Super-strong interactions between multivalent anions and graphene∗
Xing Liu(刘星) and Guosheng Shi(石国升),2,†
1Shanghai Applied Radiation Institute,State Key Laboratory Advanced Special Steel,Shanghai University,Shanghai 200444,China
2Division of Interfacial Water and Key Laboratory of Interfacial Physics and Technology,Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
Keywords: graphene,multivalent anions,anion–π interaction,density functional theory
1. Introduction
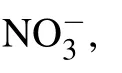

Recently,graphene/graphene oxide(GO)-based materials have been employed for the development of DNA biosensors and are widely used in genetics,clinical medicine,pathology,and many other fields.[19–23]These applications are related to the interaction between graphene/GO and DNA.[24–26]However, most researches focused on the interaction between nucleic acid bases and graphene,[27–30]the interaction of backbone phosphate groups are ignored. As an important component of DNA/RNA backbone,phosphate groups played important roles in biochemistry.[31]In the backbone of DNA/RNA,phosphoric group links two nucleotides and still ionized as monovalent anion. At the termini of DNA/RNA, phosphoric group is bivalent anion.[32]Thus,it is necessary to investigate the interaction between phosphate groups with different valence and graphene from the molecular level to provide assistance for the design and application of graphene-based DNA biosensor. Methyl phosphate[33]can be regarded as a simple analog of phosphate moieties in DNA/RNA to research the interaction between multivalent anions and the electron-rich graphene.
2. Methods

where Eanion@G,EG,and Eanionare the total energy of the anion adsorption on a graphene flake, the isolated anion, and a graphene flake,respectively. For different anions,the adsorption energies, average nearest oxygen–carbon distances, and transfer Mullikan charges are shown in Table 1.



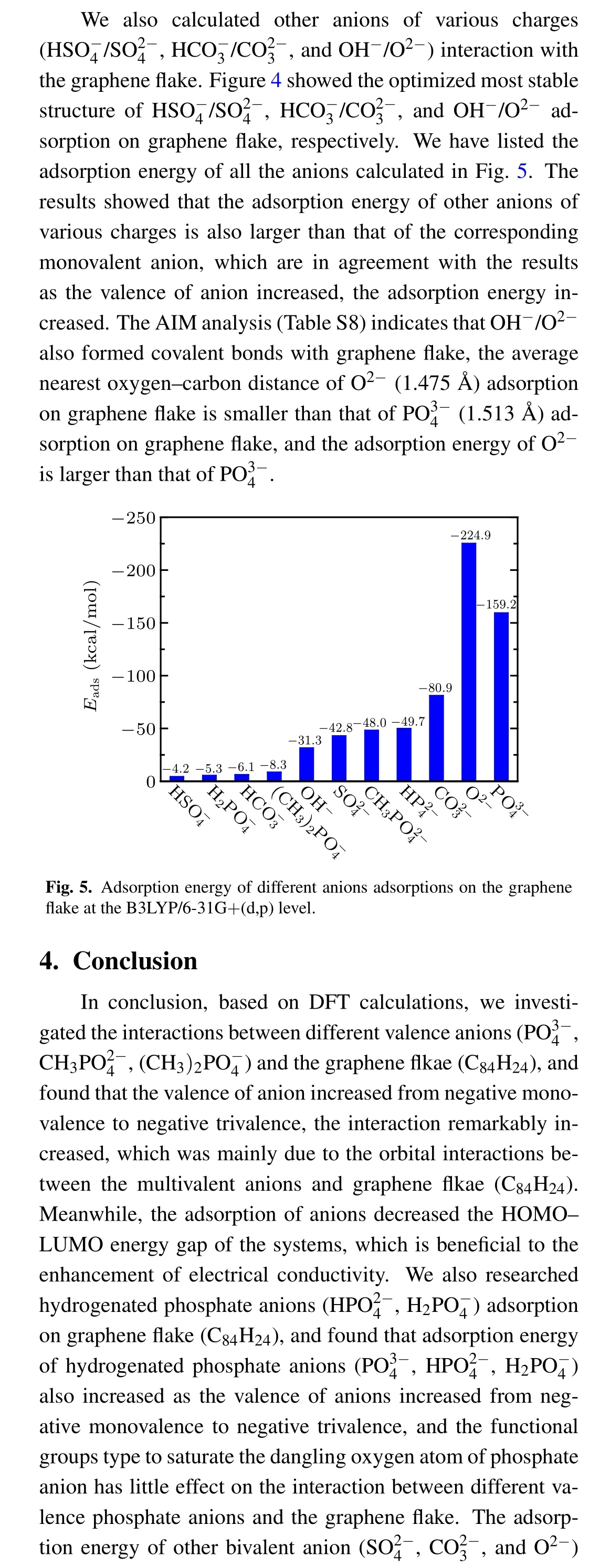

- Chinese Physics B的其它文章
- Speeding up generation of photon Fock state in a superconducting circuit via counterdiabatic driving∗
- Micro-scale photon source in a hybrid cQED system∗
- Quantum plasmon enhanced nonlinear wave mixing in graphene nanoflakes∗
- Restricted Boltzmann machine: Recent advances and mean-field theory*
- Nodal superconducting gap in LiFeP revealed by NMR:Contrast with LiFeAs*
- Origin of itinerant ferromagnetism in two-dimensional Fe3GeTe2∗

