Current status of fecal calprotectin as a diagnostic or monitoring biomarker for cow’s milk protein allergy in children:a scoping review
Li-Jing Xiong ·Xiao-Li Xie ·Yang Li ·Xiao-Zhi Deng
Abstract Background There are few approved biomarkers for diagnosis and monitoring of cow’s milk protein allergy (CMPA),thus the oral food challenge remains to be the golden diagnostic standard.A potential biomarker is fecal calprotectin,a cytosolic protein,elevating in the presence of intestinal mucosal inflammation.We aimed to undertake a scoping review of the evidence pertaining to the current status of fecal calprotectin used for diagnosis and monitoring CMPA in children,and tried to indicate the aspects needed to be concerned in the future investigations and researches.Methods A scoping review was performed using the literature searched from PUBMED,EMBASE,and Web of Science Databases until July 2019 on the studies about the application of fecal calprotectin as a biomarker of CMPA in children.Studies were examined according to the inclusion and exclusion criteria.Data were extracted,and a narrative synthesis was conducted to summarize and analyze.Results Thirteen studies with different study design embracing 1238 children were included.The age range was from infants to adolescents.Most children with CMPA presented gastrointestinal symptoms,among which hematochezia was most common.Amount of data suggested that infants with CMPA represented elevated levels of fecal calprotectin,particularly with distinct significance in non-IgE-mediated CMPA groups.Decreases of fecal calprotectin after elimination diet were demonstrated in enrolled studies.However,no matter in the CMPA positive or negative groups,the changes of fecal calprotectin before or after challenge showed no significance.Contradictory results were generated from studies on the role of fecal calprotectin in predicting allergic disease.Conclusions Available evidence is not sufficient to confirm the utilization of fecal calprotectin both in diagnosis and monitoring of CMPA and predicting for allergic disease.More clinical and bench researches with elaborate design should be conducted and the exact cut-offvalues of fecal calprotectin in different groups remain to be determined.
Keywords Cow’s milk protein allergy·Fecal calprotectin·Scoping review
Introduction
Cow’s milk protein allergy (CMPA) usually occurs in infants,and affects individuals from infancy to toddler and adolescence according to the“allergic march”theory [1].CMPA-related morbidity is increasing around the world and becoming a public health problem [2].CMPA,a complex disease with multiple immunopathogenesis,is divided into three categories:IgE-mediated type,non-IgE-mediated type and mixed type.CMPA causes various symptoms in different systems including atopic dermatitis,gastrointestinal (GI) or respiratory symptoms,even anaphylaxis.Generally,gastrointestinal symptoms are predominated but non-specific in infants with CMPA,such as hematochezia,chronic diarrhea and regurgitation.CMPA with gut involvement is mainly mediated by non-IgE mechanism including three clinical types:food protein-induced proctocolitis (FPIP),food protein-induced enteropathy (FPIE)and food protein-induced enterocolitis syndrome (FPIES)[3].For infants with CMPA,in spite of their feeding patterns,eliminating cow’s milk protein from the diet which triggers the immune reaction is the best therapy for alleviating the symptoms [4,5].
Diagnosis of CMPA is often made by clinical history that suggests the association between cow’s milk protein ingestion and symptoms.IgE-mediated CMPA can be confirmed based on the results of serum specific IgE to cow’s milk protein or a skin prick test.However,making diagnosis of non-IgE-mediated CMPA in children presenting with chronic or delayed gastrointestinal symptoms remains much more difficult and challenging [6].Therefore,the diagnostic gold standard for non-IgE-mediated CMPA is still the double-blind,placebo-controlled food challenge (DBPCFC)which is not easy to be conducted and time consuming in clinical practice.This is the reason why there is growing interest to find biomarkers serving as reliable tools for diagnosis and monitoring of CMPA,particularly for the non-IgEmediated type CMPA.
The gastrointestinal symptoms are more common and severe in children,so fecal inflammatory biomarkers of CMPA have been taken into consideration.One of the candidates is calprotectin,a calcium-and zinc-binding protein expressed in neutrophils,monocytes,macrophages and even in mucosal epithelial cells [7].It is a protein biomarker of inflammation and can be detected in body fluid and stool [8].The range of its value varies referring to the site of collection and diseases,which suggests it as an appropriate biomarker of local inflammation [9].Calprotectin can be easily detected in feces and elevates with the presence of intestinal mucosal inflammation [10].In children with GI symptoms,mucosal inflammation can be found in intestinal tract caused by infiltration of variant inflammatory cells including neutrophils,monocytes and eosinophils,which have been confirmed by endoscopy and pathology studies [11].In animal models,fecal calprotectin with other inflammation-associated cytokines were found to promote the gut inflammation in food allergy [12].Therefore,fecal calprotectin is considered potentially as a non-invasive,reliable and easily available fecal biomarker for estimating enteric inflammation in food allergy.Clinically,it has already been applied in inflammatory bowel disease (IBD) as a sensitive indicator for following-up and monitoring the remission [13].Researchers also have assessed fecal calprotectin in pediatric GI diseases including active allergic colitis,and calculated an optimized cut-offvalue for distinguishing patients with active organic/inflammatory disorders from healthy subjects and functional bowel disorder patients [14].
Recently,some researchers have examined the levels of fecal calprotectin in children with CMPA,on the assumption that inflammation or aggregation of inflammatory cells presenting in the intestinal mucosa.Since various studies have heterogeneities including methods,ages of the individuals,and the cut-offvalues,whether fecal calprotectin is effective and reliable for CMPA as a biomarker is unclear.Therefore,we aimed to undertake a scoping view of the evidence pertaining to the current status of fecal calprotectin utilized for diagnosing and monitoring CMPA in children,and tried to indicate the aspects which needed to be concerned in the future investigations and researches.
Methods
This scoping review was conducted based on the previously described methodological framework by Arksey and O’Malley [15]and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis for Scoping Reviews (PRISMA-ScR) [16].
Search strategy
The Literature search was undertaken to identify the studies about the current status of fecal calprotectin as a diagnostic or monitoring biomarker for CMPA in children.We searched PUBMED,EMBASE and Web of Science (from inception to July 2019) to find relevant articles.The following keywords were used in the developed search strategy including“fecal calprotectin”,“cow’s milk protein allergy”,“cow milk protein allergy”,“cow’s milk allergy”and”food allergy”.
Inclusion and exclusion criteria were established and revised based on the increasing familiarity with the literature.Inclusion criteria of articles for this scoping review included:(1) studies about the role of fecal calprotectin used in children with CMPA,(2) the diagnosis of CMPA should be stated,(3) quantitative value of fecal calprotectin should be stated.All types of study designs and publications,all age group of children,gender,health-care settings,feeding pattern,and treatment modalities were considered for further reviewing.Exclusion criteria:(1) study on the animal model,(2) CMPA children with other diseases which would affect the levels of fecal calprotectin like inflammatory bowel disease and intestinal infectious disease.
Study selection
All the identified literature was examined with rough initial screening of titles.All publications with titles clearly ineligible and duplicated were removed by one author.Subsequently,potentially eligible titles and abstracts were reviewed independently by two reviewers to determine the inclusion or exclusion of studies for full-text reviewing.Disagreements were discussed referring to the inclusion and exclusion criteria.In case of irreconcilable disagreements,a third reviewer was consulted.Full-text articles were examined with the focus on the role of fecal calprotectin in children with CMPA.
Data extraction and synthesis
Two reviewers extracted the following information from the selected studies using charting forms:authors,title,year of publication,journal,study design,age and gender of study subjects,methods and units of fecal calprotectin assay,levels of the protein (mean and SD or range),types of CMPA,the number of CMPA and control subjects,gastrointestinal symptoms of CMPA in children,whether diagnosis of CMPA was established by oral food challenge,therapeutic intervention against CMPA,as well as duration and type of the elimination diet.And all the studies were categorized according to the different objectives of using fecal calprotectin for diangosis or monitoring of CMPA in children.Data were collated and presented for narrative qualitative synthesis.
Results
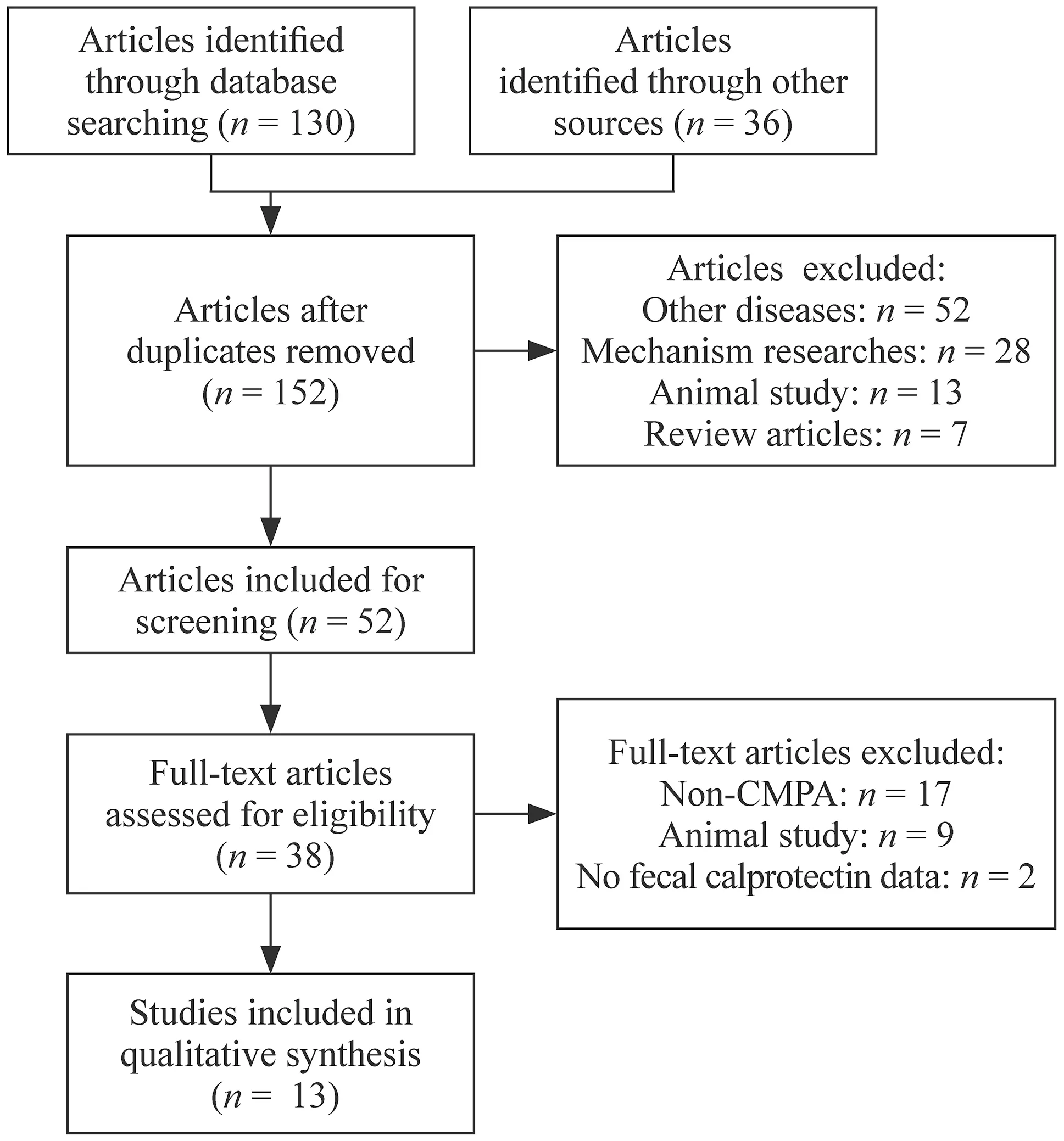
Fig.1 PRISMA flow diagram
The initial search yielded 166 potentially relevant studies.After removing duplicates and irrelevant studies,52 records were retained,of which 38 were reviewed in the full text or abstracts (Fig.1).In the end,13 studies [17— 29]with different study design embracing 1238 children were included for this scoping review (Table 1).Most patients with CMPA [17— 20,22,24]who were detected for fecal calprotectin presented with GI symptoms among which hematochezia was the most common.Only one study [29]applied fecal calprotectin as a biomarker for evaluating the clinical severity of atopic dermatitis in food allergy children who had no GI symptoms.ELISA was the most widely-used method for detection of fecal calprotectin.The age spectrum was from infants to adolescents.Moreover,CMPA children varied in their feeding pattern,elimination therapies and eliminating durations.Studies involved could be mainly stratified by the aims of each study (Table 1):(1) fecal calprotectin used as a biomarker for diagnosis of CMPA,(2)fecal calprotectin used as a biomarker for CMPA monitoring after elimination therapy,(3) fecal calprotectin used as a biomarker for assessing gut response to oral food challenge,(4)fecal calprotectin used as a biomarker for predicting CMPA later in the life or evaluating allergic disease.
Fecal calprotectin used as a biomarker for CMPA diagnosis and monitoring
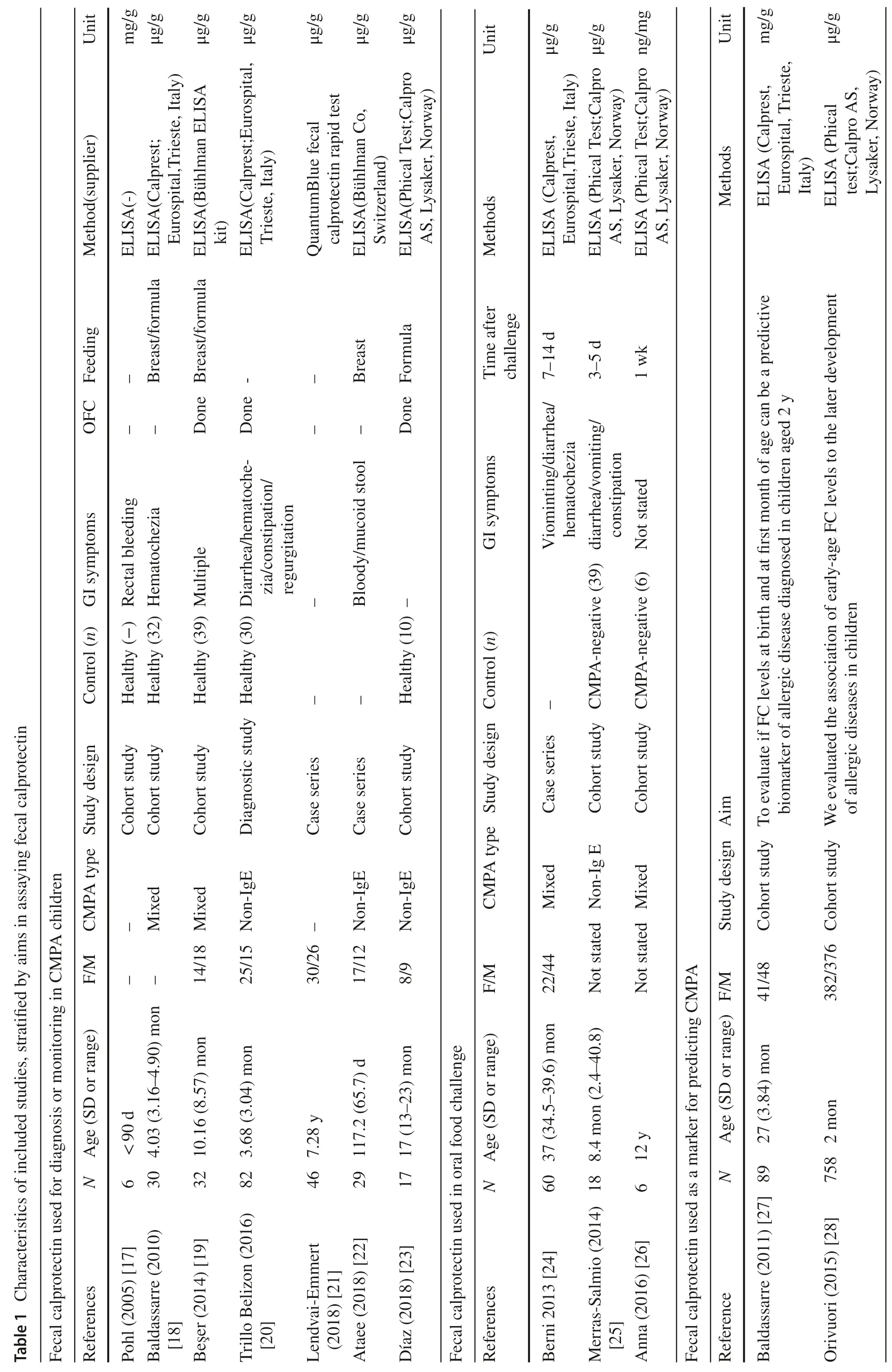
Researchers have detected fecal calprotectin in children with CMPA and compared with healthy controls.Most data suggested that CMPA in children were associated with elevated levels of fecal calprotectin (Table 2),which were consistent with the assumption that CMPA children with GI symptoms had intestinal inflammation.However,in the group of children whose mean ages were older than 12 months [19,23,26],there were no statistical significances between CMPA group and controls.Type of CMPA was another factor impacting the results of fecal calprotectin detection.Beşer ÖF [19]compared the mixed group,IgE-mediated group and non-IgE-mediated group with healthy control group,respectively,and the results indicated that there was a distinct discrepancy with statistical significance in non-IgE-mediated CMPA group.Those data suggested that fecal calprotectin might be more suitable for non-IgE-mediated CMPA infants with GI symptoms particularly the proctocolitis.However,most studies [17,18,21,22]did not state the diagnosis of CMPA confirmed by the gold standard of DBPCFC or open oral food challenge.Moreover,because of the variant sensitivity and specificity among different kits,the normal range and cut-offvalue did not state clearly,although the methods of ELESA were indicated in articles.Therefore,considering those apparent heterogeneities,it was hard to evaluate the diagnostic performance of fecal calprotectin in clinical applications.Only one study with English abstract,but published in Spanish,reported the diagnostic efficacy of fecal calprotectin in children with non-IgE-mediated CMPA with a sensitivity of 95%,a specificity of 78.57% and the cut-offvalue of 138 μg/g compared with oral food challenge [20].

After diagnosed as CMPA,patients were given elimination diets tailored to their feeding patterns.Then fecal calprotectin was detected at different time points after clinical remission.Data indicated obvious decreases in the levels of fecal calprotectin after elimination diet (Table 3).Controversially,in the study of Ataee P [22],they found fecal calprotectin was not suitable as a biomarker for evaluating the resolution of diarrhea,bloody stool and mucus.Moreover,there was no statistical significance of fecal calprotectin with different elimination duration after clinical remission.However,we noticed that the inlcuded individuals in their prospective cohort study were breastfeeding infants with suspected CMPA,which could result in an unreliable evaluation about the effect of elimination therapy.Therefore,when conducting researches for assessing the role of fecal calprotectin as a biomarker for monitoring after treatment,the diagnosis criteria of CMPA,whether the elimination diet is strict enough and the duration of elimination therapy should be stated clearly.
Since fecal calprotectin is a sensitive marker for enteric inflammation,the relationship between fecal calprotectin level and local inflammation appeared to be influenced by many factors,including age,feeding pattern,testing method,gut microbiota and developmental stage [30,31].Age was one of the most important factors which would determine the cut-offvalue of fecal calprotectin [32].It was different in different age groups and declined with growth.Furthermore,for CMPA children,more complex factors including intestinal pathophysiology mechanism,duration of elimination and choices of elimination formula needed to be taken into consideration if discussing the role of fecal calprotectin in following-up the effect of elimination therapy.Unfortunately,the data collected at present were not sufficient to evaluate the effect of each factor by subgroup analysis.Hence,although decrease of fecal calprotectin after elimination diet was demonstrated in enrolled studies,it was not profound enough to confirm the role of fecal calprotectin as a laboratory biomarker for mucosa healing of CMPA after elimination diets.
Fecal calprotectin used as a biomarker for assessing intestinal response to food challenge
Two studies [25,26]examined fecal calprotectin as a gut biomarker for assessing CMP challenge by comparing the value of fecal calprotectin before and after oral food challenge,respectively in challenge-positive and challengenegative groups.From the included studies,no matter in the CMPA positive or negative group,the changes of fecal calprotectin levels before or after challenge were both not statistically significant (Table 4).A potential reason explained by the authors was the small sample size of study (n=6) which rendering low statistical power and the large natural variance in the level of fecal calprotectin[26].We assumed that another reason might be the short interval of provocation and detection.Because CMPA with GI symptoms are mainly caused by non-IgE-mediated mechanism which is usually delayed-onset hypersensitivity and the time of immune reactions varied in individuals.Therefore,the data available are not sufficient to implicatefecal calprotectin as a sensitive biomarker for assessing the intestinal response after oral food challenge.Bernic et al.[24]utilized fecal calprotectin for evaluating intestinal tolerance to a new commercial amino-acid formula,and the level of fecal calprotectin remained stable within the normal range during the challenge.Therefore,from another aspect,whether fecal calprotectin could be more suitable for using as a negative predictive marker deserves further investigations.
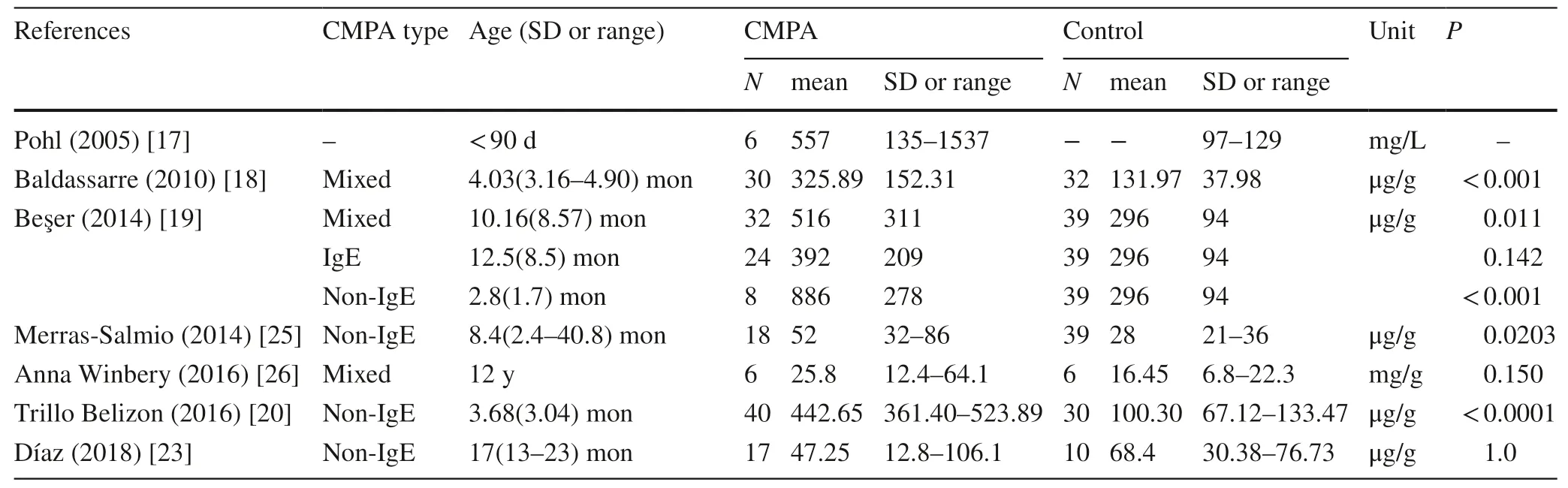
Table 2 Fecal calprotectin detected in children with cow’s milk protein allergy (CMPA) and control
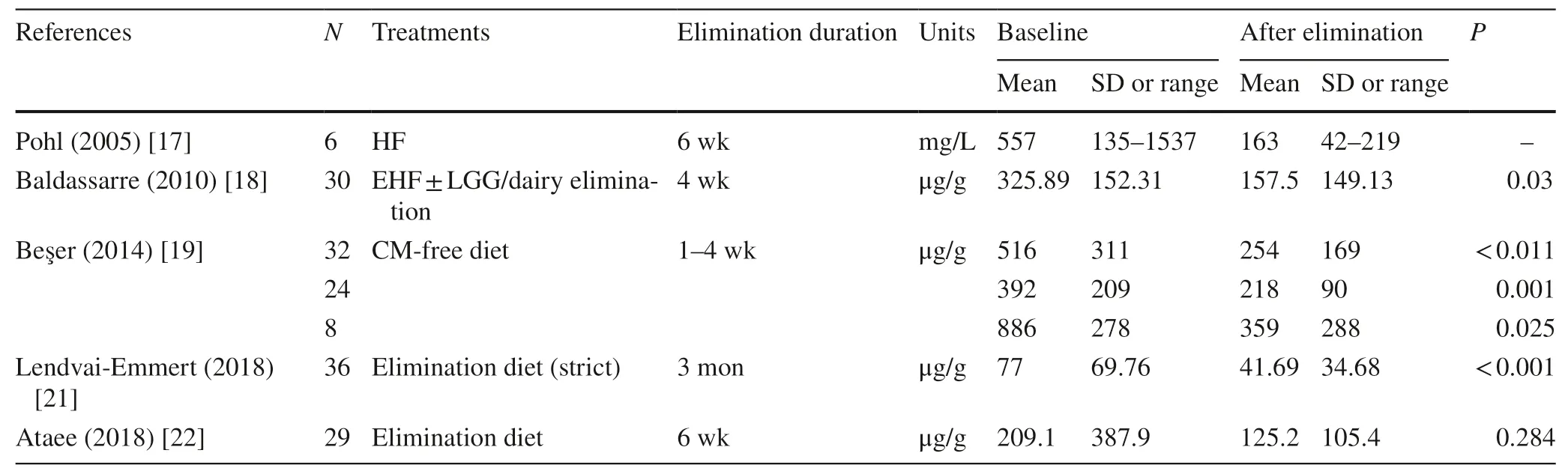
Table 3 Fecal calprotectin used for monitoring cow’s milk protein allergy (CMPA) after elimination diet
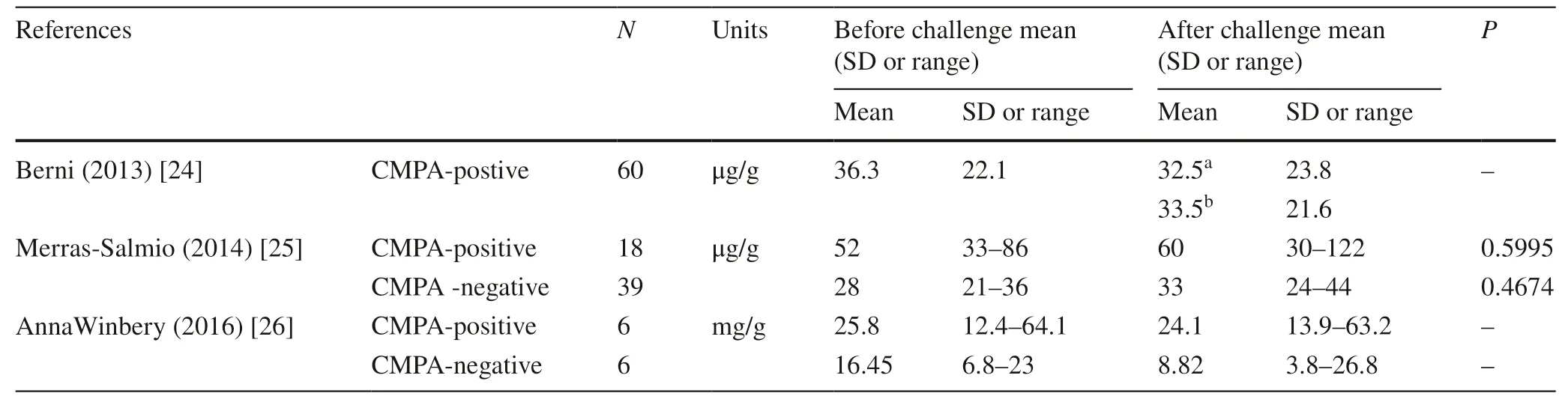
Table 4 Fecal calprotectin used in oral food challenge
Fecal calprotectin used as a predictor or indicator in allergic disease
When using fecal calprotectin as a predictor for CMPA in children’s later life,contradictory results were yielded from two studies.Baldassarre et al.[27]detected fecal calprotectin in neonates,and compared them in allergic and non-allergic groups 2 years later.No link could be found between fecal calprotectin early in life and the development of allergic disease.However,in a prospective birth cohort study with 758 children conducted in Austria,Finland,France,Germany and Switzerland named PASTURE (Protection against Allergy-Study in Rural Environments),higher levels of fecal calprotectin (> 90th percentile) in infants aged 2 months were related to the increased risk of developing allergic disease,like asthma and atopic dermatitis,at the age of 6 years.This association might come from the different development of gut microbita [28].Another study [29]which used fecal calprotectin as a biomarker in food allergy patients without GI symptoms was to investigate the relationship between fecal calprotectin and clinical severity of atopic dermatitis in children above 3 years old.Researchers divided patients with atopic dermatitis into two groups according to the different values of fecal calprotectin,and compared serum IgE,Scoring Atopic Dermatitis index (SCORAD),and blood eosinophils between the two groups;the results suggested that fecal calprotectin might be utilized for assessment of disease severity in atopic dermatitis children.
Discussion
Searching,testing,and applying of biomarkers should be based on profound researches about the pathogenesis and their relations to clinical feature.CMPA is a kind of disease with multiple complicated immunopathogenesis and distinct clinical heterogeneity.Therefore,more researches focusing mechanism of CMPA should be conducted to find out a proper biomarker or the combination of biomarkers to provide objective evidences in the diagnosing,monitoring,predicting and evaluating of CMPA.
Fecal calprotectin is a gut inflammation biomarker with the advantages of non-invasion,high sensitivity,easy conduction and availability,and it is more suitable to be applied in infants and children.However,the role of fecal calprotectin in the occurrence and development of CMPA remains unclear.Narrative synthesis analysis showed that elevated levels of fecal calprotectin were detected in CMPA children,and declined after elimination diet.Therefore,it was believed that fecal calprotectin might be a potential biomarker for diagnosing CMPA in children.However,the results were influenced by many factors including age,feeding pattern,gut mirobiota,and mucosa permeability.Therefore,those confounding factors should be taken into consideration at the time of interpreting the changes and discrepancy of fecal calprotectin values.Furthermore,when evaluating the reliability of fecal calprotectin in diagnosing and monitoring,the gold standard for diagnosis of CMPA,duration and type of elimination therapy,comorbidity of intestinal bacterial infections or other diseases must be stated clearly.Different cut-offvalues of fecal calprotectin should be calculated and analyzed according to age,feeding patterns,clinical symptoms,and even immune mechanism type.Moreover,as a biomarker indicating the local inflammation in gastrointestinal tract,it should be more cautious to evaluate the results when using fecal calprotectin in food allergy children without GI symptoms.
In summary,we have reviewed and assessed the application of fecal calprotectin as a potential biomarker for diagnosis,monitoring,predicting in CMPA children.However,the available evidence is not profound enough to confirm the use of fecal calprotectin in clinical practice.More researches with elaborate design should be conducted and the exact cut-offvalues of fecal calprotectin in different groups remain to be determined.
Author contributionsLJX and YL contributed to the literature searching,analysis,and drafting the article;XLX and XZD contributed to conception and revising it critically for important intellectual content,and final approval of the version to be published.
FundingThis study was supported by Science and Technology Project of the Health Planning Committee of Sichuan Province,China(17PJ278).
Compliance with ethical standards
Ethical approvalNot required for this review article.
Conflict of interestAll authors read and approved the manuscript for publication.No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
 World Journal of Pediatrics2021年1期
World Journal of Pediatrics2021年1期
- World Journal of Pediatrics的其它文章
- Why COVID-19 is less frequent and severe in children:a narrative review
- Laboratory diagnosis of COVID-19 in secondary care hospitals in India:will standalone serology suffice?
- Winter is coming:care of the febrile children in the time of COVID-19
- Addressing e-cigarette health claims made on social media amidst the COVID-19 pandemic
- Social responsibilities of a pediatric journal:considerations and future directions
- Surrogate markers and predictors of endogenous insulin secretion in children and adolescents with type 1 diabetes
