Extrahepatic cancer risk after liver transplantation for hepatocellular carcinoma: incidence, risk and prevention
Claire M. Vajdic, Marina T. van Leeuwen, Geoffrey W. McCaughan
1Centre for Big Data Research in Health, University of New South Wales, Sydney 2052, Australia.
2Centenary Research Institute, Australian National Liver Transplant Unit, Royal Prince Alfred Hospital, University of Sydney,Sydney 2006, Australia.
Abstract This article synthesises the current evidence on the risk of de novo extrahepatic cancer in people living with a liver transplant after hepatocellular carcinoma, the risk factors for cancer, and the recommended approaches to cancer prevention and surveillance. People living with a transplanted liver have an elevated risk of cancer and cancer death, and the indication for transplantation does not markedly alter the cancer risk. The excess risk of cancer is double that of the age- and sex-matched general population. Virus-related cancers, especially non-Hodgkin lymphoma, Kaposi sarcoma, Merkel cell carcinoma, oral, and anogenital cancers occur at increased risk, as do cancers causally associated with high prior sun exposure, smoking and excessive alcohol consumption, including skin, oesophageal, larynx, lung, kidney, and bladder cancer. The risk of incident breast and prostate cancer is not increased. Cancer-related deaths largely mirror that for cancer incidence, and extend to include the more common malignancies such as breast, colorectal, prostate cancer and non-melanoma skin cancer. As medical immunosuppression is the principal risk factor for cancer, the regimen should be reviewed on a regular basis to achieve immunosuppression minimisation. An individual, risk-based approach to cancer screening according to test characteristics and personal and family cancer history, medical history, lifestyle factors, and life expectancy is recommended. Multicomponent interventions may achieve the best results in supporting the adoption and maintenance of cancer risk-reducing behaviours. Regular, empowering patient counselling and education is a cornerstone for the care of people living with a liver transplant.
Keywords: Cancer, risk, prevention, screening, surveillance, cohort
INTRODUCTION
Advances in surgical techniques, patient selection and immunosuppressive regimens have improved survival outcomes for liver transplant recipients. However, people living with a transplanted liver continue to have an excess risk of cancer and death from cancer. This excess cancer risk is predominantly attributed to exposure to medical immunosuppression[1], but other known carcinogenic exposures before and after transplantation such as infection with an oncogenic virus, smoking, heavy alcohol consumption and sun exposure, are also contributing factors[2]. Recipients of liver transplants have a lower risk of cancer compared to recipients of cardiothoracic organs and kidneys, due to generally lower overall doses of immunosuppression[3-5].
Liver transplantation is an effective therapeutic option for select patients diagnosed with hepatocellular carcinoma (HCC)[6]. Improved patient/tumour selection algorithms have resulted in similar survival outcomes for people living with a transplanted liver with and without a history of HCC[6-8]. HCC is a relatively common indication (40%-50%) for transplantation in countries with a higher incidence of HCC and is an increasing indication for liver transplantation globally. In the United States in 2018, HCC was the primary indication for 10.5% of waitlist candidates, and the third most common indication for transplantation[9]. Importantly in terms of cancer risk after transplantation, the proportion of older candidates (age ≥ 65 years) has also increased over time; such individuals comprised 24% of the United States waitlist candidates in 2018[9].
This review presents the current evidence on the risk ofde novoextrahepatic cancer in people living with a liver transplant after HCC, the risk factors for cancer, and the recommended approaches to cancer prevention and surveillance. There is limited reliable evidence about cancer risk after liver transplantation for HCC. However, international population-based evidence indicates the indication for transplantation does not markedly alter the cancer risk profile, and thus much can be inferred from such studies of all liver transplant recipients as well as other solid organ transplant recipients. This article synthesises populationbased evidence on the epidemiology of cancer after liver transplantation for any indication and, where it exists, transplantation for HCC.
CANCER RISK
Historically, most high-quality evidence on the cancer incidence and mortality profile of people living with a liver transplant has been generated from Western populations, due to their long-established population cancer registries and record linkage infrastructure. Such infrastructure is needed to perform populationbased studies, defined as studies of all transplant recipients that capture all incident cancers, regardless of the setting in which they were diagnosed. These studies are considered the highest quality of observational evidence. However, the findings may not be entirely generalisable to other populations with different characteristics, particularly if they vary with respect to the prevalence of infection with oncogenic viruses,especially hepatitis B (HBV), hepatitis C (HCV), human immunodeficiency virus (HIV), human papillomavirus (HPV) and Epstein-Barr virus (EBV)[10], and the predominant indications for transplantation[11]. Furthermore, most of these studies have reported on all liver transplant recipients, not just those transplanted for HCC, and in Western populations the proportion of recipients with a history of HCC is comparatively small (~10%) compared to many Eastern populations (40%-50%)[3,12-14].
The population-based evidence from Western, predominantly Caucasian populations shows a 2- to 3-fold excess risk of cancer in adults living with a liver transplant [Table 1][3,12,13,15-17]. These national studies have typically shown an excess cancer risk regardless of the indication for transplantation, with the highest risk usually observed for people living with a liver transplant after alcoholic cirrhosis and primary sclerosing cholangitis[14]. Evidence also shows a high short-term cancer risk, driven mostly by the occurrence of non-Hodgkin lymphoma in the early post-transplant period[12]. However, cancer risk is not confined to the early post-transplant period, and continues to increase with increasing time from transplantation and exposure to immunosuppression. Some[14], but not all[11,18], studies that have examined cancer risk by year of liver transplantation suggest a lower risk of cancer in people transplanted in more recent eras. This is likely to be associated with transplant clinical practice recommendations to minimise the overall dose of immunosuppression.
Non-Hodgkin lymphoma is the most common non-skin cancer in people living with a liver transplant. In solid organ transplant recipients, high short-term risk is associated with more intense immunosuppression and lack of immunological control of EBV infection in the early period[5,19]. However, an excess risk is also observed many years after transplantation. In Western populations, the most common solid malignancy after liver transplantation is skin cancer, and an excess skin cancer risk is observed throughout the followup period. This includes melanoma, keratinocyte cancer [basal cell carcinoma (BCC) and squamous cell carcinoma (SCC)] and Merkel cell carcinoma, although these malignancies are less common among recipients of liver transplants than among those of cardiothoracic transplants[3].
Virus-related cancers, especially non-Hodgkin lymphoma, Kaposi sarcoma, Merkel cell carcinoma, oral(head and neck), cervical and other anogenital cancers occur at increased risk in liver transplant recipients[2], as do cancers causally associated with smoking and excessive alcohol consumption, including oesophageal, larynx, lung, kidney, and bladder cancer. The risk of breast and prostate cancer is not increased in people living with a liver transplant. The increased risk of colorectal cancer observed in liver transplant cohorts is confined to those receiving a liver transplant with a history of primary sclerosing cholangitis with and without inflammatory bowel disease[20-22]. Evidence is emerging from very large-scale cancer registry linkage studies of solid organ transplant recipients that they are also at excess risk of numerous rare cancers (incidence < 6 per 100,000 person-years), including cancers of the eye and adnexa,salivary gland, nasal cavity and anus, and neuroendocrine tumours and specific subtypes of sarcoma[23]. The risk of several, but not all, of these cancers was shown to increase with increasing time since transplantation.
More recently, evidence on the risk of cancer in people living with a liver transplant has been published for Taiwanese and Korean populations. Although the source of transplant recipients and cancer outcomes in these studies is health insurance databases, and is thus not population-based, this evidence also demonstrates a moderate excess risk of cancer[24-26]. A range of viral- and non-viral cancers were observed to occur at rates higher than background rates, including skin cancer in some cohorts.
The whole-of-population evidence regarding cancer risk after liver transplantation for HCC is less extensive, with few studies having sufficient power to generate reliable estimates for individual cancers[Table 2]. The largest study showed a 1.6-fold excess risk of any cancer, and a similar site-specific cancer risk profile to that observed for all people living with a transplanted liver[14]. Non-population based evidence from South Korean patients transplanted for HCC has also generated a similar cancer profile to that described above, as well as an excess risk of leukemia, myeloma, and thyroid cancer[27]. Interestingly, this study found a substantially higher short-compared to long-term risk of cancer, with most cancers diagnosed within the first 12 months of transplantation.
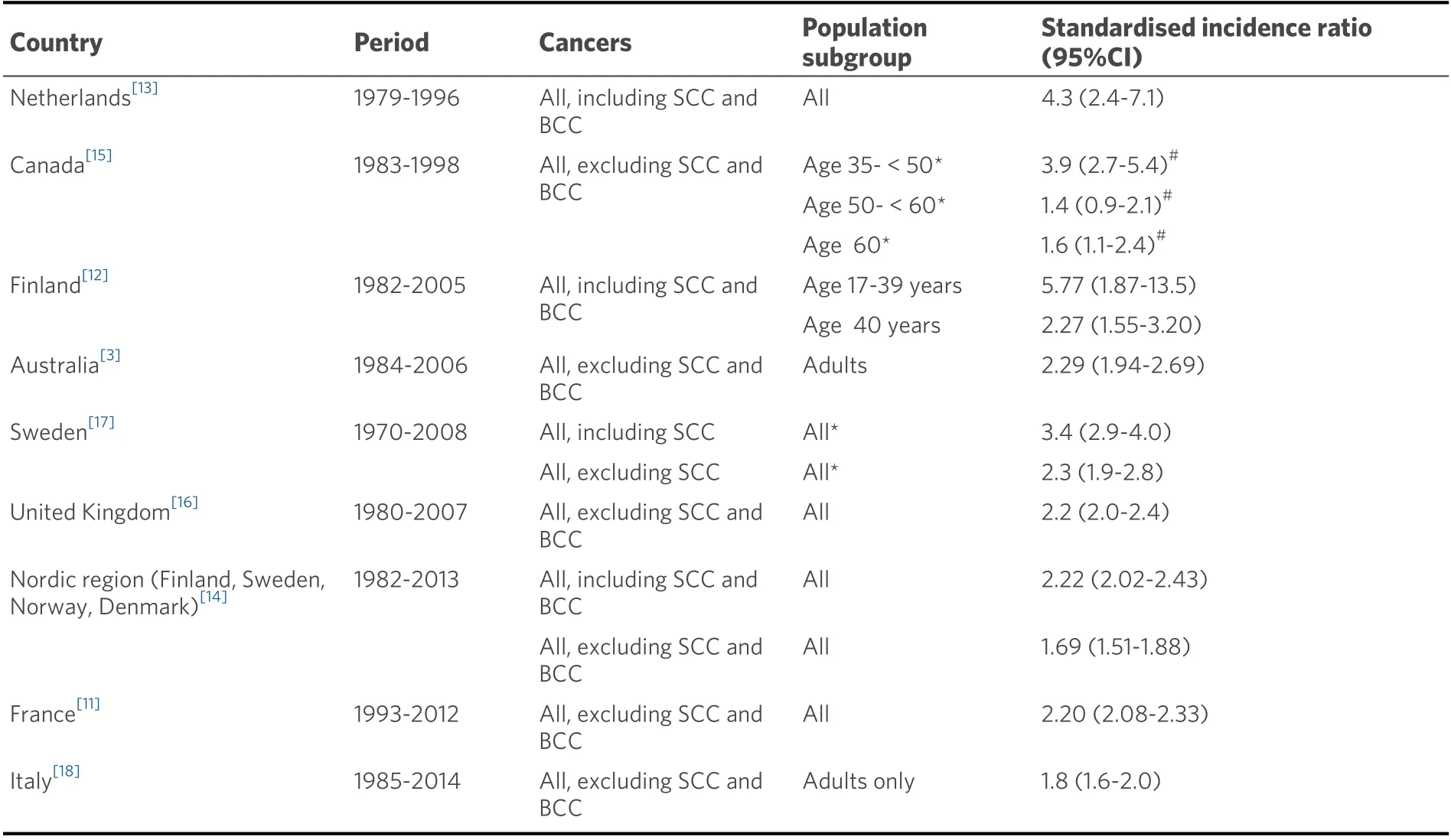
Table 1. Risk of any cancer in national population-based cohort studies of people living with a liver transplant, irrespective of indication
CUMULATIVE CANCER INCIDENCE
Large-scale data from the United States Scientific Registry of Transplant Recipients (SRTR) database, based on physician-reported cancer diagnoses, estimate the cumulative incidence ofde novoextrahepatic cancer after liver transplantation for any indication to be 1.3% (95%CI: 1.3-1.4) at 1 year post-transplantation,increasing to 6.2% (6.0-6.4) at 5 years, 11.5% (11.3-11.8) at 10 years, 15.8% (15.5-16.2) at 15 years, 18.8%(18.4-19.3) at 20 years and 20.5% (19.9-21.1) at 25 years[28]. Estimates from German single-institution studies vary; 12.9% at 10 years and 23% at 25 years in one study[29], and 10% at 5 years, 26.4% at 10 years and 34.7%at 15 years in another[30].
CANCER MORTALITY RISK
Thirteen percent of deaths overall[31]and up to 20% of long-term deaths in people living with a liver transplant[32], are attributed to cancer. Population-based evidence indicates a 2-fold excess risk of death from cancer in Western populations transplanted predominantly for indications other than HCC[11,33,34]. In one Canadian study of solid organ transplant recipients, the highest risk of cancer mortality was observed in recipients who underwent transplantation for cancer[34]. The cancer mortality profile largely mirrors that for cancer incidence, and extends to include non-melanoma skin cancer[18,33], as well as breast, colorectal and prostate cancer[18,35,36].
The excess risk of cancer death after liver transplantation is related to the increased incidence of cancer, and may also reflect the more advanced stages of cancer at diagnosis and likely more biologically aggressive cancers in the context of immunosuppression[35,37,38]. Furthermore, it appears that solid organ transplant recipients may receive less aggressive cancer therapy, either on account of their frailty, or to minimise therisk of graft rejection. In a United States study, transplant recipients with cancer were less likely to undergo surgery and radiotherapy compared with non-transplant recipients with cancer[35].

Table 2. Risk of any cancer and specific cancer in national population-based cohort studies of people living with a liver transplant after HCC or liver tumour
An analysis of United States SRTR data on adults transplanted for HCC showed that malignancy was more prevalent as a cause of late- (> 5 years) compared to early-mortality (1st year)[39].
RISK FACTORS FOR ANY CANCER
Another analysis of the United States SRTR database, which includes longitudinal measures of immunosuppressive therapy up to 12 months after liver transplantation, found risk of any cancer increased with older age at transplantation, male sex, white race, multiorgan transplant, history of any cancer, and alcoholic liver disease, autoimmune disease, non-alcoholic steatohepatitis and primary sclerosing cholangitis compared with hepatitis C viral infection[28]. This study found no association between cancer risk and type of immunosuppression. Another interrogation of the United States SRTR data showed an increased cancer risk in those with a history of cutaneous SCC, but not BCC[40].
Most population-based studies do not have data on recipients’ smoking or tobacco history. However, singlesite studies, with well characterised liver transplant cohorts, have shown a higher risk of extrahepatic cancer,for current and previous smokers compared to non-smokers[41,42]. In addition to smoking, a large-scale single-site study in Germany observed that older age and use of cyclosporine-A compared to tacrolimus therapy increased the risk ofde novocancer[29]. In contrast, another single-centre German study that did not have smoking data found increasedde novocancer risk in association with older age, male gender and tacrolimus-based immunosuppression at transplantation[30]. In this study, HCC as an indication for transplantation was not associated with cancer risk in multivariable modelling.
A population-based United Kingdom Transplant Registry study examined all solid organ transplant recipients and found no evidence of an association between risk of cancer and cytomegalovirus infection[43].
RISK FACTORS FOR THE MOST COMMON CANCERS
Non-Hodgkin lymphoma
The incidence of post-transplant lymphoproliferative disorders/non-Hodgkin lymphoma after liver and other solid organ transplantation follows a bimodal pattern[19,44,45]. In one population-based study of kidney transplant recipients, early non-Hodgkin lymphoma (< 2 years after transplantation) was associated with EBV seronegativity at transplantation and receipt of antibody therapy[19]. The association of early non-Hodgkin lymphoma with EBV seronegativity at transplantation is a consistent finding and explains the markedly higher risk of early non-Hodgkin lymphoma in paediatric compared to adult transplant recipients[46], in the absence of prophylaxis and monitoring of EBV viral load. Late non-Hodgkin lymphoma(≥ 2 years after transplantation) was associated with older age at transplantation, increasing time since transplantation and current use of calcineurin inhibitors. Another population-based study of liver and cardiothoracic transplant recipients found an increased risk of early- and late-non-Hodgkin lymphoma in association with higher mean daily doses of azathioprine[5]. Azathioprine is not currently recommended in liver transplantation, regardless of indication.
Head and neck cancer
A multi-centre Italian study identified an association between older age at transplantation, greater time since transplantation, history of heavy smoking and history of alcoholic liver disease and the incidence of head and neck cancer after liver transplantation, with risk markedly increased in those with a history of both smoking and alcoholic liver disease[47].
Keratinocyte skin cancer (BCC and SCC; non-melanoma skin cancer)
Population-based evidence in solid organ transplant recipients indicates increased risk of keratinocyte cancer in association with older age at transplantation, white compared to black race, longer time since transplantation, history of skin cancer prior to transplantation, occurrence of precancerous skin lesions after transplantation, and male sex[16,48,49]. Several of these factors are proxies for high prior sun exposure,information that is not usually available at the population-level. A United Kingdom single-centre study of ethnically diverse organ transplant recipients reported that light skin phototype (Fitzpatrick skin type I-IV),history of sun exposure, older age at transplant and longer time since transplantation increased the risk of keratinocyte cancer[50]. A French single-centre study of liver transplant recipients observed a very similar risk profile, and no association with indication for transplantation or immunosuppressive agent[51]. A Korean single-centre study reported that risk of skin cancer was associated with older age at transplantation and treatment with more than two immunosuppressive agents; no associations were observed for individual immunosuppressive agents[52].
A recent retrospective study in two French transplant centres found that risk of keratinocyte cancer in liver transplant recipients with a history of SCC, BCC or Bowen’s disease was significantly reduced after withdrawal of calcineurin inhibitors[53]. Furthermore, a retrospective population-based Irish study of liver transplant recipients observed a significant reduction in keratinocyte risk after conversion of maintenance immunosuppression from calcineurin inhibitors to mTOR inhibitors for clinical indications[54]. However, a meta-analysis of studies of non-renal organ transplant recipients found no significant protective effect of mTOR inhibitors on risk of primary or secondary keratinocyte cancer[55]. Nevertheless, the complete risk profile must always be considered, as demonstrated by a meta-analysis of individual patient data from randomised trials of kidney transplant recipients that found sirolimus use was associated with a reduced risk of any malignancy and non-melanoma skin cancer, but an increased risk of all-cause death[56].
Lip cancer
In an Australian population-based study of liver and cardiothoracic transplant recipients, the risk of lip cancer was associated with older age at transplantation, earlier transplantation era, greater time since transplantation, history of smoking and higher current and mean dose of azathioprine[4].
RISK FACTORS FOR CANCER-RELATED DEATH
A study of United States SRTR recipients transplanted for HCC with linked national pharmacy claims data found no relationship between cancer-specific mortality and use of sirolimus in the first 3 months after transplantation[57]. This finding mirrored those for all-cause mortality, and is thus at odds with the metaestimate from randomised trials of kidney transplant recipients[56].
TRANSMISSION OF DE NOVO CANCER
On account of rigorous donor evaluation and inspection of organs prior to transplantation, the risk of transmittingde novocancer from a liver donor to a recipient is exceedingly low (0.03%-0.06%). A recent systematic review of case reports identified a total of 92 confirmed transmitted cancers, the most frequent being lymphomas (33%), melanomas (9%) and neuroendocrine tumours (9%)[58]. Most (80%) transmitted cancers were diagnosed within the first year, and 90% were diagnosed within 2 years.
Then he will bring forward a whole troop of women, and cause them to pass before you, in order that you may pick out the one that you take for his daughter
CANCER PREVENTION AND SURVEILLANCE
There is consistent evidence that cancers diagnosed in people living with a transplanted organ are at a more advanced stage than those in the general population, and that transplant recipients with cancer have worse outcomes than the general population with cancer. The bulk of this evidence comes from population-based studies of kidney transplant recipients[35,37,38], but there is also evidence from large single-site studies of liver transplant recipients[20]. It is therefore critical that cancer prevention and surveillance are built-into the care of people living with a liver transplant, to reduce the risk of cancer and to detect cancers at an early stage of development.
Immunosuppression
There is uncontested evidence from organ transplant recipients and people living with HIV infection linking immunosuppression and cancer occurrence[1,2]. The mechanism is believed to be impaired immune surveillance of neoplastic cells. The risk of cancer in people living with a liver transplant can be reduced by tapering the dose of immunosuppression whilst balancing this risk against the risk of loss of the allograft,renal toxicity and metabolic syndrome. It is therefore recommended that the immunosuppression regimen be reviewed on a regular basis to achieve immunosuppression minimisation. Innovative techniques are being explored to reduce the need for lifelong high-dose immunosuppression, including treatment with natural regulatory T cells (nTregs)[59]. In select people living with a transplanted liver for at least 3 years,immunosuppression can be stopped without graft rejection or subclinical alloimmune damage, a state known as “operational tolerance”[60]. Operational tolerance is more likely to be achieved in older recipients(> 60 years) and those without autoimmune liver disease, but it is not generally recommended for HCC patients.
The most appropriate immunosuppressive agent(s), and whether immunosuppressive drugs can be directly oncogenic are contentious. Most observational cancer risk factor studies have assessed the immunosuppressive regimen at transplantation and have not taken into account changes over time, and nor have they controlled for the duration of immunosuppression. The few population-based studies with detailed longitudinal data on immunosuppressive agents are inconsistent.
Infection with oncogenic viruses
Hepatitis B and tetravalent HPV vaccination are recommended for HPV-negative and paediatric patients,ideally before transplantation. Antiviral drugs for HBV, HCV and HIV infection must be administered as early as possible after diagnosis.
Cancer screening
An individual, risk-based approach to cancer screening according to patient characteristics including personal and family cancer history, medical history, lifestyle factors, and life expectancy is recommended for people living with a liver transplant. It is also important to consider the screening test characteristics, and to acknowledge that the cancer screening recommendations for the general population have not been extensively tested in people living with a liver transplant.
As there is sufficient evidence of an excess risk of cervical cancer in liver transplant recipients, health professionals are advised to follow the guidelines for another immunosuppressed group, HIV-infected women[61]. This means annual cervical cytology after transplantation, and if 3 consecutive cytology results are normal, moving to cytology every three years. Co-testing with cytology and HPV testing is preferred; if the cytology is normal and HPV is negative, then co-testing can be performed every 3 years. The risk of cervical cancer extends for many years after transplantation, necessitating lifelong screening beyond age 65 years. Cervical screening should only be discontinued on the basis of a shared agreement between the physician and patient, taking into account the quality and duration of life. For male and female transplant recipients, an annual anogenital examination is recommended.
Melanomas diagnosed in people living with a transplanted organ are at a more advanced stage than those in the general population, and the survival outcomes are worse[37,62]. Physicians should conduct an annual total skin assessment for people at low risk of melanoma or non-melanoma skin cancer, and refer those at high risk for an annual total skin assessment by a dermatologist, preferably experienced in the management of people with immunosuppression. Prophylactic excision of truncal nevi has been advised on the basis of high-quality Swedish data[62], while in an Australian setting, referral to a specialised transplant dermatology clinic resulted in the early detection of melanomas and markedly improved outcomes[63]. Similarly,adherence to annual dermatology assessments has been shown to reduce keratinocyte cancer-related morbidity or death[64]. People living with a liver transplant should also perform regular skin checks themselves and seek urgent medical opinions if they notice a change. Harwoodet al.[50]have generated an algorithmic, evidence-based risk stratification approach to skin cancer screening of an ethnically diverse transplant population that includes skin type, age at transplant and sunburn history; annual surveillance is recommended for the higher risk group, and modifications are necessary after the first SCC or BCC.
As there is no excess risk of breast, bowel or prostate cancer after liver transplantation (other than bowel cancer for those with inflammatory bowel disease), patients should be advised to participate in national screening programmes for these malignancies, where available, according to the guidelines for the general population, or as indicated by their family history. Some argue, however, that given the excess risk of death for these cancers, and the consistent evidence of biological aggressiveness in the context of immunosuppression, transplant recipients should receive more intensive screening, including colonoscopy for all those over 50 years of age[29,36]. It may also be advisable to start bowel cancer screening earlier than population-based recommendations for those with a history of alcoholic or viral cirrhosis.
Given their higher risk of lung cancer, people living with a liver transplant who are former or current smokers may benefit from annual lung cancer screening using low-dose chest computed tomography (CT).Clinical trial evidence in non-transplant populations indicates lower lung cancer mortality in screened individuals than that in unscreened individuals[67,68], although false positives are common and screening is not yet routinely implemented in all settings. A recent small French hospital-based study (n= 147)retrospectively examined the efficacy of an intensive cancer screening program for people living with a liver transplant for alcoholic liver disease who actively smoked[69]. The screening program consisted of an annual health check, clinical examination by an otorhinolaryngologist, a chest CT scan and an upper digestive endoscopy. Overall, this program did not result in higher rates of curative treatment over a median followup of 10 years, but when a lung cancer diagnosis was made by CT scan compared to symptoms, curative treatment was statistically significantly more likely.
Given the excess risk of oral cancers in people living with a liver transplant, good oral hygiene and annual dental examinations, with a full examination of the oral cavity, are strongly recommended. Annual ear, nose and throat examinations by a head and neck specialist should be performed for current and former smokers and people with a history of alcohol-related liver disease.
Liver transplant recipients in countries with a high background incidence of gastric cancer may benefit from screening with esophagogastroduodenoscopy (EGD) more frequently than recommended for the general population (i.e., every 2 years for people aged 40 years in South Korea)[70].
Lifestyle interventions
Patient counselling is a cornerstone for the care of people living with a liver transplant. In addition to maintaining their immunosuppressive medications, transplant recipients must be actively supported to stop smoking, including with the assistance of a specialist counsellor, nicotine patches, and/or pharmacological agents.
Transplant recipients must also be counselled and supported to avoid sun exposure and sunburns, and if sun exposure is unavoidable, then they must wear a wide-brimmed hat, full-length clothing and high sun protection factor sunscreen, and the sunscreen must be reapplied regularly. They must also be advised never to use a sunbed or sunlamp to achieve a suntan.
Similarly, advice, encouragement, education and support should be directed towards abstinence from alcohol consumption, maintaining a healthy diet and healthy weight, and pursuing regular physical activity.Patients with excessive alcohol use should be referred to addiction services, where appropriate. Surgical and medical solutions available to the general population to manage obesity are not contraindicated in liver transplant recipients. Individuals at high risk of keratinocyte cancer may benefit from targeted nutritional management, specifically advice and support to maintain a relatively high intake of long-chain omega-3 polyunsaturated fatty acid and -linolenic acid, such as via the consumption of oily fish[71].
Self-efficacy and accountability to the medical team are motivating factors to follow medical advice, and multicomponent interventions including personalised care plans, education, psychosocial support, decision aids and self-monitoring tools may achieve the best results[72]. Clinicians should facilitate honest and open discussions with people living with a liver transplant, and their family, regarding lifestyle behaviours that increase, and decrease the risk of cancer. A holistic approach is essential, being mindful of an individual’s financial constraints, and other relevant social and cultural factors. Empathy and an exploration of patient attitudes to certain behaviours, including discussing the benefits of those behaviours, may translate to less risky behaviours[73]. Patients would likely benefit from a coordinated medical approach, with sharing of information, strategies and outcomes with their primary care clinician[74].
Chemoprevention
A retrospective study in United Kingdom organ transplant recipients with a history of at least one SCC found that low-dose systemic retinoids reduced the incidence of new SCCs[75]. Since this study, the evidence has grown, and oral retinoids are recommended for the prophylaxis of keratinocyte cancer and actinic keratoses in organ transplant recipients. Chemoprophylaxis with other agents, including oral nicotinamide and capecitabine, may also be effective at reducing the incidence of melanoma and keratinocyte cancers,although supportive evidence in organ transplant recipients is limited.
CONCLUSION
Liver transplant recipients have a 2-fold excess risk of cancer and cancer-related death after transplantation,regardless of the indication for transplantation. HCC is an increasing indication for liver transplantation.The most common incident cancers are those causally associated with oncogenic viruses, and those associated with sun exposure, smoking and alcohol consumption. The cancer mortality profile includes most cancers. Cancer risk can be reduced by minimising the extent of immunosuppression, by performing risk-stratified cancer screening and surveillance, and by giving regular, empowering support and education to recipients so they can adopt and maintain cancer risk-reducing behaviours.
DECLARATIONS
Authors' contributions
Made substantial contributions to the conception and content of the report: Vajdic CM, van Leeuwen MT,McCaughan G
Drafting the work or revising it critically for important intellectual content: Vajdic CM, van Leeuwen MT,McCaughan G
Final approval of the version to be published: Vajdic CM, van Leeuwen MT, McCaughan G
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2021.
- Hepatoma Research的其它文章
- Prevention of hepatitis B virus recurrence
- GNMT: a multifaceted suppressor of hepatocarcinogenesis
- Systemic therapies for hepatocellular carcinoma: an evolving landscape
- Gut microbiome profiles associated with steatosis severity in metabolic associated fatty liver disease
- Steatohepatitic hepatocellular carcinoma
- Epidemiology and aetiology of hepatocellular carcinoma in Sub-Saharan Africa