Dixon P F,Raley M E,Levine J F. 2007. Phylogene-tic analysis of spring virema of carp virus reveals distinct subgroups with common origins for recent isolates in North America and the UK[J]. Diseases of Aquatic Organisms,76(3):193-204. doi:10.3354/dao076193.
Misk E,Garver K,Nagy E,Isaac S,Tubbs L,Huber P,Al-Hussinee L,Lumsden J S. 2016. Pathogenesis of spring viremia of carp virus in emerald shiner Notropis atherinoides Rafinesque,fathead minnow Pimephales promelas Rafinesque and white sucker Catostomus commersonii (Lacepede)[J]. Journal of Fish Diseases,39(6):729-739. doi:10.1111/jfd.12405.
Miyazaki T,Isshiki T,Katsuyuki H. 2005. Histopathological and electron microscopy studies on sleepy disease of koi Cyprinus carpio koi in Japan[J]. Diseases of Aquatic Organisms,65(3):197-207. doi:10.3354/dao065197.
Monaghan S J,Thompson K D,Adams A,Kempter J,Bergmann S M. 2015. Examination of the early infection sta-ges of koi herpesvirus(KHV) in experimentally infected carp,Cyprinus carpio L. using in situ hybridization[J]. Journal of Fish Diseases,38(5):477-489. doi:10.1111/jfd.12260.
Nakajima T,Hudson M J,Uchiyama J,Makibayashi K,Zhang J Z. 2019. Common carp aquaculture in Neolithic China dates back 8,000 years[J]. Nature Ecology and Evolution,3(10):1415-1418. doi:10.1038/s41559-019-0974-3.
Oh M J,Jung S J,Choi T J,Kim H R,Rajendran K V,Kim Y J,Park M A,Chun S K. 2001. A viral disease occurring in cultured carp Cyprinus carpio in Korea[J]. Fish Pathology,36(3):147-151. doi:10.3147/jsfp.36.147.
Oyamatsu T,Matoyama H,Yamamoto K Y,Fukuda H. 1997. A trial for the detection of carp edema virus by using polymerase chain reaction[J]. Aquaculture Science,45(2):247-251. doi:10.11233/aquaculturesci1953.45.247.
Perelberg A,Ronen A,Hutoran M,Smith Y,Kotler M. 2005. Protection of cultured Cyprinus carpio against a lethal viral disease by an attenuated virus vaccine[J]. Vaccine,23(26):3396-3403. doi:10.1016/j.vaccine.2005.01.096.
Pikarsky E,Ronen A,Abramowitz J,Levavi-Sivan B,Hutoran M,Shapira Y,Steinitz M,Perelberg A,Soffer D,Kotler M. 2004. Pathogenesis of acute viral disease induced in fish by carp interstitial nephritis and gill necrosis virus[J]. Journal of Virology,78(17):9544-9551. doi:10.1128/ JVI.78.17.9544-9551.2004.
Rodák L,Pospí?il Z,Tomanek J,Vesel? T,Obr T,Valí?ek L. 1993. Enzyme-linked immunosorbent assay (ELISA) for the detection of spring viraemia of carp virus (SVCV) in tissue homogenates of the carp,Cyprinus carpio L.[J]. Journal of Fish Diseases,16(2):101-111. doi:10.1111/ j.1365-2761.1993.tb00853.x.
Ronen A,Perelberg A,Abramowitz J,Hutoran M,Tinman S,Bejerano I,Steinitz M,Kotler M. 2003. Efficient vaccine against the virus causing a lethal disease in cultured Cyprinus carpio[J]. Vaccine,21(32):4677-4684. doi:10.1016/ s0264-410X(03)00523-1.
Rosenkranz D,Klupp B G,Teifke J P,Granzow H,Fichtner D,Mettenleiter T C,Fuchs W. 2008. Identification of envelope protein pORF81 of koi herpesvirus[J]. The Journal of General Virology,89(4):896-900. doi:10.1099/vir. 0.83565-0.
Sano M,Ito T,Kurita J,Takanori Y,Watanabe N,Miwa S,Iida T. 2004. First detection of koi herpesvirus in cultured common carp Cyprinus carpio in Japan[J]. Fish Pathology,39(3):165-167. doi:10.3147/jsfp.39.165.
Sano T,Fukuda H,Furukawa M. 1985. Herpesvirus cyprini:Biological and oncogenic properties[J]. Fish Pathology,20(2/3):381-388. doi:10.3147/jsfp.20.381.
Schroder L,Klafack S,Bergmann S M,Fichtner D,Jin Y,Lee P Y,H?per D,Mettenleiter T C,Fuchs W. 2019. Generation of a potential koi herpesvirus live vaccine by simultaneous deletion of the viral thymidine kinase and dUTPase genes[J]. The Journal of General Virology,100(4): 642-655. doi:10.1099/jgv.0.001148.
Shimahara Y,Kurita J,Nishioka T,Kiryu I,Yuasa K,Sakai T,Oseko N,Sano M,Dixon P. 2016. Development of an improved RT-PCR for specific detection of spring viraemia of carp virus[J]. Journal of Fish Diseases,39(3):269-275. doi:10.1111/jfd.12357.
Stone D M,Kerr R C,Hughes M,Radford A D,Darby A C. 2013. Characterisation of the genomes of four putative vesiculoviruses:Tench rhabdovirus,grass carp rhabdovirus,perch rhabdovirus and eel rhabdovirus European X[J]. Archives of Virology,158(11):2371-2377. doi:10. 1007/s00705-013-1711-x.
Swaminathan T R,Kumar R,Dharmaratnam A,Basheer V S,Sood N,Pradhan P K,Sanil N K,Vijayagopal P,Jena J K. 2016. Emergence of carp edema virus in cultured ornamental koi carp,Cyprinus carpio koi,in India[J]. The Journal of General Virology,97(12):3392-3399. doi:10. 1099/jgv.0.000649.
Teng Y,Liu H,Lü J Q,Fan W H,Zhang Q Y,Qin Q W. 2007. Characterization of complete genome sequence of the spring viremia of carp virus isolated from common carp(Cyprinus carpio) in China[J]. Archives of Virology,152(8):1457-1465. doi:10.1007/s00705-007-0971-8.
Waltzek T B,Kelley G O,Stone D M,Way K,Hanson L,Fukuda H,Hirono I,Aoki T,Davison A J,Hedrick R P. 2005. Koi herpesvirus represents a third cyprinid herpesvirus(CyHV-3) in the family Herpesviridae[J]. The Journal of General Virology,86(6):1659-1667. doi:10.1099/vir.0.80982-0.
Warg J V,Dikkeboom A L,Goodwin A E,Snekvik K,Whitney J. 2007. Comparison of multiple genes of spring viremia of carp viruses isolated in the United States[J]. Virus Genes,35(1):87-95. doi:10.1007/s11262-006-0042-3.
Way K. 1991. Rapid detection of SVC virus antigen in infec-ted cell cultures and clinically diseased carp by the enzyme-linked immunosorbent assay (ELISA)[J]. Journal of Applied Ichthyology,7(2):95-107. doi:10.1111/j.1439- 0426.1991.tb00515.x.
Zhang Q Y,Gui J F. 2015. Virus genomes and virus-host interactions in aquaculture animals[J]. Science China. Life Sciences,58(2):156-169. doi:10.1007/s11427-015-4802-y.
Zhou J X,Xue J D,Wang Q J,Zhu X,Li X W,Lü W L,Zhang D M. 2014. Vaccination of plasmid DNA enco-ding ORF81 gene of CJ strains of KHV provides protection to immunized carp[J]. In Vitro Cellular and Deve-lopmental Biology. Animal,50(6):489-495. doi:10.1007/s11626-014-9737-2.
(責任编辑 兰宗宝)

