Factors associated with change in frailty scores and long-term outcomes in older adults with coronary artery disease
S. Michael Gharacholou✉, Joshua P. Slusser, Ryan J. Lennon, Carolyn R. Flock,Leslie T. Cooper, Patricia A. Pellikka, Jorge Brenes Salazar, Mandeep Singh
1. Department of Cardiovascular Medicine, Mayo Clinic, Jacksonville, FL, USA; 2. Department of Cardiology, Mayo Health System, La Crosse, WI, USA; 3. Department of Biostatistics, Mayo Clinic, Rochester, MN, USA; 4. Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, USA
ABSTRACT OBJECTIVE Older adults with coronary artery disease (CAD) are at risk for frailty. However, little is known regarding transition in frailty measures over time or its impact on outcomes. We sought to determine the association of temporal change in frailty with long-term outcome in older adults with CAD. METHODS We re-assessed for phenotypic frailty using the Fried index (0=not frail; 1-2=pre-frail; ≥ 3 frail) in a cohort of CAD patients ≥ 65 years old at 2 time points 5 years apart. Factors associated with frailty worsening were assessed with scatterplots and outcomes estimated using the Kaplan-Meier method. Cox models were used to assess the risk of worsening frailty on outcome. RESULTS There were 45 subjects that completed both baseline and 5-year Fried frailty assessment. Mean age was 74.6 ± 5.9 and 30 (67%) were men. Frailty incidence increased over time: baseline (3% frail, 37% pre-frail); 5 years (10% frail, 40% pre-frail).Baseline factors were not predictors of worsening frailty score, while both slower walk time (r=0.46; P=0.004) and diminishing grip strength (r=-0.39; P=0.01) were associated with worsening frailty transitions. In follow-up (median 5.2 years), long-term major adverse cardiac event (MACE) free survival (P=0.12) or hospitalization (P=0.98) was not different for those with worsening frailty score (referent: improved/unchanged frailty). Frailty worsening had a trend towards increased risk of MACE (HR =1.86; 95% CI: 0.65-5.27, P=0.25). CONCLUSIONS Frailty transitions, specifically, declines in walk time and grip strength, were strongly associated with worsening frailty score in a cohort of older adults with CAD than were baseline indices, though frailty change status was not independently associated with MACE outcomes.
The geriatric syndrome of frailty is characterized by diminished resilience to physiologic stressors due, in part, to vulnerability in health reserves. It is postulated that the diminished homeostatic reserves place the susceptible older adult at increased risk for loss of independence, increased health utilization, diminished quality of life, and disability.[1]Frailty is not a binary phenomenon, but rather a continuum of functional and physiologic factors, such that even early elements of frailty have been shown to be associated with higher rates of cardiovascular events.[2]Although increasing age has been associated with frail features, ageper sedoes not define frailty and differences between normal physiologic aging and frailty have distinct biological underpinnings.[3]Despite the variability in definitions for frailty across studies,[4-6]the phenotype of frailty has been associated with adverse outcomes in communitydwelling populations[7]and in patients with coronary artery disease (CAD).[8-10]A limitation of prior studies evaluating the relationship between frailty and outcomes has been measurement of frailty only as an exposure variable at baseline,[7,11]rather than evaluating how frailty features may change over time. The factors associated with incident frailty, or development of new frail features over time, is less understood and may potentially be identified as a target for intervention. In addition, identification of factors that have a protective effect (i.e., fewer frailty features over time) may also have important implications for management. Finally, the trajectory of change in frailty status has not been studied with respect to its association with important clinical outcomes.
In this study, our objective was to determine which frailty factors change over time to better characterize the frailty phenotype, given that frail features are neither static nor binary variables. To accomplish this, we re-measured frailty features in community dwelling patients with known CAD that had participated in an initial frailty prevalence study several years prior. By utilizing a 2ndwave assessment, we were uniquely able to study not only the relationship between baseline characteristics and frailty change status, but correlate change in frailty indices over time with long-term outcomes.
METHODS
Patient Population
This study received approval from the Institutional Review Board of the Mayo Clinic. Patients 65 years or older with a history of CAD, defined as a prior history of percutaneous or surgical coronary artery revascularization, prior myocardial infarction (MI), ischemia on prior functional study, or ≥50% stenosis in an epicardial vessel at invasive angiography, were prospectively enrolled in an observational study evaluating functional status, frailty,and sarcopenia at Mayo Clinic Health System-Franciscan Healthcare in La Crosse, WI (USA) from 2008 through 2012. Patients with residual neurological deficits after a stroke, severe Parkinson's disease, or dementia were excluded from the study given the concern regarding validity of functional assessments in these patients. Baseline demographic and functional characteristics were collected as part of an initial (1stwave) observational study, which included assessment of the frailty phenotype by trained personnel. We used the 5-component Fried frailty score which included assessment for unintended weight loss (>10 lb in the preceding year),physical activity, exhaustion, gait speed (walking time over 15 feet), and grip strength.[4]Physical activity was measured by the short version of the Minnesota Leisure Time Activity questionnaire.Low physical activity was defined as < 383 Kcal/week for men and < 270 Kcal/week for women. Exhaustion was measured by the Center of Epidemiologic Studies-Depression's subscale. The two items in this scale are: how often in the past week did the patient feel the following: (a) I felt that everything I did was an effort, and (b) I could not get going.Subjects who answered “a moderate amount of time (3-4 days)” or “most of the time” to either of the statements were categorized as meeting the exhaustion criteria for frailty. Gait speed was assessed as walking time across a 15-foot distance in an unobstructed, well-lit hallway. Participants walking with an assist device were permitted to do so for the test. Hand-grip strength was tested in the subject's dominant hand using a Jamar®handgrip dynamometer (Bolingbrook, IL). Participants were told to squeeze as hard as possible and results were scored as kilograms. Cutoffs for values that constituted abnormal frailty measures are reported (Supplementary Table 1). A patient was defined as frail if they were found to have deficits in three or more core elements, pre-frail if they had deficits in 1-2 core elements, or non-frail if no deficits were identified.[4]In 2015, patients originally participating in the 1stwave study were contacted by mail for an inperson re-assessment of frailty features, which was approximately 5 years after initial study participation, allowing for assessment in temporal change of the frailty phenotype. The Fried frailty domains were performed from 1/2015-12/2015 as part of the 2ndwave assessment, which occurred several years after the initial frailty assessment. Follow-up was performed through review of medical records for important clinical outcomes, including death,myocardial infarction (MI), percutaneous coronary intervention (PCI), coronary artery bypass grafting(CABG), stroke or transient ischemic attack, or hospitalizations. A composite outcome of major adverse cardiac event (MACE) included all of the individual outcome components with the exception of hospitalization.
Statistical Analysis
Continuous variables were summarized as means ±SD and categorical variables were presented as frequencies (percentages). For ease in data interpretation, a “frailty worsening score” was developed to signify change in frailty from baseline (1stwave) to follow-up (2ndwave), where a positive score identified worsening frailty features while a negative score characterized fewer frailty features. Associations were tested with the frailty worsening score using scatterplots and using a trend test for binary variables and a correlation coefficient for continuous variables. Scatterplot smoothing lines were used for testing relationships between continuous variables. The occurrence of outcomes was estimated using the Kaplan-Meier method. Individual comparisons of the Kaplan-Meier estimates were completed using the log-rank test. Univariate Cox proportional hazard models were constructed for outcomes of interest, adjusting for selected covariates. Based upon these results, multivariate Cox proportional hazard models were then constructed,adjusting first for age and gender, then for age,gender and worsening frailty, and finally adjusting for age, gender, worsening frailty and any other covariates with a univariateP-value ≤ 0.15. Statistical significance is defined as a 2-tailedPvalue of less than 0.05. Statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS

Table 1 Patient characteristics.
From the original cohort of 144 patients participating in the baseline frailty assessment, 47 patients responded to the mailed contact letter, consented to study participation, and underwent in-person reassessment of frailty. Complete baseline data was unavailable in two subjects, leaving a final cohort of 45 patients. Baseline characteristics between patients that responded to the contact letter and those that did not return for frailty reassessment were similar(data not shown). Table 1 lists the demographic characteristics of the study cohort. There were high rates of cardiovascular risk factors among study participants, including hypertension (89%), hyperlipidemia (66%), and diabetes (23%). Characteristics of frailty features at baseline and follow-up are shown in Table 2. Few patients were frail at baseline,consistent with known low prevalence of frailty in community dwelling participants. However, reassessment for frailty features several years later demonstrated higher rates of exhaustion, weight loss, low physical activity, and slower walk time as compared to baseline. Physical activity was significantly lower at follow-up as compared to baseline (P=0.008). Frailty classifications showed fewer non-frail adults at follow-up with higher rates of pre-frail and frail phenotypes. Composite scores also confirmed accrual of frailty features as compared to baseline, with shifting of the frailty composite score towards greater deficits. There were 11 patients(28%) whose frailty classification worsened from baseline to follow-up, while only five patients (13%)had improvement.

Table 2 Summary of frailty features and composite score: baseline to follow-up.
Figure 1A and 1B shows that changes in walk time (i.e, slower walking time) and grip strength(i.e, poorer grip strength) over time were associated with worsening frailty status as assessed in follow-up, respectively. Both slower walk time (r=0.46;P=0.004) and decreased grip strength (r=-0.39;P=0.01) were associated with worsening frailty change score, while increase in BMI showed trend towards an improving frailty score but was not statistically significant (r=-0.26;P=0.11) (Figure 1C).Table 3 shows clinical events over long term followup by frailty change status. There was a trend toward higher rates of all clinical events, including death, MACE, and hospitalizations, in patients with worsening frailty status as compared to patients whose frailty status remained unchanged or had improved.
Figure 2A shows the survival curve for MACE by frailty change status (improved/unchanged frailty vs. worsened frailty) over a median follow-up of 5.2 years. Although there was an observed trend toward worse survival free of MACE among those worsening frailty change status, it was not statistically significant (P=0.12 by log-rank). Hospitalization events over long-term follow-up were not different between frailty change status groups (Figure 2B).Factors independently associated with MACE were studied using a multivariable model (Supplementary Table 2). Frailty change status had nearly 2-fold increased risk of MACE, but did not reach significance (HR=1.86; 95% CI: 0.65-5.29). A history of hypertension was associated with significantly lower risk of MACE (HR=0.17; 95% CI: 0.04-0.65) while a history of heart failure was strongly associated with MACE (HR=4.10; 95% CI: 1.36-12.40).
DISCUSSION
In a cohort of older adults with CAD, repeat measures of frailty indices provided important information on frailty status change over time. Most Fried frailty features were more prevalent during 2ndwave assessments, though only decreased physical activity was significantly lower. Serial assessment demonstrated that nearly 1/3 (28%) of subjects show worsening frailty classification over followup. No baseline factors were associated with worsening frailty status at five years, however, declining walk time and grip strength identified worsening frailty classification over time. We observed a trend towards less frailty progression by increased BMI, which was not evident when only baseline BMI was considered. Despite higher crude rates of adverse outcomes in patients with worsening frailty vs. those with unchanged/improved frailty, MACE and hospitalization rates were not significantly different between groups. These observations suggest that baseline only assessments of frailty may be incomplete, and that functional,physiologic decline in older adults occurs across a continuum. Hallmarks of worsening frailty may be better identified by looking at change in walk time and grip strength rather than relying on their values at baseline alone.
Newman,et al.[12]noted that baseline frailty,without subsequent re-measurement, was associated with markers of cardiovascular disease, such as heart failure and peripheral artery disease. A potential link between frailty and both manifest cardiovascular disease and biomarkers of CAD was felt to be related to chronic inflammation. Interestingly,the authors acknowledged that a key limitation of their findings related to only a baseline assessment of frailty criteria, rather than an understanding of changes to frailty features over time. A consensus work group on data elements and outcomes in frailty research has emphasized the need to better understand how commonly used frailty measures or instruments are sensitive to change, especially with respect to clinical outcomes.[13]In addition, the recognition that the phenotype of frailty reflects reduced intrinsic capacity over time, serial measurement of functional, physiologic, and vitality indices in older adults may further help define the continuum of frailty. Unfortunately, few studies published in the frailty space have re-assessed frailty indices over time in a cohort of community dwelling patients.

Figure 1 Association calculations. (A): association between the change in walk time as assessed at baseline and follow-up time points and worsening frailty score (P=0.004). Longer walk times equated with slower gait speed and greater frailty deficit. (B): association between the change in grip strength as assessed at baseline and follow-up time points and worsening frailty score(P=0.01). The higher changed grip strength equated with grip strength improvement and fewer frailty deficits. (C): association between change in BMI and worsening frailty score. There was no significant relationship between change in BMI and worsening frailty score. BMI: body mass index.
In our cohort, walk time at baseline was generally normal, with fewer than 7% classified as slow walkers. However, follow-up assessment of walk time demonstrated that the prevalence of slow walkers had increased by 2-fold (15%). In addition,baseline walk times did not correlate with frailty worsening scores; however, change in walk time was significantly associated with frailty worsening(r=0.46;P=0.004). This underscores the dynamic nature of mobility assessment in the geriatric population, suggesting that baseline cross-sectional type aging assessments have limitations. This observation also comports with clinical comprehensive geriatric assessment that often entails serial evaluations and re-measurement over time. Gait speed change, rather than baseline walk speed, has been used in different populations but rarely studied in patients with CAD. Gait speed decline in older adults has been associated with changes in brain volume and white matter hyperintensity on neuroimaging,[14]with cognition influencing gait speed change during physical reconditioning.[15]Improved gait speed change after first stroke was associated with improved walking ability as rated by therapists and subjects.[16]In a large prospective cohort study of community-dwelling elders, Duan-Porter,et al.[17]showed that hospitalization was associated with decreased gait speed and nearly 2-fold increased odds of new disability or new mobility limitation. Decline in serial gait speed may function as a barometer of cognitive and health reserves,and may identify a vulnerable population at risk for adverse outcomes. However, as suggested in post stroke recovery, walk time improvement may herald improved reserves, reconditioning, and functional resilience.
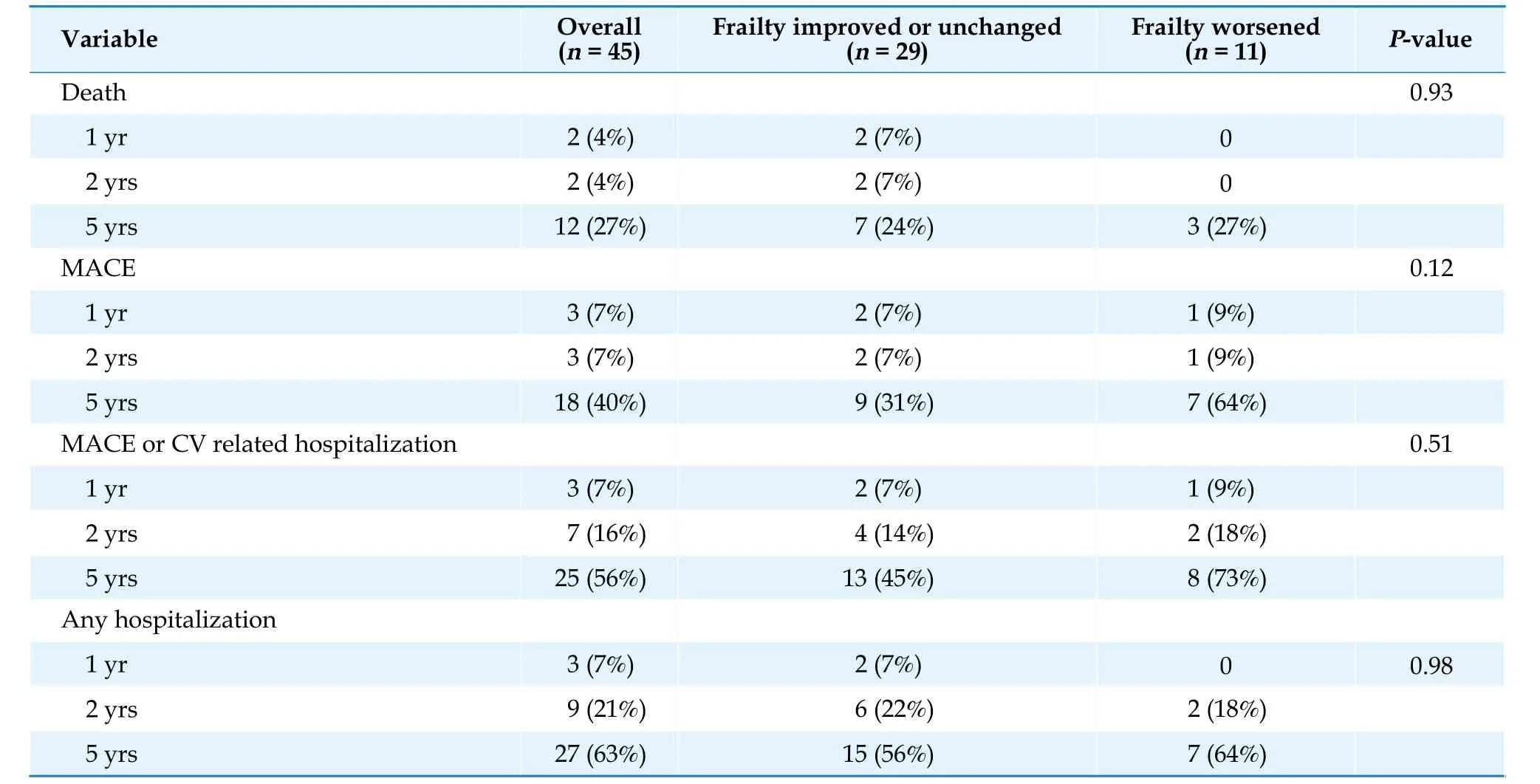
Table 3 Long term outcomes by frailty change status.

Figure 2 Survival free of MACE (A) and hospitalization (B) by frailty change score status over long-term follow-up. MACE: major adverse cardiac event.
Baseline grip strength was not associated with frailty worsening score in our study, though change in grip strength was (r=-0.39;P=0.01). In a large cohort of adults ≥ 60 years old, a 1 kg improvement in grip strength over 1-year was associated with 13% lower mortality.[18]Similar observations have been noted over long-term follow-up among those ≥85 years old, with some suggestion that women with declining grip strength may be at greater mortality risk than men.[19]Declining grip strength change may also correlate with changes in skeletal health, bone loss, fracture risk, and other adverse health outcomes.[20,21]These data support serial assessment of grip strength as both a biomarker of functional abilities and identifier of vulnerability in older adults with geriatric syndromes. Intervention trials aimed at attenuating grip strength decline through strength training may have the added benefit of impacting the frailty phenotype. Given that lower grip strength has been associated with higher cardiovascular mortality,[22]the implications of declining grip strength in a CAD population deserves further study.
Few studies have looked at frailty change and its relationship to outcome. Wang,et al.[23]used the Fried frailty index to show that both baseline frailty and a graded risk between frailty transition states were observed with respect to mortality. A study from the Chinese Longitudinal Healthy Longevity Survey aimed to study transitions between frailty states and mortality.[24]In addition to noting worse survival among frailty change worsening status,these patients were also more likely to have greater suffering at end of life based on survey responses from next of kin. Similar to our study, both showed that frailty transitions were more likely to show frailty worsening than frailty improvement. Both studies highlight the dynamic process of change that occurs in the frailty phenotype and its association with outcome, underscoring that baseline indices incompletely characterize age-associated impairments and that frailty trajectory may provide additive information with respect to risk of adverse outcome.
Our study adds to the literature by reporting frailty change over a 5-year period, followed by outcome assessment for another subsequent five years, representing one of the longest incident frailty studies to date. In addition, we studied MACE in addition to mortality which are relevant endpoints for patients with CAD. We identified that specific frailty components, namely functional performance with grip strength and gait speed, are responsible for the majority of transitions. The independent association of heart failure with MACE after accounting for demographics and frailty change status shows the prognostic significance of this comorbidity in older adults. This finding extends prior observations of the relationship between frail heart failure patients and outcome.[25]
Limitations of our study included a modest sample size returning for the 2ndwave assessment,which likely affected our power to detect significant differences between frailty worsening status and MACE outcomes. Characteristics of responders vs non-responders in our study were similar, reducing potential for non-response bias. We defined frailty using the validated Fried index at both baseline and follow-up, though other forms of frailty assessment that are more streamlined, incorporate other features (i.e., cognition), and are, perhaps, less intensive have been used. Our study demonstrated that baseline assessments for Fried frailty classification change over time, with walk time and grip strength predominately affecting Fried frailty classification. An increase in BMI had a trend towards being mildly protective from worsening frailty. There was no baseline single-item measure correlated with worsening Fried frailty status at 5-year follow-up. Efforts to improve understanding of age-related impairments may be supplemented through repeated measures to evaluate change in phenotype over time. Baseline abnormalities for Fried indices are less indicative of frailty change over long term follow-up.
ACKNOWLEDGMENTS
This study was supported by Mayo Clinic Foundation for Education and Research.
 Journal of Geriatric Cardiology2021年3期
Journal of Geriatric Cardiology2021年3期
- Journal of Geriatric Cardiology的其它文章
- Journal of Geriatric Cardiology
- Hybrid coronary revascularization vs. percutaneous coronary interventions for multivessel coronary artery disease
- Effects of the total physical activity and its changes on incidence, progression, and remission of hypertension
- Association of changes in waist circumference with cardiovascular disease and all-cause mortality among the elderly Chinese population: a retrospective cohort study
- Associations between low-density lipoprotein cholesterol and haemorrhagic stroke
- Optimal dual antiplatelet therapy strategy in elderly patients with acute coronary syndrome
