Genetic and biochemical aspects of quorum sensing in the bacterial lifestyle and pathogenesis
Nafisa Tabassum,Syed Abdullah Ibn Asaduzzaman,AKM Ashik Ullah,Afia Ibnat Ava,Rahmatur Rob Kawnain,Rabib Hasan Mahin,Faiqa Samrose Sezen,Rashed Noor*
1Department of Life Sciences (DLS),School of Environment and Life Sciences (SELS),Independent University,Bangladesh(IUB),Dhaka 1229,Bangladesh.
Key words:Bacteria,Ecology,Communication,Quorum sensing,Antibiotic resistance
Background
In the last few decades,the general perception on bacterial adaptation within a range of environments and their poly-microbial communities has changed dramatically due to raising concerns on bacterial quorum sensing (QS) which is preliminarily known to manage the interaction and communication among the adjacent cells via the multi-complex cell-cell networking [1,2].QS has been noticed in an array of physiological processes including in the development of biofilm,facilitating the plasmid mediated conjugation,imparting motility,and conferring resistance against a range of antibiotics [3] (Figure 1).The advanced molecular innovative approaches have been found useful for studying the bacterial growth dynamics,interactions among the mixed microbial communities,and certainly,the progressive research on the complex QS system triggered by the microbial consortia [1–3].These advanced experiments described the contribution of QS in the rise of the resistance ability of microorganisms by triggering the regulation of the QS system [4–7].
The mechanism behind the drug resistance acquired by the employment of the QS system greatly relies on the cellular signaling cascade,commonly consisting of the acyl-homoserine lactone (AHL) molecules,the auto inducing peptides (AIPs),the autoinducer-2 (AI-2),etc.that regulate the steps in bacterial pathogenesis [5,6].These signals also participate in the synthesis of virulence factors; for example (1) lectin,exotoxin A,pyocyanin,and elastase during the growth and infection phases ofPseudomonas aeruginosa,and (2) production of hemolysins,protein A,enterotoxins and lipases in case ofStaphylococcus aureusinfection [8–12].The cell-to-cell communication usually depends on the population-density; and relies upon the regulation of pheromone-like biochemical molecules called auto inducers [5,6].
Bacterial QS is promoted by these auto inducers which regulate virulent genes and other proteins involved in basic metabolic processes [6] (Figure 2).
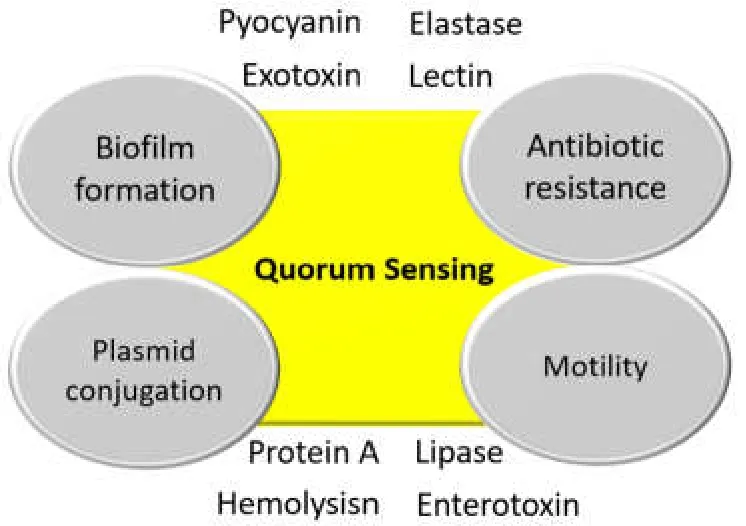
Figure 1 Effects of QS on the physiological process of bacteria.
The anti-QS agents can be considered as the alternatives to antibiotics because of their potential in lowering the bacterial virulence with a concomitant elimination of pathogenic microorganisms [4].The QS mechanism employed by the pathogens not only regulates the generation of virulence factors but also helps to adapt to the diverse metabolic demands of different ecosystems.Previous studies proved that the QS systems may influence about 4%–10% bacterial genome and approximately 20% of the proteomes [13–17].As QS is continually reported to be involved in developing the resistance ability of various pathogenic microorganisms and promoting bacterial infection,researchers in laboratory,hospital or industry are trying their level best in formulating the antimicrobial agents to inhibit or inactivate cell density-dependent QS signaling.
Based on the large arena of the bacterial QS network as observed especially in case of different environmental,genetic and clinical issues,the study of the signals involved in the QS system,as well as the anti-QS components,have been conducted by many groups.Present review compiled the knowledge on such QS procedure focusing on the mechanism of bacterial QS and their variations,bacterial (both inter- and intraspecies) communications,the QS signals,formation of biofilm for the bacterial growth and survival,and finally the impact of such QS system on food spoilage.The profound insightful knowledge on QS will certainly help to expand the understanding of the microbial molecular ecology which in turn would go a long way to study the drug-resistance spectrum of bacteria and the associated virulence mechanisms.
Bacterial communications via QS
Besides the extracellular signals,the QS system also largely depends on the microbial gene expression profile; i.e.,the production of the corresponding proteins to sense the appropriate signal(s) [17,18].Thus,the signal molecules or the auto-inducers (as stated above),when elevated,may instigate a positive feedback loop which orchestrates the overall QS mechanisms [19].The mechanism of QS follows initially the production of biochemical signals by the bacterial cells,and after that the bacterial consortium continually generates the signal(s) primarily at a minimal concentration followed by the subsequent release into the surrounding system,and such accumulation of these signals boosts as the population density increases.As these molecules are secreted in specific acceptable concentration,the other cells recognize them and such signal-receptor interaction leads to changes in genetic regulation or the expression of new gene(s) according to the demand to maintain the cellular homeostasis (Figure 3).The QS signals (both intra-species and inter-species) vary among the bacterial population depending on the cell type,surrounding environment,the internal or external stimuli,and on the survivability and the drug-resistance ability of cells[20–23].For instance,many proteobacteria utilize AHL-type signals while the Gram-positive bacterial species employ the shorter oligopeptide signals.Several other bacteria employ the QS mechanism to produce the anti-microbial peptides or exotoxins,develop biofilm and evolve the mechanism of nitrogen fixation [24–26].
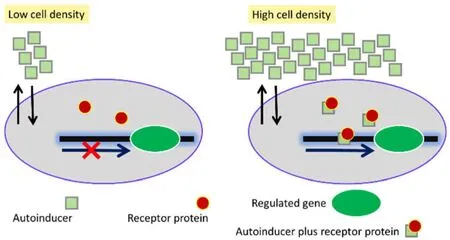
Figure 2 Linkage between auto-inducer and cell density.
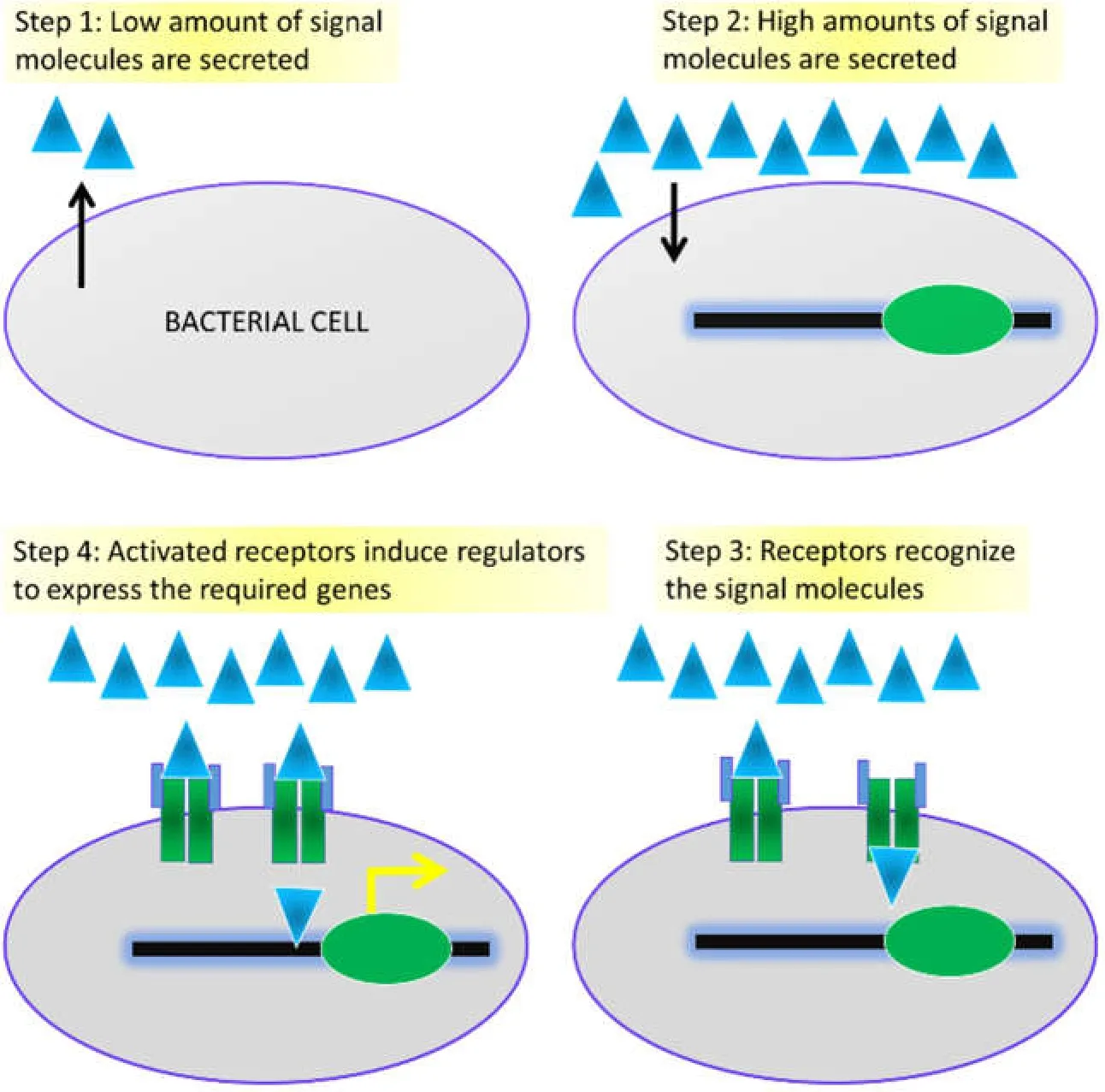
Figure 3 Major steps in QS.
Variations in QS among Gram-positive and Gramnegative bacteria
The AHL signa ling molec ules hav e be en noticed in the Gram-negativebacteriawhileinGram-positive bacteria,the AIP signaling molecules are dominant [27,28].However,both are able to produce and sense the AI-2 signal,liberated by LuxS enzyme [29].In the Gram-negative bacteria,the S-adenosyl methionine(SAM) together with an acyl-acyl carrier protein aids in the synthesis of AHLs with the help of an enzyme of the LuxI family of the AHL synthases,followed by sensing withluxR gene products (the response transcriptional regulators).This is to be noted that the LuxR/AHL complex regulates the expression of multiple-target genes; and the Lux-I type AHL synthase circuit plays a role in the QS signaling mechanism [30,31].Pseudomonas aeruginosahas been reported to produce multiple short chained AHLs which gather in the extracellular environment as a result of QS signaling[32–35].The signal molecules diffuse across the cell membrane,bind to the specific QS transcriptional regulators,and trigger the expression of the target genes required for cellular survival with a consequence of integration by the small RNAs,required for the associated feedback loop mechanism as a trait of QS[36–39].Additionally,tRNA-dependent cyclodipeptide synthases ofP.aeruginosacontrol the regulation and production of another autoinducer named cyclic dipeptides (2,5-diketopiperazines; DKPs).Thus,the genetic information like the RNA promotes the signaling fidelity,the temporal control and flexible input–output dynamics by controlling the synthesis of virulence factors [39].Furthermore,several chemical factors including temperature,pH,NaCl,growth medium,inoculum size,etc.govern the concentration and type of AHLs; and in such process the cellular growth phase (whether they are in the exponential state or in stasis) are of significance (Figure 4) [40–42].Generally,the Gram-positive bacteria synthesize AIPs(bind to a by-component histidine kinase sensor if the signaling molecules reach the threshold level) which are secreted by membrane transporters-ATP binding cassette transporter needed for the intercellular communication [44].S.aureususes an accessory gene regulator which is associated with AIPs secretion,production of toxins and degradable exoenzymes [43,44].
Inter-species & intra-species communication
AI-2,synthesized from SAM,is abundant in more than 70 bacterial species; and hence are considered as a universal signaling language among the diverse bacterial community [45].The synthesis of AI-2 can be influenced by the temperature and the growth medium constituents [1,2,45,46].For example,production and transportation of the AI-2 signal into the cell of S.enterica serovar Typhimurium can be increased in response to high glucose and osmotic stress which may also trigger the activation of the lsr operon that is involved in the AI-2 transportation within the cells in the biofilm,possibly for imparting the traits as shown in Figure 1 [45].
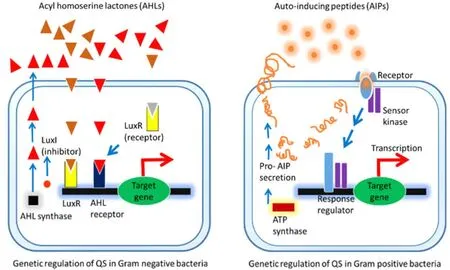
Figure 4 Different mechanism of AHL and AIP signaling.
The lux-type QS system and the AHL signaling in Gram negative bacteria
In Gram-negative bacteria,the lux-type QS system and AHL are intently linked since the former one is dependent on the AHLs [47].The pre-eminent lux-type QS system consists of two major components: (1) LuxI,the autoinducer synthase (synthesizes AHLs from SAM); and (2) LuxR,a transcriptional regulator which facilitates AHL to diffuse across the cell membrane smoothly [3,47].Eventually,as the population rises as well as the signals released by the cells reach to the threshold point,the intracellular AHL tends to bind to the LuxR-like receptor within the cytosol to persuade differential gene expression (Figure 4).P.aeruginosa has been shown to become virulent by using 2 distinct lux-type QS systems: las and rhl [48].Some molecular studies demonstrated the central role for AHL QS system in P.aeruginosa pathogenesis [48–50].Such findings were confirmed when the genetically modified las and rhl system mutants had been noticed to significantly reduce the virulence [51–53].
Common signaling molecules involved in the QS system
Depending on cellular structure,intra- and inter-species communication,and origin/evolution of species,microorganisms apply a number of signaling molecules(like the AI-1 and AHLs by Gram-negative bacteria and the AI-2 and furanosyl borate diesters by both Grampositive and Gram-negative bacteria) as stated above[54–56].The autoinducer-3,responsible for host intestinal colonization with the formation of attaching and effacing lesion,is involved in Escherichia coli QS signaling [53,54,56].The AIPs are utilized only by the Gram-positive bacteria [58].PQS (2-heptyl-3-hydroxy-4-quinolone),cyclic dipeptides or the di-ketopiperazines are used by P.aeruginosa [59,60].Vibrio cholerae utilizes the cholera autoinducer-1 that is responsible for adherence in course of QS mechanism[61,62].
Gene transfer via QS
QS upholds a multi-complex networking between interor intra-species levels by means of horizontal gene(consisting of QS components) transfer [63–65].A phylogenetic analysis of 76 individual LuxI and LuxR homologues present in diverse members of the Gramnegative proteobacteria showed that these homologues had been inherited horizontally.In P.aeruginosa,a transferred QS system has been observed whereby the LuxI/R family in Pseudomonas species conserved the mechanism of lateral gene transfer,the ancestral duplication as well as loss of genes [66].Interestingly,it has been recently determined that the existence of the mobile genetic elements (i.e.the transposon) is also possible to notice in the QS system as a functional AHL system within a mobile transposon in Serratia marcescens [67].One recent example of QS mediated transfer of gene may be drawn through the work of Wu et al.(2021) whereby the communication between the host intestinal bacteria under normal physiological or pathological conditions have been well explained as the gene transfer output [64].While several QS signal molecules are engaged in the crosstalk between host cell and the infecting pathogens under the normal physiological condition; under pathological conditions,AI-2 has been reported to promote the TNFSF9 gene expression (Tumor Necrosis Factor Ligand Superfamily Member 9) in macrophages which in turn results in host protective immunity.
Biofilm formation triggered by QS and its clinical complication
Biofilm formation is an essential requirement for the bacterial growth and survival in some of the unusual domestic environment by producing extracellular polymer and adhesion matrix [65–68].Within a biofilm,bacteria become more resistant against antibacterial agents and radiation by changing their gene expression[69–71].Biofilm development initially involves the attachment on the surface,followed by stalkless growth instigated by the QS factors with the maturation of the biofilm [70].It is worth to note that several clinically important pathogens like Pseudomonas aeruginosa,Staphylococcus aureus,Streptococcus mutans,Fusobacterium nucleatum,Lactobacillus casei and Actinomyces naeslundii can facilitate infection within the host through biofilm formation [70].Interestingly,the blockage in QS signal pathway prevents the biofilms formation as well as renders the pathogenic bacteria sensitive towards the anti-microbial agents [72,73].For example,Terminalia bellerica,a medicinal plant,works as an anti-QS and anti-biofilm agent to control infections caused by Pseudomonas spp.[73].Furthermore,N-2-pyrimidyl-butanamide,another QS inhibitor,and also known as C11,possesses the potential to hinder the biofilm formation capacity of P.aeruginosa [73].However,conclusively the most interesting feature of the microbial consortium within the biofilm is revealed through their adaptive responsiveness to the varying environment with nutrient depletion [70].
QS contribution in food spoilage
The ubiquitous bacteria likeP.aeruginosaorS.aureusare of prime importance for food spoilage since these species can be easily disseminated from the surrounding environments.Studies have shown that the production of virulence factors by these bacteria (pyocyanin,elastase,lectin,and exotoxin produced byP.aeruginosa; and hemolysin,proteinA,lipase,and enterotoxin produced byS.aureus) are completely dependent on the QS system which may influence the food quality especially during food preservation [74–78].The autoinducer signal production capacity by the food borne pathogenic bacteria (for example,the AI-2 expression byE.coliO157:H7 and bySalmonella entericserovar Typhimurium) are of important concern regarding their survival within the food items [79,80].Moreover,several QS signals like AI-1 and AI-2(enhancing the survival ofE.coliO157:H7 in foods)have been shown to be present in different microbiologically contaminated food items [81–86].This is to be pondered that the quorum-sensing inhibitors target synthesis of the cell signaling molecules and blocks the signaling systems to prevent food spoilage and biofilm formation by food-related bacteria [81,86].Thus,understanding the mechanism of QS disruption would be useful to maintain the food quality as well.
QS disruption
Studies revealed that QS disruption follows some strategies such as (1) the inactivation of receptor,(2)inhibition of the synthesis of the QS signals as well as their degradation,(3) signaling blockage by the antibodies,and (4) employing antibiotics to convey the potential of QS as the therapeutic target for bacterial diseases (Figure 5).Various receptor inhibitors such as the AHL signals reduce the production of virulence factors byP.aeruginosa[87–89].Moreover,the metabromo-thiolactone has been shown to decrease the pyocyanin production inP.aeruginosaas well its biofilm formation capacity [98].Additionally,immucillin A and triclosan can reduce the AHLs synthesis which in turn may block the microbial metabolism [90–92].The major QS degradative signals include several enzymes like lactonase,acylase,oxidoreductases,AHL lactonase,etc.which can hinder both the antibiotic resistance and the biofilm formation capacity inP.aeruginosa[93–95].It is interesting to note that the AHL and AI-2 signaling can induce the programmed cell death,thereby influencing the host immunity [96,97].Indeed,combination of the antibiotic(s) and the anti-QS agent would be the most effective therapeutic option for preventing bacterial diseases [98,99].
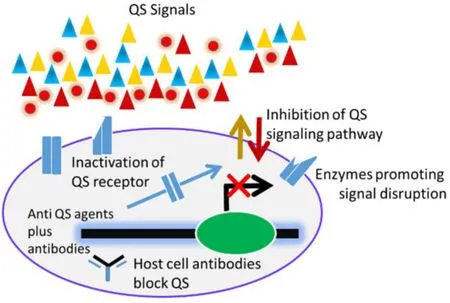
Figure 5 Ways of disrupting QS.
Conclusion
Current review portrayed the general information about the QS system especially focusing on its genetic and biochemical basis of imparting pathogenic properties within the bacteria.QS and its complex functional activity and the diverse behaviors of its bacterial population have been widely studied in the past halfdecade.These poly-microbial communities of this complex QS are associated with various neurodegenerative diseases,respiratory diseases or food borne diseases as they promote the virulence factors of microorganisms as well as their resistance ability.
- Life Research的其它文章
- Pathogenesis of the Chikungunya virus and the host immunity response
- Diabetic neuropathy: What now? What’s next?
- Prevalence of influenza vaccination coverage amongst the first and third generation Asian adults in California,United States of America
- Prevalence and factors associated with low birth weight among neonates in Rwanda: a prospective cross-sectional study
- Changes in muscle activity in patients with primary chronic headache
- Study on the medication rule of herbs for uterine bleeding in ancient Chinese medicine

