Surrogate markers and predictors of endogenous insulin secretion in children and adolescents with type 1 diabetes
Jin-Na Yuan·Jian-Wei Zhang·Wayne S.Cutfield·Guan-Ping Dong·You-Jun Jiang·Wei Wu·Ke Huang·Xiao-Chun Chen·Yan Zheng·Bi-Hong Liu·José G.B.Derraik,·Jun-Fen Fu
Abstract Background No studies have examined endogenous insulin secretion in pediatric patients with type 1 diabetes in China using the gold-standard mixed-meal tolerance test.Because the latter is labor-intensive,we examined simpler surrogate markers of endogenous insulin secretion in Chinese youth,as previously reported for a European population.Methods Participants were 57 children and adolescents with type 1 diabetes aged 4.4—16.8 years (56% females).We performed 120-minute mixed-meal tolerance tests with serum C-peptide (CP) measurements every 30 minutes.Severe insulin deficiency (SID) was defined as CP peak < 0.2 nmol/L.Urine CP and creatinine levels were measured at 0 and 120 minutes.Results Twenty-five (44%) patients had SID.Fasting CP levels missed one case (96% sensitivity) with no false positives (100% specificity).While the 120-minute urine CP/creatinine had 100% sensitivity,it yielded markedly lower specificity (63%).Every 1-year increase in diabetes duration and 1-year decrease in age at diagnosis were associated with 37% (P < 0.001) and 20% (P =0.005) reductions in serum CP area-under-the-curve,respectively.Thus,86% of children aged < 5 years had SID compared to none among patients aged ≥ 11 years.Conclusions Simple fasting CP measurements could be used to detect most SID cases in Chinese youth with type 1 diabetes.Fasting CP is a far more reliable measure of endogenous insulin secretion than the more commonly used insulin dose.Therefore,it could more precisely determine insulin secretory capacity to target those who could benefit,if treatments to preserve residual insulin secretion are developed.
Keywords Endogenous insulin secretion · Mixed-meal tolerance test (MMTT) · Type 1 diabetes mellitus
Introduction
There are approximately 425 million people living with diabetes worldwide,5—10% of whom have type 1 diabetes [1].This is the most common form of diabetes in children,and the incidence is on the rise in China and globally [2,3].Type 1 diabetes is an autoimmune disease characterized by T-cell mediated destruction of insulin-producing β-cells in the pancreatic islets [4,5].At the time of type 1 diabetes diagnosis,most patients may have a residual β-cell function that continues to produce some insulin.However,there is a progressive decline in endogenous insulin secretion with increasing diabetes duration [6— 8].Retention of endogenous insulin secretion leads to improved glycemic control and decreases the risk of micro-vascular complications and diabetic ketoacidosis [9— 12].Therefore,the evaluation of endogenous insulin secretion in patients with type 1 diabetes is important to improve health outcomes.
C-peptide (CP) is co-secreted with insulin by the pancreatic β-cells as a by-product of the enzymatic cleavage of proinsulin,and stimulated secretion of CP is considered a measure of endogenous insulin secretion [13].The mixedmeal tolerance test (MMTT) is the gold-standard technique to measure endogenous insulin secretion in type 1 diabetes[6,13].During the MMTT,the fasting and postprandial CP levels in serum are collected over 120—240 minutes.Although commonly used in clinical research,it rarely adopted in routine clinical practice as the blood sampling is labour intensive [14].Therefore,the identification of accurate surrogate markers of endogenous insulin secretion would be useful.
Besser et al.reported that fasting CP,90-minute CP,and urine CP to creatinine ratio (CP/Cr) were all highly correlated with the standard MMTT in a group of 72 European patients with type 1 diabetes (including 21 children and adolescents in Sweden) and could be used to estimate β-cell reserve [15].Because the standard MMTT is invasive to perform,we aimed to assess the accuracy of simpler surrogate markers to estimate endogenous insulin secretion,as proposed by Besser et al.[15],in children and adolescents with type 1 diabetes in China.The simpler markers could be easier to adopt in clinical practice and in large-scale cohort studies.To our knowledge,no previous studies have assessed endogenous insulin secretion in pediatric patients with type 1 diabetes in China using MMTT.As a result,we also examined the associations between endogenous insulin secretion and demographic factors.
Methods
Study population
Pediatric patients with type 1 diabetes were recruited into the study between January 2013 and August 2016 at the Children’s Hospital,Zhejiang University School of Medicine (Hangzhou,China).All patients had previously been diagnosed with type 1 diabetes based on clinical and biochemical features:(1) elevated blood glucose at presentation (random measurement ≥ 11.1 mmol/L and/or fasting blood glucose ≥ 7.1 mmol/L);AND (2) classical symptoms of diabetes;AND (3) either:(1) antibodies present;OR (2) if antibodies were negative,the serum CP and insulin should be at severely low levels,and insulin must have been started at or shortly after diagnosis and used continually thereafter.
Tests were performed for autoantibodies to insulin,islet cell,glutamate decarboxylase,and islet antigen-2.Participants were recruited based on windows of opportunity (mostly during the summer holidays) to make involvement in the study more agreeable to patients.All patients were being treated with insulin at the time of study,and had normal renal function.Exclusion criteria were:(1) ongoing treatment with drugs that influence insulin secretory capacity;and (2) known renal impairment (i.e.,estimated glomerular filtration rate < 60 mL/min/1.73 m 2).
Mixed-meal tolerance test
Participants underwent a 120-minute MMTT at the Department of Endocrinology at the Children’s Hospital of Zhejiang University School of Medicine.The MMTT was performed at approximately 8 a.m.following an overnight fast of at least 10 hours.Patients on the insulin pump had it paused at least 3 hours before the MMTT.The use of longacting or intermediate-acting insulin was paused at least 24 hours before the test,and no insulin bolus was taken in the morning before the MMTT.No patients had diabetic ketoacidosis during the test.
Patients were given an oral solution (6 mL/kg,with a maximum of 360 mL) containing carbohydrate (33%),protein (17%),and lipids (50%) (Glucerna TPF-D,ABBOTT,Zwolle,Netherlands).Serum CP measurements were taken every 30 minutes.Severe insulin deficiency was defined as a serum CP peak from all samples during the 120-minute MMTT < 0.2 nmol/L,which is a threshold associated with clinical outcomes [16,17].Endogenous insulin secretion was assessed by the serum CP area under the curve(CPAUC).Urine samples were also collected immediately before the MMTT start (0 minute) and after 120 minutes.Urine CP and creatinine levels were measured,with CPpeakand CP/Cr ratio calculated.
Assays
All blood and urine samples were sent to our hospital’s laboratory for analyses soon after collection (within an hour).CP was measured by a two-site immunometric assay on the Roche Cobas e602 analyser (Roche Diagnostics,Mannheim,Germany).Creatinine was measured on a Beckman Coulter AU5800 (Beckman Coulter Inc.,Brea,CA,USA) as per manufacturer’s protocol,with a coefficient of variation ≤ 3%.
Ethics
This study was approved by the Medical Ethics Committee of the Children’s Hospital,Zhejiang University School of Medicine (No.2020-IRB-034),and informed consent was obtained from a parent and/or legal guardian of the patients.All of the reported investigations were performed in accordance with the principles of the Declaration of Helsinki as revised in 2000.
Statistical analyses
CPAUCwas calculated using Prism v7.03 (GraphPad Software Inc,San Diego,CA,USA).CP data from serum and urine were log-transformed to approximate normality.The associations between serum CP peak,serum CP AUC ,and urine measurements were examined using Pearson’s correlation coefficients.The associations between serum CPpeakand specific predictors (i.e.,sex,age at diagnosis,and diabetes duration) were examined using general linear regression models.The urine CPpeakand CP/Cr thresholds that were equivalent to 0.2 nmol/L from the serum CPpeakwere derived using simple linear regressions.Overall and pairwise differences in SID rates among strata according to diabetes duration or age at diagnosis were assessed using Fisher’s exact tests.The ability of individual serum and urine parameters to accurately detect patients with severe insulin deficiency was examined as follows:Sensitivity=ntrue positives/(ntrue positives+nfalse negatives);Specificity=ntrue negatives/(ntrue negatives+nfalse positives);Positive predictive value=ntrue positives/(ntrue positives+nfalse positives);Negative predictive value=ntrue negatives/(ntrue negatives+nfalse negatives).Statistical analyses were performed in Minitab v.16 (Pennsylvania State University,State College,PA,USA) and SPSS v.26 (IBM Corp,Armonk,NY,USA).All tests were two-tailed,with statistical significance set atP< 0.05 and without adjustment for multiple comparisons.There was no imputation of missing values.
Results
We studied 57 patients,including 32 (56%) females,whose demographic characteristics and clinical information are described in Table 1.Twenty-one (37%) of these patients were on insulin pump therapy.
Timing of CPpeak
Seven (12%) patients had very severe insulin deficiency,so that their serum CPpeakwas below detectable levels(i.e.,< 0.003 nmol/L).From the 50 remaining patients,40%(n=20) had a serum CP peak at 90 minutes,24% (n=12 each)at 60 or 120 minutes,and 12% (n=6) at 30 minutes.
Predictive ability
Of the 57 patients,25 had severe insulin deficiency with a serum CPpeak< 0.2 nmol/L throughout the MMTT.Fasting CP accurately detected all but one case of severe insulin deficiency (i.e.,sensitivity 96%) and with no false positives(100% specificity) (Table 2).Conversely,serum CPpeakat 30,60,and 90 minutes had 100% sensitivity,but lower specificity ranging from 84 to 94% (Table 2).
Urine CP/Cr at 120 minutes had 100% sensitivity,but much lower specificity at 63% with 11 false positives (Table 2).Urine CPpeakhad nearly perfect sensitivity at 96%,and its specificity was better than that of CP/Cr the ratio at 87%.The higher urine CP/Cr cut-off proposed by Besser et al.’s [15]performed at a similar level to our urine CP/Cr at 120 minutes,with 100% sensitivity and 60% specificity (Table 2).
Predictors of endogenous insulin secretion
There was a weak inverse correlation between insulin dose(adjusted for weight) and fasting CP (r=− 0.27;P=0.044).Furthermore,there was no observed association between glycated haemoglobin (HbA1c) and fasting CP,either in simple correlation (r=0.02;P=0.88) or covariate-adjusted analysis [β=− 0.073 (95% CI − 0.220,0.074);P=0.34].
Increasing diabetes duration was associated with a lower serum CPAUC[β=− 0.37 (95% CI − 0.56,− 0.18);P< 0.001],so that every 1-year increase in duration was associated with a 31% decrease in serum CPAUC.As a result,there was a steady increase in the rate of severe insulindeficiency over time (Table 3).This change was particularly marked after 2 years since diabetes diagnosis when the rate of severe insulin deficiency increased from 13% (2/16) to 67% (6/9) (Table 3;P=0.010 for a pairwise comparison).
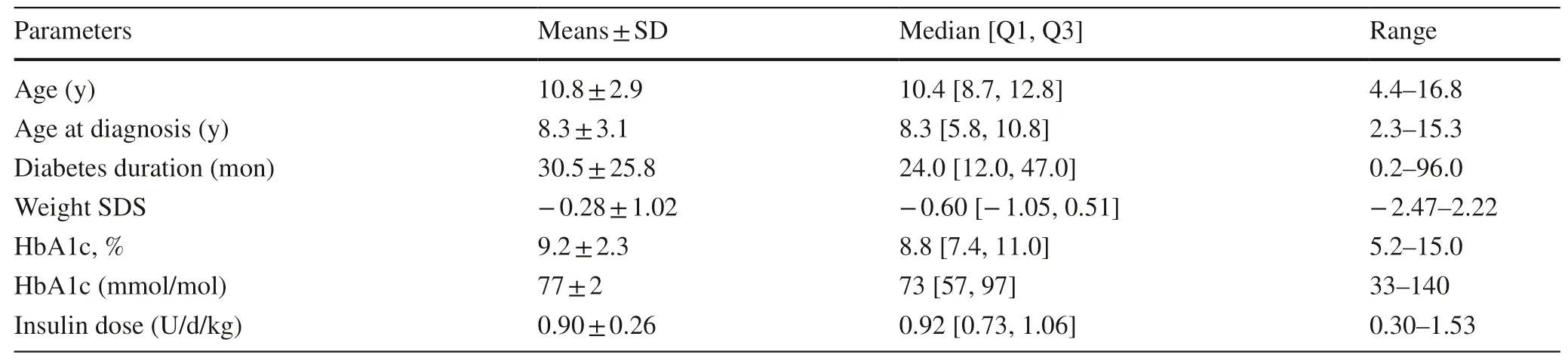
Table 1 Demographic and clinical data on 57 children and adolescents with type 1 diabetes mellitus in Hangzhou,China
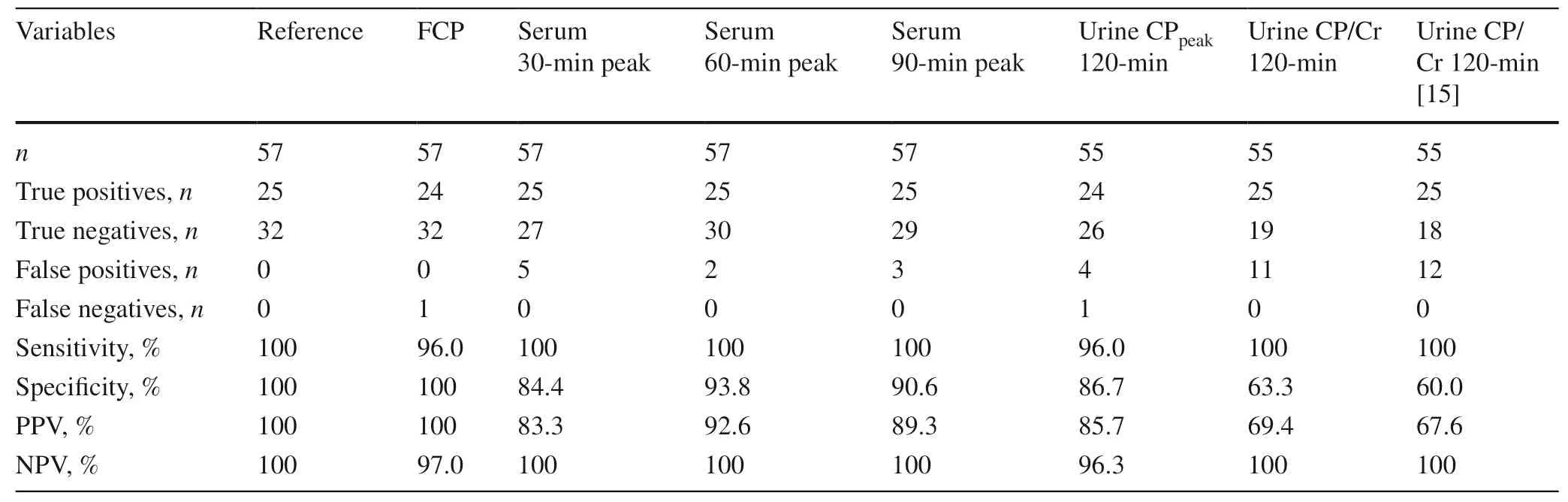
Table 2 The predictive ability of serum and urine parameters from a 120-min mixed-meal tolerance test (MMTT) to identify children and adolescents with type 1 diabetes mellitus who had severe insulin deficiency
In addition,every 1-year increase in age at type 1 diabetes diagnosis was associated with a 22% increase in serum CPAUC[β=0.20 (95% CI 0.73,0.33);P=0.005].There was a consequent decline in the rate of severe insulin deficiency with increasing age at diagnosis (Table 3,with a 86% (6/7)prevalence among children under 5 years of age compared to no cases among children aged 11 or older (0/9) (P=0.001 for a pairwise comparison).Note that there were no differences in serum CPAUCbetween boys and girls (P=0.45).
Associations between diagnostic measures
All diagnostic measures from the MMTT or urine test examined were highly correlated (Table 4).Fasting CP was almost perfectly correlated with serum CPpeakand CPAUC,with urine CPpeakalso highly correlated with thesetwo serum parameters;urine CP/Cr was also highly correlated with CPpeakand CPAUC,but not as strong as the other two (Table 4).These strong linear associations are illustrated in Fig.1.
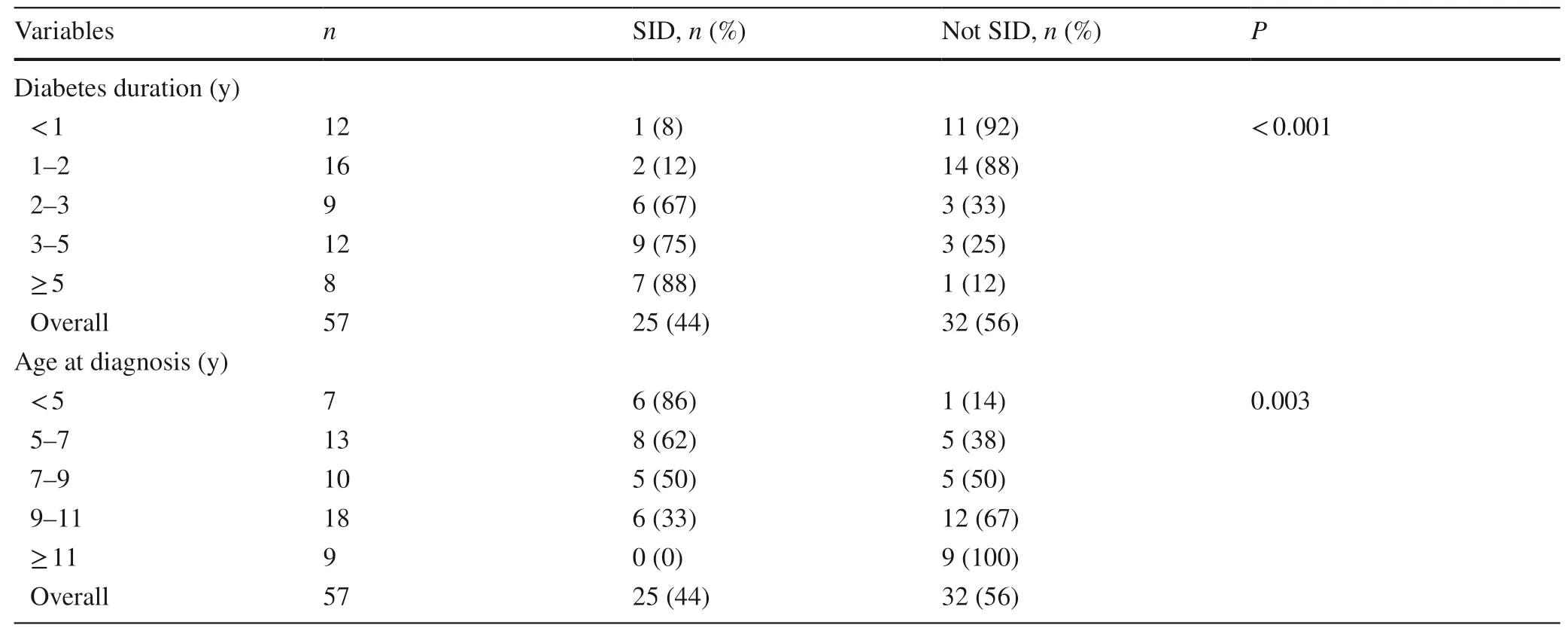
Table 3 Rates of severe insulin deficiency (SID) among children and adolescents with type 1 diabetes in Hangzhou,China,according to diabetes duration and age at diagnosis
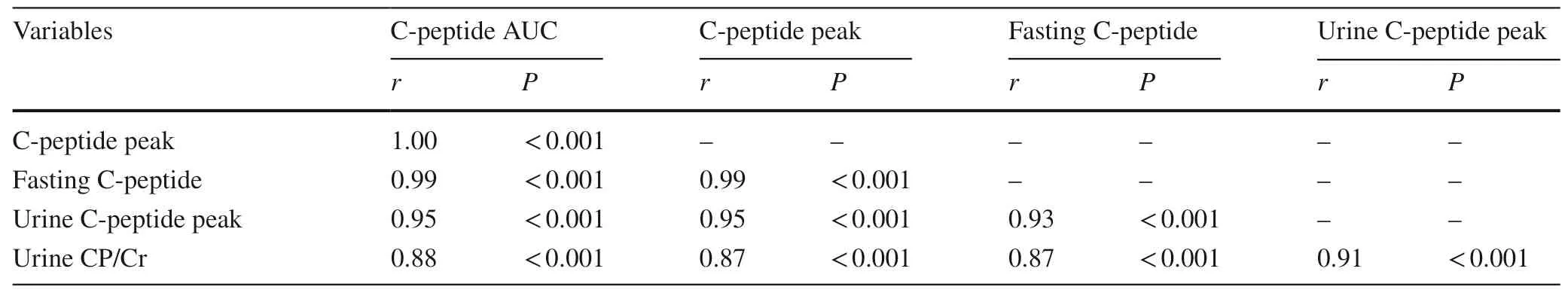
Table 4 Correlations between measures of endogenous insulin secretion in children with type 1 diabetes mellitus in Hangzhou,China
Discussion
The MMTT is recognized as the gold-standard method to evaluate endogenous insulin secretion,being more sensitive,more reproducible,and better tolerated than the glucagonstimulation test [6].However,the MMTT requires blood samples every 30 minutes for at least 2 hours,which hinders its use in clinical practice.Besser et al.have previously shown that the mean 90-minute CPAUCwas strongly correlated with the overall AUC across 120 minutes (r=0.96),suggesting its adoption instead of the full MMTT [18].Importantly,fasting CP measurements had high accuracy to detect cases of severe insulin deficiency,with 96% sensitivity and 100% specificity.Fasting CP is considered a typical indicator that is well-correlated to stimulated CP [19],and we observed that fasting CP was almost perfectly correlated with both serum CPAUCand CPpeakin our patients (r=0.99).Fasting CP is a far more reliable measure of endogenous insulin secretion than the more commonly used insulin dose adjusted for weight.Furthermore,HbA1c is not a useful guide to residual insulin secretion,so that a lower HbA1c does not necessarily mean there is appreciable insulin secretion when there is good glycaemic control [20].
As a result,the protective effect of endogenous insulin secretion for long-term complications in children with type 1 diabetes can be more precisely determined with accurate measures,such as fasting CP.In addition,the development of future interventions that could preserve insulin secretory capacity may target those with higher CP levels.Individuals with severe insulin deficiency tend to get less treatment benefits from insulin therapy,with an increased incidence of hypoglycaemia,worse glycaemic control,and more complications associated with glucose fluctuations [10].Finally,we showed that the assessment of endogenous insulin secretion using only two blood samples (at 0 and 120 minutes) provides exactly the same accuracy as the full MMTT.While it does not reduce the overall duration of the test,this simpler method would eliminate the need of the three additional blood tests,being also more accurate than the 90-minute serum CPAUC.
Recently,stimulated urine CP/Cr has been evaluated as a measure of endogenous insulin production in diabetes [21].Urine CP/Cr has been shown to be well correlated with serum CP levels and CPAUC[21,22].Besser et al.proposed an MMTT urine CP/Cr cut-offof 0.53 nmol/mmol for significant endogenous insulin secretion,with 94% sensitivity and 100% specificity [15].In this study,urine CP/Cr and urine CPpeakalso correlated well with serum CPAUC.The 120-minute urine CPpeakhad 96% sensitivity and 87% specificity,and performed better than the urine CP/Cr.While Besser et al.’s urine CP/Cr cut-offperformed at a similar level to our lower 120-minute urine CP/Cr threshold,the specificity of both 120-minute urine thresholds was lower (60%and 63%,respectively) compared to that reported in Besser et al.’s study (100%) [15].This difference likely resulted from the fact that Besser et al.’s patients were mostly adults(n=51 vs 21 children and adolescents),in contrast to our study population consisting solely of 55 pediatric patients.In addition,Besser et al.’s adult patients were from the UK and pediatric ones from Sweden,compared to our group of Han Chinese,and,although less likely,one cannot disregard potential differences associated with ethnicity or countryrelated environmental factors.
Several studies have investigated the relationship between age at diagnosis and residual endogenous insulin secretion in type 1 diabetes.Bruno et al.found that fasting CP values were twofold lower in patients aged 0—14 years than in those aged 15—29 years [23].Picardi et al.also observed higher fasting CP levels in association with increasing age at diagnosis [24].Similarly,we found a 22% increase in serum CP AUC with every 1-year increase in age at diagnosis.There is evidence that younger children have a less functional β-cell mass [25],and the above findings suggest that a younger age at type 1 diabetes diagnosis compounds this problem leading a more marked loss in endogenous insulin secretion.
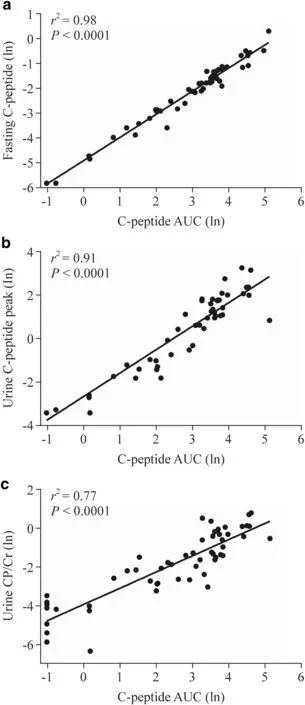
Fig.1 Scatter plots showing the associations between serum C-peptide area under the curve (AUC) from a mixed-meal tolerance test and other serum and urine parameters.a Fasting C-peptide levels; b urine C-peptide peak levels from two samples at 0 and 120 minutes; c urine C-peptide to creatinine ratio (CP/Cr) at 120 minutes.The regression lines and the respective coefficients of determination (r 2) and P values are shown.All data have been log-transformed
Type 1 diabetes results from a chronic immune-mediated destruction of β-cells that begins long before clinical diagnosis and continues afterwards [26].There was a progressive decrease in endogenous insulin secretion with increasing duration of diabetes in our patients,as previously observed[6— 8].However,Davis et al.’s data also suggest that residual secretion is present in approximately one out of three individuals at 3 or more years from type 1 diabetes diagnosis [8].The rate of decline in CP concentrations during the initial years after diagnosis is highly variable and does not seem to decrease in a simple linear manner [27].The reason for this marked heterogeneity in the rate of CP decay remains unknown.CP is co-secreted with insulin,being consequently an index (i.e.,a surrogate marker) of insulin secretion.Therefore,any measures to maintain endogenous insulin secretion in patients with type 1 diabetes are important.
In conclusion,our study shows that a simple fasting serum CP measurement could be used to detect most cases of severe insulin deficiency in Chinese children and adolescents with type 1 diabetes.Fasting CP is a far more reliable measure of endogenous insulin secretion than the more commonly used insulin dose.Therefore,it could more precisely determine insulin secretory capacity to target those who could benefit,if treatments to preserve residual insulin secretion are developed.
Author contributionsYJN and ZJW contributed equally to this work as first authors.DJGB and FJF contributed equally to this work as senior authors.YJN conceptualized and designed the clinical study,compiled the data.ZJW,DGP,JYJ,WW,HK,CXC,ZY,and LBH carried out the clinical assessments.DJGB compiled and analysed the data.FJF conceptualized and designed the clinical study.YJN,ZJW,DJGB and FJF wrote the manuscript with critical input from CWS and DGP.All authors approved the final version of the manuscript.
FundingYJN is supported by National Key Research and Development Program of China (No.2016YFC1305301),National Natural Science Foundation of China (No.81570759),Research Fund of Zhejiang Major Medical and Health Science and Technology and National Ministry of Health (WKJ-ZJ-1804).JGBD was supported by a travel fellowship from the New Zealand-China Non-Communicable Diseases Research Collaboration Center.
Compliance with ethical standards
Ethical approvalThis study was approved by the Medical Ethics Committee of the Children’s Hospital,Zhejiang University School of Medicine (No.2020-IRB-034),and informed consent was obtained from a parent and/or legal guardian of the patients.All of the reported investigations were performed in accordance with the ethical principles of the Declaration of Helsinki.
Conflict of interestThis was an investigator-initiated study.The funders had no role in study design,data collection and analysis,decision to publish,or preparation of this manuscript.The authors have no financial or non-financial conflicts of interest to disclose that may be relevant to this work.
 World Journal of Pediatrics2021年1期
World Journal of Pediatrics2021年1期
- World Journal of Pediatrics的其它文章
- Why COVID-19 is less frequent and severe in children:a narrative review
- Laboratory diagnosis of COVID-19 in secondary care hospitals in India:will standalone serology suffice?
- Winter is coming:care of the febrile children in the time of COVID-19
- Addressing e-cigarette health claims made on social media amidst the COVID-19 pandemic
- Social responsibilities of a pediatric journal:considerations and future directions
- Efficacy of oral magnesium therapy in the treatment of chronic constipation in spastic cerebral palsy children:a randomized controlled trial
