TiO2对中空硅减反射涂层硬度的影响
黄粉超,焦剑,程皓,王瑾,王佳
TiO2对中空硅减反射涂层硬度的影响
黄粉超1,焦剑2,程皓1,王瑾2,王佳2
(1.西安超码科技有限公司,西安 710025;2.西北工业大学,西安 710129)
提高中空硅减反射(AR)涂层的硬度。采用溶胶-凝胶法制备中空二氧化硅纳米微球(HSNs)胶体溶液,通过异丙醇钛(TTIP)的水解缩合作用,在HSNs表面沉积纳米TiO2后,制备HSNs@TiO2胶体溶液。将HSNs@TiO2胶体溶液与酸性硅溶胶(ACSS)复合,制备HSNs@TiO2/ACSS减反射液,通过旋涂法在玻璃基板上制备相应的AR涂层。通过特高分辨率场发射扫描电子显微镜、高分辨透射电子显微镜和原子力显微镜对HSNs和HSNs@TiO2纳米粒子的形貌进行分析,通过紫外-可见分光光度计和纳米压痕仪对HSNs/ACSS AR涂层和HSNs@TiO2/ACSS AR涂层的透射率、硬度和弹性模量分别进行分析。纳米TiO2沉积在HSNs表面后,减反射液中HSNs@TiO2纳米粒子的粒径较HSNs粒径增大1~30 nm不等。由HSNs@TiO2/ACSS减反射液制备的AR涂层表面颗粒及团簇明显,表面粗糙度(RMS)可达9.61 nm,远高于HSNs/ACSS AR涂层的3.62 nm。含有较大粒径HSNs@TiO2纳米粒子的HSNs@TiO2/ACSS AR涂层使玻璃基板在550 nm波长处的透射率增加1.3%,低于HSNs/ACSS AR涂层的增加值2.8%。纳米TiO2沉积之前,HSNS/ACSS AR涂层的硬度和弹性模量分别为2.3 GPa和56.3 GPa,纳米TiO2沉积之后,HSNs@TiO2/ACSS AR涂层的硬度和弹性模量分别为3.3 GPa和55.2 GPa,AR涂层的硬度显著提高。溶胶-凝胶法在HSNs上沉积纳米TiO2后,可有效提高AR涂层的硬度,因此AR涂层的环境适用性有望得到进一步提高。
减反射涂层;溶胶-凝胶;中空SiO2;纳米TiO2;硬度;透射率
随着太阳能与电子科学技术领域的发展,具有高透射率、机械性能优异、使用方便及成本低的减反射(AR)涂层在太阳能光伏[1-2]、集热器[3-5]、探测器[6]、高功率激光[7-8]、显示器件[9-11]等领域受到了国内外学者的广泛关注。AR涂层可以有效降低光的反射,提高光的透射[12]。在户外恶劣环境中使用时,摩擦、物体打击等都会对AR涂层造成损伤,使AR涂层的机械性能变差,影响其使用寿命[13-14]。因此,研制一种具有优异机械性能的高透射率AR涂层,对太阳能的有效收集利用和电子器件的性能提升都具有重要的现实意义。
近年来,SiO2AR涂层因其综合性能优异[15],受到国内外学者的广泛关注。其中,中空SiO2AR涂层因其低折射、高透射的特点,已成为SiO2AR涂层研究领域的热点之一。Tao等人[16]用甲基三乙氧基硅烷(MTES)和正硅酸乙酯(TEOS)作为前驱体,通过一步碱催化溶胶-凝胶法,制备了HSNs胶体溶液,将K9玻璃在该胶体溶液中浸渍后,得到了中空二氧化硅(HSNs)AR涂层,使玻璃的透射率达到97.65%。Guo等人[17]制备了苯乙烯-丙烯酸酯乳液@有机-无机二氧化硅前体(SA@OISP)核/壳分级纳米结构,通过浸涂和煅烧处理,该SA@OISP纳米球可形成中空闭孔二氧化硅减反射涂层(CHAR),CHAR在380~1100 nm波长范围内的平均透射率为97.64%,接近理想单层AR涂层的最高透射率98.09%。
高透射的中空SiO2AR涂层实质是由中空SiO2纳米粒子在基底上密切堆积而成,与基底之间主要通过范德华力结合,机械性能较差[18-19],这在一定程度上缩短了其使用寿命,限制了其使用范围。提高AR涂层机械性能的方法有很多[20-21],向AR涂层中加入无机纳米颗粒,是一种提高AR涂层机械性能的有效方法,如添加纳米TiO2[22-23]、纳米SiO2[24-25]等。Guo等人[26]采用单浸渍溶胶-凝胶法,制备了二氧化硅-中空纳米微球(SiO2-HNS)混合的SiO2涂层,该AR涂层的压痕硬度约为2.0 GPa。Zhang等人[27]制备了一种由HSNs和ACSS组成的闭孔纳米复合涂层,此涂层不添加无机纳米颗粒,硬度约为1.6 GPa,低于添加纳米颗粒的其他AR涂层。Miao等人[28]制备了双层SiO2-TiO2涂层,第一层由杂化甲基功能化纳米多孔SiO2组成,第二层是沉积在SiO2AR层顶部的超薄TiO2纳米多孔层,在0.025 kg载荷下,SiO2-TiO2涂层的显微硬度为598HV。由此可见,在具有高透射率AR涂层中引入无机纳米粒子,可有效提高AR涂层的硬度。
本文针对中空硅AR涂层机械性能较差的问题,考虑向其中引入TiO2成分以提高AR涂层的硬度,创新性地提出了制备具有核-壳结构的HSNs@TiO2纳米粒子,并以该纳米粒子作分散相制备AR涂层,希望在保留AR涂层中的大量空隙以提高其透射性能的同时,利用TiO2提高AR涂层的硬度。通过溶胶-凝胶法,成功合成了具有核-壳结构的HSNs@TiO2纳米粒子,并将其与少量的酸性硅溶胶(ACSS)复合,制备了HSNs@TiO2/ACSS减反射液。由此制备的AR涂层不仅有良好的透射性,而且相对于HSNs/ACSS AR涂层,硬度有明显提高。同时,作为粘合剂存在的ACSS,可以使最终制备的AR涂层的致密性提高,有望进一步提高该AR涂层的环境适应性。
1 试验
1.1 HSNs/ACSS及HSNs@TiO2/ACSS减反射液的制备
选用正硅酸乙酯(TEOS,质量分数为99%,分析纯)作为SiO2前驱体,购自成都市科龙化工试剂厂;异丙醇钛(TTIP,99%,分析纯)作为TiO2前驱体,聚丙烯酸(PAA,W≈5000,50%)作为HSNs制备前的核材料,购自上海麦克林生化科技有限公司;催化剂为氨水(25%水溶液)和浓盐酸(HCl,36%~38%的水溶液,分析纯),分别购自广东省化学试剂工程技术研究开发中心和成都市科隆化学品有限公司。
HSNs/ACSS减反射液的制备:HSNs胶体溶液和ACSS的制备参考文献[27]、[29]曾报道的方法,将两者按一定比例混合均匀即可制备得到HSNs/ACSS减反射液。
HSNs@TiO2/ACSS减反射液的制备如下:1)在2 h内分5次将0.05 g异丙醇钛(TTIP,Ti(OC3H7)4)加入50 mL上述HSNs胶体溶液中;2)以500 r/min的搅拌速率继续搅拌使其反应6 h,即可制得HSNs@TiO2胶体溶液;3)将HSNs@TiO2胶体溶液和ACSS按3∶1的质量比混合,混合后持续搅拌4 h使其反应完全;4)将该混合胶体溶液在室温下老化1 d后备用,即得到HSNs@TiO2/ACSS减反射液。
1.2 HSNs/ACSS及HSNs@TiO2/ACSS AR涂层的制备
所用胶粘剂为自制的ACSS,玻璃基板为25 mm× 25 mm×1 mm的载玻片。
AR涂层的制备步骤如下:1)将载玻片置于去离子水和乙醇中分别超声清洗10 min;2)采用KW-4A型旋涂仪以4000 r/min的旋涂速率在载玻片上旋涂HSNs/ACSS或HSNs@TiO2/ACSS减反射液;3)将涂覆AR涂层的载玻片在室温下晾干,然后置于450 ℃的马弗炉中焙烧1.5 h;4)焙烧后的载玻片自然冷却至室温,得到涂覆HSNs/ACSS或HSNs@TiO2/ ACSSAR涂层的玻璃基板。
1.3 性能测试及组织观察
采用FEI Talos F200X型场发射高分辨透射电镜(HRTEM)观察HSNs@TiO2纳米粒子。将HSNs@ TiO2纳米粒子用无水乙醇稀释到0.5%左右,超声分散10 min,然后滴加到铜网上,红外灯干燥后进行HRTEM观测,加速电压为200 kV。采用Verios G4型特高分辨率场发射扫描电镜(FESEM)观察HSNs@TiO2纳米粒子和HSNs@TiO2/ACSS减反射膜的表面形貌,测量前作喷金处理,喷金时间60 s。采用Dimension Fastscan and Dimension Icon型原子力显微镜(AFM)观察HSNs@TiO2/ACSS减反射膜的表面形貌和粗糙度。原子力测试模式为非接触模式,频率为5 Hz,针尖上的力为0.1 nN。采用UV-3100型紫外-可见(UV-Vis)分光光度计测量HSNs/ACSS减反射膜的透过率。仪器采用空气为背景校零,样品表面与测量光线保持垂直,波长范围为300~800 nm,扫描步长为2 nm。采用TI980型纳米压痕仪(Hysitron公司)测量AR涂层的硬度和损耗模量r。测试的热漂移率低于0.05 nm/s,每个样品的测试范围为50 μm×50 μm的1×5阵列。
2 结果及分析
2.1 HSNs及HSNs@TiO2纳米粒子的微观结构
图1为HSNs及HSNs@TiO2纳米粒子的微观形貌。由图1a、b可知,HSNs表面光滑,具有明显的中空结构,形状呈规则球形且粒径分布均一,平均粒径约50 nm。由图1c、d可知,HSNs@TiO2纳米粒子表面凹凸不平,存在明显的颗粒状小粒子,内部中空结构明显,粒径大多在50~80 nm范围内。与HSNs相比,HSNs@TiO2纳米粒子表面的颗粒明显,且粒径明显增大,表明纳米TiO2成功沉积在HSNs表面。从HSNs@TiO2纳米粒子的EDS图(图1e)中可获得单个粒子的元素组成,C、O、Si、Ti四种元素的定量结果如表1所示。其中Ti元素在HSNs@TiO2纳米粒子中的质量分数为14.59%,进一步证明了纳米TiO2已成功沉积在HSNs表面。Ti元素出现的两个峰则是由核外电子的不同跃迁造成。纳米TiO2与HSNs、纳米TiO2与纳米TiO2之间的结合力主要为范德华力。
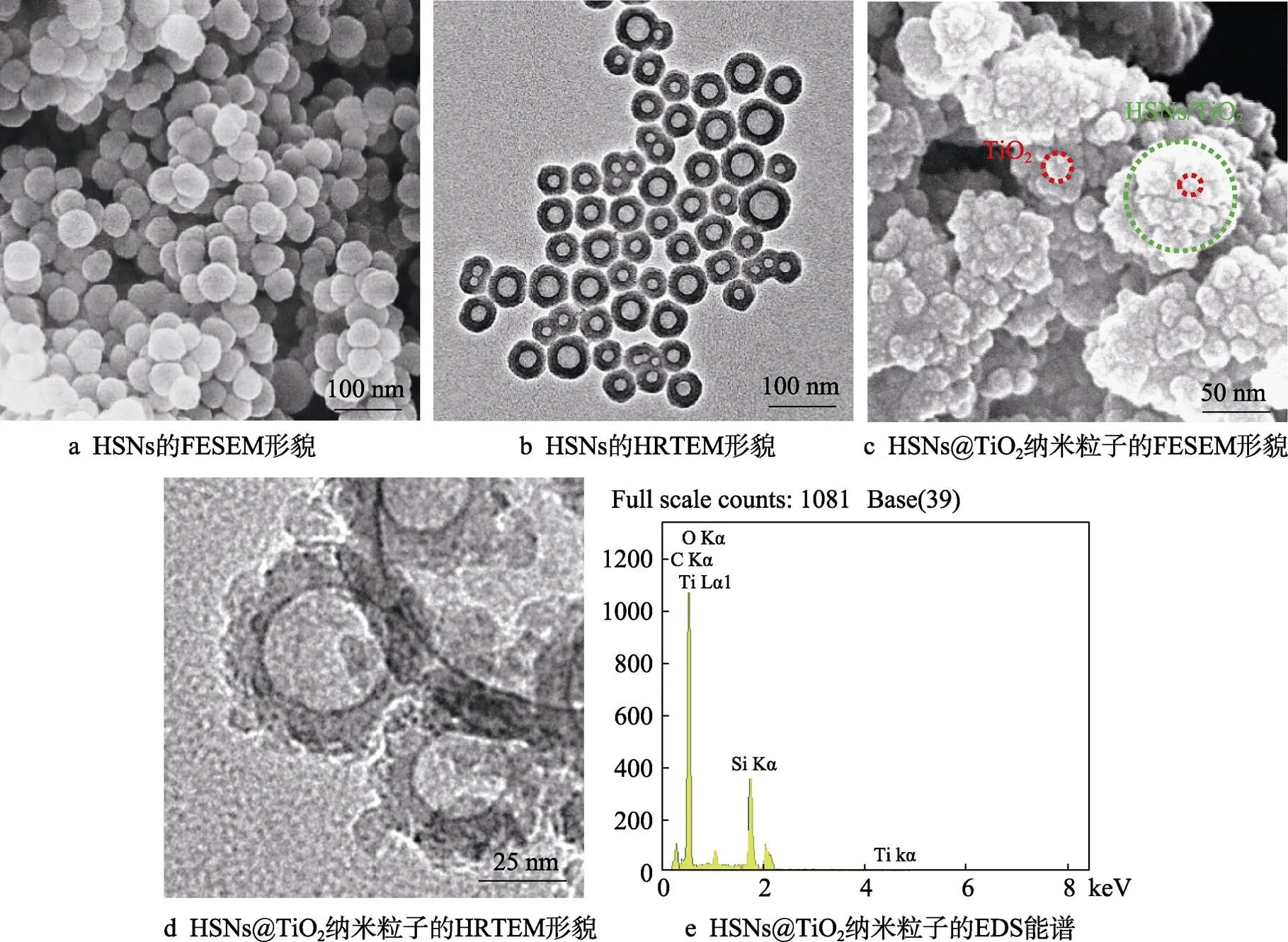
图1 HSNs及HSNs@TiO2纳米粒子的微观形貌图
表1 单个HSNs@TiO2纳米粒子的EDS测量结果
2.2 HSNs/ACSS AR及HSNs@TiO2/ACSS AR涂层的微观结构
图2为HSNs/ACSS AR涂层及HSNs@TiO2/ACSS AR涂层的微观形貌图。由图2a、b可以看出,HSNs/ACSS AR涂层是由连续的ACSS相和非连续的HSNs颗粒相组成。其成形原理为,ACSS以胶粘剂的形式将HSNs粒子粘结起来,并在干燥的过程中进一步完成缩聚成为凝胶,而HSNs粒子则分散于ACSS形成的凝胶中。HSNs/ACSS AR涂层的表面粗糙度为3.62 nm。由图2c、d可见,HSNs@TiO2/ACSS AR涂层是由连续的ACSS相和HSNs@TiO2粒子相组成。其成形原理与HSNs/ACSS AR涂层一致,但其表面的颗粒及团簇明显,RMS值高达9.61 nm。这是因为被纳米TiO2包覆的HSNs在胶体溶液中的稳定性差,易团聚,且游离在减反射液中的未包覆在HSNS上的纳米TiO2也因范德华力作用聚集成簇。团聚的HSNs@TiO2粒子和成簇的纳米TiO2分散于ACSS中,仍保持其聚集状态,导致由该减反射液制备的AR涂层出现粒径较大的团聚颗粒。与HSNs/ACSS AR涂层相比,HSNs@TiO2/ACSS AR涂层的粗糙度明显增加。

图2 HSNs/ACSS AR涂层及HSNs@TiO2/ACSS AR涂层的微观形貌图
2.3 HSNs/ACSS AR及HSNs@TiO2/ACSS AR涂层的透射率
图3为HSNs@TiO2/ACSS AR涂层、HSNs/ACSS AR涂层和载玻片的透射图。可以看出,涂覆AR涂层的载玻片的透射率上升阶段主要集中在425~ 675 nm,HSNs@TiO2/ACSS AR涂层的透射率虽较载玻片有所提高,但低于HSNs/ACSS AR涂层。在550 nm波长处,HSNs@TiO2/ACSS AR涂层的透射率为87.0%,较载玻片的透射率85.7%提高了1.3%,而HSNs/ACSS AR涂层的透射率提高到了88.5%,较载玻片的透射率提高了2.8%。这是因为单层AR涂层的透射率曲线呈“Λ”形,这意味着涂层存在峰值透射率,仅在峰值处的透射率高,在其他处的透射率会降低,即/4光学AR涂层的增透带宽较小。本次试验的峰值透射率出现在约525 nm波长处,结合单层AR涂层透射率的曲线特征,发现其增透宽度主要集中在550 nm波长前后,即425~675 nm之间。

图3 HSNs@TiO2/ACSS AR涂层、HSNs/ACSS AR涂层和载玻片的透射图
影响AR涂层透射率的直接因素是AR涂层的折射率,根据Lorentz-Lorentz公式,薄膜的折射率与其孔隙率相关,薄膜孔隙率越大,其折射率越低。在本研究中,具有较大孔隙率的HSNs@TiO2/ACSS AR涂层折射率较低,因而提高了载玻片的透射率,但该AR涂层较高的表面粗糙度导致其表面漫反射增加,且TiO2自身具有较高的折射率[30](锐钛矿的折射率≈2.52),因此在一定程度上,透射率较HSNs/ACSS AR涂层的透射率有所降低。
2.4 HSNs/ACSS AR及HSNs@TiO2/ACSS AR涂层的力学性能
图4为HSNs@TiO2/ACSS AR涂层和HSNs/ACSS AR涂层的典型载荷-位移曲线。可以看出,在相同的载荷作用于AR涂层表面时,探针在HSNs@TiO2/ ACSS AR涂层内的位移均小于在HSNs/ACSS AR涂层内的位移,表明HSNs@TiO2/ACSS AR涂层的硬度高于HSNs/ACSS AR涂层。在施加200 μN的载荷时,HSNs@TiO2/ACSS AR涂层的位移、平均硬度和损耗模量分别为32 nm、3.3 GPa和54.1 GPa,而HSNs/ACSS AR涂层的位移、平均硬度和损耗模量分别为55 nm、2.3 GPa和56.3 GPa。200 μN的压入载荷是通过基础标准法在一个压痕点上进行一系列加载-卸载过程测试后选取而来的。AR涂层的弹性模量s可通过公式(1)计算:

图4 HSNs@TiO2/ACSS AR涂层和HSNs/ACSS AR涂层的典型载荷-位移曲线
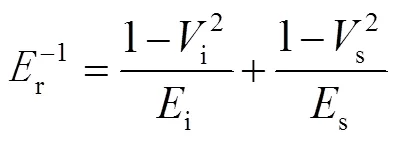
式中:r为试样材料的损失弹性模量;i和i分别为压头的弹性模量和泊松比;s和s分别为试样材料的弹性模量和泊松比。
由公式(1)计算可知,HSNs@TiO2/ACSS AR涂层和HSNs/ACSS AR涂层的弹性模量分别为57.6 GPa和55.2 GPa。与HSNs/ACSS AR涂层相比,HSNs@TiO2/ ACSS AR涂层的硬度增加,这是AR涂层内高硬度的纳米TiO2所致。
在本研究当中,纳米TiO2包覆在HSNs表面,且大部分随HSNs均匀分布于ACSS中,形成减反射液。由该减反射液制备的HSNs@TiO2/ACSS AR涂层与HSNs/ACSS AR涂层相比,表现出更好的力稳定性和牢固性,因此其硬度更高。HSNs@TiO2/ACSS AR涂层和HSNs/ACSS AR涂层在200 μN载荷下的位移、硬度和弹性模量如表2所示。
<
表2 HSNs@TiO2/ACSS AR涂层和HSNs/ACSS AR涂层的位移、硬度、损耗模量和弹性模量
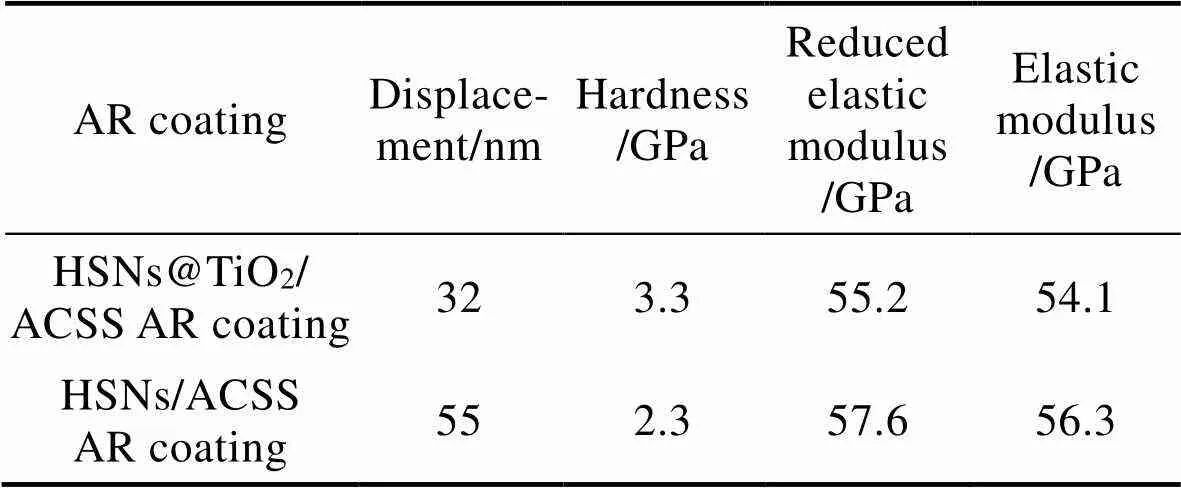
Tab.2 Displacement, hardness, reduced elastic modulus and elastic modulus of HSNs@TiO2/ACSS AR coating and HSNs/ ACSS AR coating
3 结论
1)通过溶胶-凝胶法,可成功将纳米TiO2沉积在HSNs上,从而制备得到核-壳结构的HSNs@TiO2纳米粒子。此种方法引入的纳米TiO2在AR涂层中分布均匀,有利于提高AR涂层的硬度。
2)纳米TiO2的引入显著提高了AR涂层的硬度,当HSNs@TiO2胶体溶液与ACSS的质量比为3∶1时,HSNs@TiO2/ACSS AR涂层的硬度可达3.3 GPa,同时该涂层在550 nm波长处的透射率较玻璃基板提高了1.3%。
3)纳米TiO2的引入会导致纳米HSNs@TiO2和纳米TiO2团聚粒子的出现,增加AR涂层表面粗糙度,使HSNs@TiO2/ACSS AR涂层透射率较HSNs/ ACSS AR涂层有所降低。
[1] WANG Y, HE M Y, CHEN R Y. Fabrication of mechanically robust antireflective films using silica nanoparticles with enhanced surface hydroxyl groups[J]. Materials chemistry A, 2015, 3(4): 1609-1618.
[2] GUILLEMOT F, BRUNET-BRUNEAU A, BOURGEAT- LAMI E, et al. Latex-templated silica films: Tailoring porosity to get a stable low-refractive index[J]. Chemistry of materials, 2010, 22(9): 2822-2828.
[3] CAI Shuang, XUE Qing-lan, XIA Bi-bo, et al. Hydrophobic-oleophobic antireflective film with excellent optical property prepared by a simple sol-gel route[J]. Materials letters, 2015, 156: 14-16.
[4] DOU Wen-wen, WANG Peng, ZHANG Dun, et al. An efficient way to prepare hydrophobic antireflective SiO2film by sol-gel method[J]. Materials letters, 2016, 167: 69-72.
[5] WANG Yun-bo, WU Jian, WANG Hong-ning, et al. Effective balance of antireflection and self-cleaning properties via hollow silica nanospheres-based surface coated with scattered titania nanoparticles[J]. Solar energy, 2015, 122: 763-772.
[6] ZHENG Ke-lin, WEI Peng, WANG Li-wen, et al. The study of multilayer anti-reflection coating in InSb focal plane detector[C]//Proceedings of SPIE-the international society for optical engineering. Beijing: [s. n.], 2016.
[7] LI Yuan-yang, LV Hai-bing, YE Long-qiang, et al. Preparation of porous silica films in a binary template system for double-layer broadband antireflective coatings[J]. RSC advances, 2015, 5(26): 20365-20370.
[8] SUN Jing-hua, WU Bao-hu, JIA Hong-bao, et al. Fluoroalkyl-grafted mesoporous silica antireflective films with enhanced stability in vacuum[J]. Optics letters, 2012, 37(19): 4095-4097.
[9] HO Jyh-jier, CHEN Chin-ying, HUANG Chao-ming, et al. Ion-assisted sputtering deposition of antireflection film coating for flexible liquid-crystal display applications[J]. Applied optics, 2005, 44(29): 6176-6180.
[10] KIM N Y, SON Y B, OH J H, et al. TiNlayer as an antireflection and antistatic coating for display[J]. Surface & coatings technology, 2000, 128: 156-160.
[11] MOGHAL J, KOBLER J, SAUER J, et al. High-performance, single-layer antireflective optical coatings comprising mesoporous silica nanoparticles[J]. ACS applied materials & interfaces, 2012, 4(2): 854-859.
[12] ESHAGHI A, MOJAB M. Fabrication of antireflective antifogging nano-porous silica thin film on glass substrate by layer-by-layer assembly method[J]. Non-crystalline solids, 2014, 405: 148-152.
[13] OH W, KANG B, CHOI S, et al. Evaluation of anti- soiling and anti-reflection coating for photovoltaic modules[J]. Nanoscience and nanotechnology, 2016, 16(10): 10689- 10692.
[14] YAO Lin, HE Jun-hui. Recent progress in antireflection and self-cleaning technology-from surface engineering to functional surfaces[J]. Progress in materials science, 2014, 61: 94-143.
[15] TAO Chao-you, YAN Hong-wei, YUAN Xiao-dong, et al. Sol-gel based antireflective coatings with superhydrophobicity and exceptionally low refractive indices built from trimethylsilanized hollow silica nanoparticles[J]. Colloids and surfaces A: Physicochemical and engineering aspects, 2016, 509: 307-313.
[16] TAO Chao-you, YAN Hong-wei, YUAN Xiao-dong, et al. Sol-gel preparation of moisture-resistant antireflective coatings from novel hollow silica nanoparticles[J]. Sol- gel science technology, 2016, 80(2): 538-547.
[17] GUO Zhao-long, ZHAO Hai-xin, ZHAO Wei, et al. High- quality hollow closed-pore silica antireflection coatings based on styrene-acrylate emulsion@organic-inorganic silica precursor[J]. ACS applied materials & interfaces, 2016, 8(18): 11796-11805.
[18] DENG Xu, MAMMEN Lena, ZHAO Yan-fei, et al. Transparent, thermally stable and mechanically robust superhydrophobic surfaces made from porous silica capsules[J]. Advanced materials, 2011, 23(26): 2962-2965.
[19] REN Hong-bo, ZHU Jia-yi, BI Yu-tie, et al. Assembly of methylated hollow silica nanospheres toward humidity- resistant antireflective porous films with ultralow refractive indices[J]. Porous materials, 2018, 25(1): 55-62.
[20] CHI Fang-ting, YAN Liang-hong, LV Hai-bing, et al. Novel pathways for the preparation of silica antireflective films: Improvement in mechanical property[J]. Materials letters, 2011, 65(7): 1095-1097.
[21] 许羽冬. 光伏玻璃增透膜的制备及其性能[D]. 广州: 华南理工大学, 2013. XU Yu-dong. Preparation and properties of antirefractive films for solar energy application[D]. Guangzhou: South China University of Technology, 2013.
[22] VIOLETA Purcar, VALENTIN Rădiţoiu, ANCA Dumitru, et al. Antireflective coating based on TiO2nanoparticles modified with coupling agents via acid-catalyzed sol-gel method[J]. Applied surface science, 2019, 487: 819-824.
[23] KHAN Sadaf-Bashir, ZHANG Zheng-jun, LEE Shern- long. Single component: Bilayer TiO2as a durable antireflective coating[J]. Alloys and compounds, 2020, 834: 155137.
[24] SUN Xiao-yu, XU Xiao-zhuang, SONG Guan-yu, et al. Preparation of MgF2/SiO2coating with broadband antireflective coating by using sol-gel combined with electron beam evaporation[J]. Optical materials, 2020, 101: 109739.
[25] JIANG Xiao-long, LIAO Wei, LI Bo, et al. Removal of antireflection sol-gel SiO2coating based on Ar ion beam etching[J]. Fusion engineering and design, 2020, 156: 111578.
[26] GUO Z Q, LIU Y, TANG M Y, et al. Super-durable closed-surface antireflection thin film by silica nanocomposites[J]. Solar energy materials and solar cells, 2017, 170: 143-148.
[27] ZHANG Xian-peng, LAN Pin-jun, LU Yue-hui, et al. Multifunctional antireflection coatings based on novel hollow silica-silica nanocomposites[J]. ACS applied materials & interfaces, 2014, 6(3): 1415-1423.
[28] MIAO Lei, SU Li-fen, TANEMURA S, et al. Cost-effective nanoporous SiO2-TiO2coatings on glass substrates with antireflective and self-cleaning properties[J]. Applied energy, 2013, 112: 1198-1205.
[29] ZHANG Jing, LAN Pin-jun, LI Jia, et al. Sol-gel derived near-UV and visible antireflection coatings from hybridized hollow silica nanospheres[J]. Sol-gel science and technology, 2014, 71(2): 267-275.
[30] LI Xiao-yu, HE Jun-hui. Synthesis of raspberry-like SiO2- TiO2nanoparticles toward antireflective and self-cleaning coatings[J]. ACS applied materials & interfaces, 2013, 5(11): 5282-5290.
Effect of TiO2on the Hardness of Hollow Silica Antireflection Coating
1,2,1,2,2
(1.Xi’an ChaoMa Technology Co., Ltd, Xi’an 710025, China; 2.Northwestern Polytechnical University, Xi’an 710129, China)
The purpose is to improve the hardness of hollow silica antireflection (AR) coatings. In this paper, a colloidal solution of hollow silica nanospheres (HSNs) is prepared by sol-gel method, and HNSs@TiO2colloidal solution is prepared by depositing nano-TiO2on the surface of HSNs through hydrolysis and condensation of titanium isopropoxide (TTIP). The HSNs@TiO2/ACSS AR solution is prepared by mixed the HSNs@TiO2colloidal solution and acidic silica sol (ACSS). The morphology of HSNs and HSNs@TiO2nanoparticles are analyzed by ultra-high resolution field emission scanning electron microscope, high-resolution transmission electron microscope and atomic force microscope. The transmittance, hardness and elastic modulus of HSNs/ACSS AR coating and HSNs@TiO2/ACSS AR coating are analyzed by UV-visible spectrophotometer and nanoindenter respectively. After the nano-TiO2is deposited on the surface of HSNs, the particle size of the HSNs@TiO2nanoparticles in antireflection liquid increased by 1~30 nm compared with the particle size of the HSNs; Particles and clusters on the surface of AR coating that prepared by HSNs@TiO2/ACSS AR liquid are obvious, and the surface roughness (RMS) of the AR coating could reach 9.61 nm, which is much higher than 3.62 nm of HSNs/ACSS AR coating; HSNs@TiO2/ACSS AR coating with larger HSNs@TiO2nanoparticles increased the transmittance of glass at 550 nm by 1.3%, which is lower than 2.8% of HSNs/ACSS AR coating; Before the nano-TiO2deposited, the hardness and elastic modulus of the HSNS/ACSS AR coating are 2.3 GPa and 56.3 GPa, respectively, the hardness of the AR coating is significantly improved after the nano-TiO2deposited, the hardness and elastic modulus of the HSNs@TiO2/ACSS AR coating are 3.3 GPa and 55.2 GPa, respectively. The nano-TiO2deposited on HSNs by sol-gel method could effectively improve the hardness of AR coatings, so the environmental applicability of AR coatings is expected to be further improved.
AR coatings; sol-gel; hollow SiO2; nano-TiO2; hardness; transmittance
2020-03-23;
2020-05-26
HUANG Fen-chao(1994—), Female, Master, Assistant engineer, Research focus: nano functional coating.
焦剑(1970—),女,博士,教授,主要研究方向为纳米复合材料和介孔材料。邮箱:jjiao@nwpu.edu.cn
Corresponding author:JIAO Jian (1970—), Female, Doctor, Professor, Research focus: nanocomposite and mesoporous materials. E-mail: jjiao@ nwpu.edu.cn
黄粉超, 焦剑, 程皓, 等. TiO2对中空硅减反射涂层硬度的影响[J]. 表面技术, 2021, 50(4): 191-197.
TB332
A
1001-3660(2021)04-0191-07
10.16490/j.cnki.issn.1001-3660.2021.04.018
2020-03-23;
2020-05-26
黄粉超(1994—),女,硕士,助理工程师,主要研究方向为纳米功能涂层。
HUANG Fen-chao, JIAO Jian, CHENG Hao, et al. Effect of TiO2on the hardness of hollow silica antireflection coating[J]. Surface technology, 2021, 50(4): 191-197.

