Genetic variants associated with endometriosis patients: a systematic review
Endometriosis is defined as the presence of endometrium-like tissue outside the uterus, 80% of which occur in the ovaries. It is characterized by an estrogen-dependent produces periodic and repeated bleeding, and may be accompanied by clinical symptoms such as dysmenorrhea, fatigue, dysuria, deep dyspareunia, and infertility. Due to the complex etiology and the yet-unknown pathogenesis of endometriosis, and the treatment effect is not ideal,causing significant physical and mental harm to reproductive-age women; thus, it has become a hot research topic.Endometriosis is still a mysterious disease of unknown origin and pathogenesis. Genetic factors are known to affect the manifestation and progression of endometriosis. A selection of genetic studies revealed genetic mutations and polymorphisms of endometriosis and their effects on the risk of developing this disease. This paper aimed to discuss the genetic variants associated with the risk of endometriosis and provided information to enrich the gene spectrum of endometriosis.
Key words: Endometriosis, Genetic variants, Single nucleotide polymorphism
Background
Endometriosis (EM) is a common chronic gynecological disease characterized by an estrogen-dependent, which is estimated to affect 10%–15% of reproductive-age women; that is, at least approximately 190 million women are affected worldwide [1–4]. In recent years, its incidence has been increasing. The most common locations for ectopic endometrial implants are the ovaries, the fossa ovarica, the uterosacral ligaments, the posterior cul-de-sac, the rectum and sigmoid (20%) and more rarely in the pericardium, pleura and even the brain[5–7]. Until today, EM is still a mysterious disease of unknown origin and pathogenesis. It may be accompanied by a series of clinical symptoms, such as dysmenorrhea, fatigue, dysuria, deep dyspareunia,infertility, et al [8–11]. These symptoms can affect physical, mental, and sexual, so the cost of care to manage symptoms is much greater [10]. Current evidence indicates that whether EM can be formed after the endometrial ectopic may relate to genetic,immunological, inflammatory, and environmental characteristics of the ectopic endometrium itself[12–15]. The occurrence and development of EM could be regulated by genetic variations. Several studies indicate that EM has a certain familial genetic tendency and familial aggregation. Based on a study of 3,096 twins, the heritability of EM, or the proportion of disease variance due to genetic factors, has been estimated at 52% [16]. The incidence of first-degree relatives of EM patients is sevenfold that of those without a family history, which may be affected by multiple genes and multifactorial inheritance [17].
Although the cause of EM remains unclear, genetic factors are considered as risk factors. With the use of advanced technological applications, a better understanding of the genetic risk factors associated with EM has been achieved. Much evidence suggests that there is an association between mutations and genetic polymorphisms and the risk of EM [18–20].Besides, mutations and polymorphisms in several genes may be involved in the pathophysiological process that results in EM [21]. The genetic risk for EM results from a large number of genetic variants.However, each variant has exerted a little effect, all of the multiple genetic, epigenetic, and environmental factors interacting with each other lead to yield EM phenotype. In addition, EM has a certain familial genetic tendency and familial aggregation. This review summarized and discussed the currently genetic variants associated with the risk of EM and briefly explains the mechanism of various factors on the genetic variations of EM. In the future, these alterations may constitute therapeutic targets for pharmacological compounds able to modify the epigenetic code.
There were variants on nineteen autosomes and X chromosomes except for the chromosomes 8, 21, 22 and Y have no variants from the 135 variants we collected (Table 1, Figure 1). It is not difficult to find that more than ten variants on chromosomes 1, 2 and 6,which shows that these three chromosomes are highly related to the occurrence of EM. The next are chromosomes 3, 5, 9, 10, 11, 14, 15, and 19, which have 5–10 variants related to EM. Lower associations with EM are showed on the chromosomes 4, 7, 12, 13,16, 17, 18, 20 and X, which have up to 3 variants. The genes where these EM-associated variants are distributed widely cover some protein family genes,cell factors genes, enzyme genes, and receptor genes,et al. They affect the occurrence and development of EM through different pathways, promoting or inhibiting. This data may help us find more genetic markers on these high relationship chromosomes with EM to provide new markers for genetic diagnosis and precision treatment.
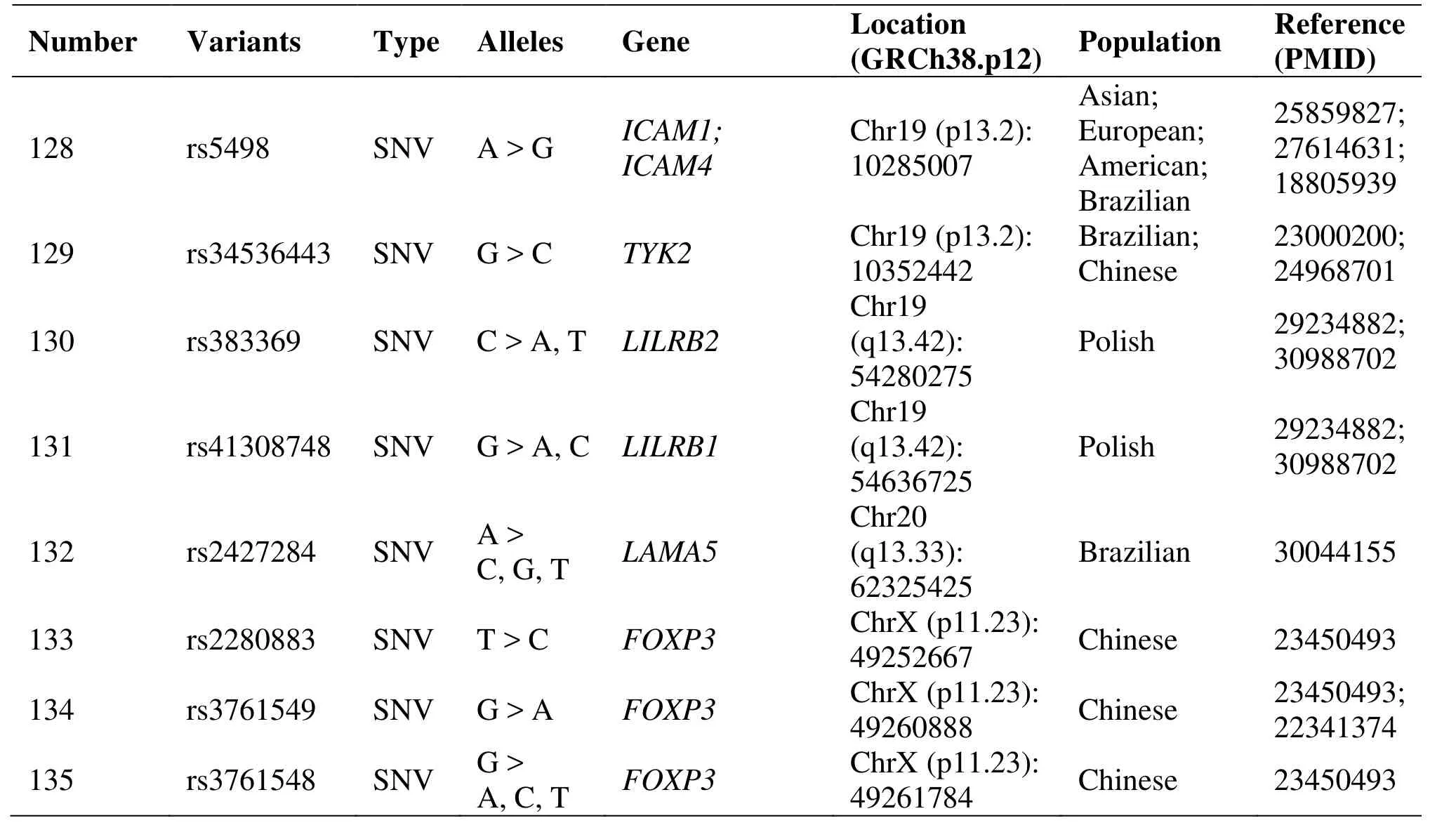
Table 1 Genetic polymorphisms related to the development of endometriosis (Continued)
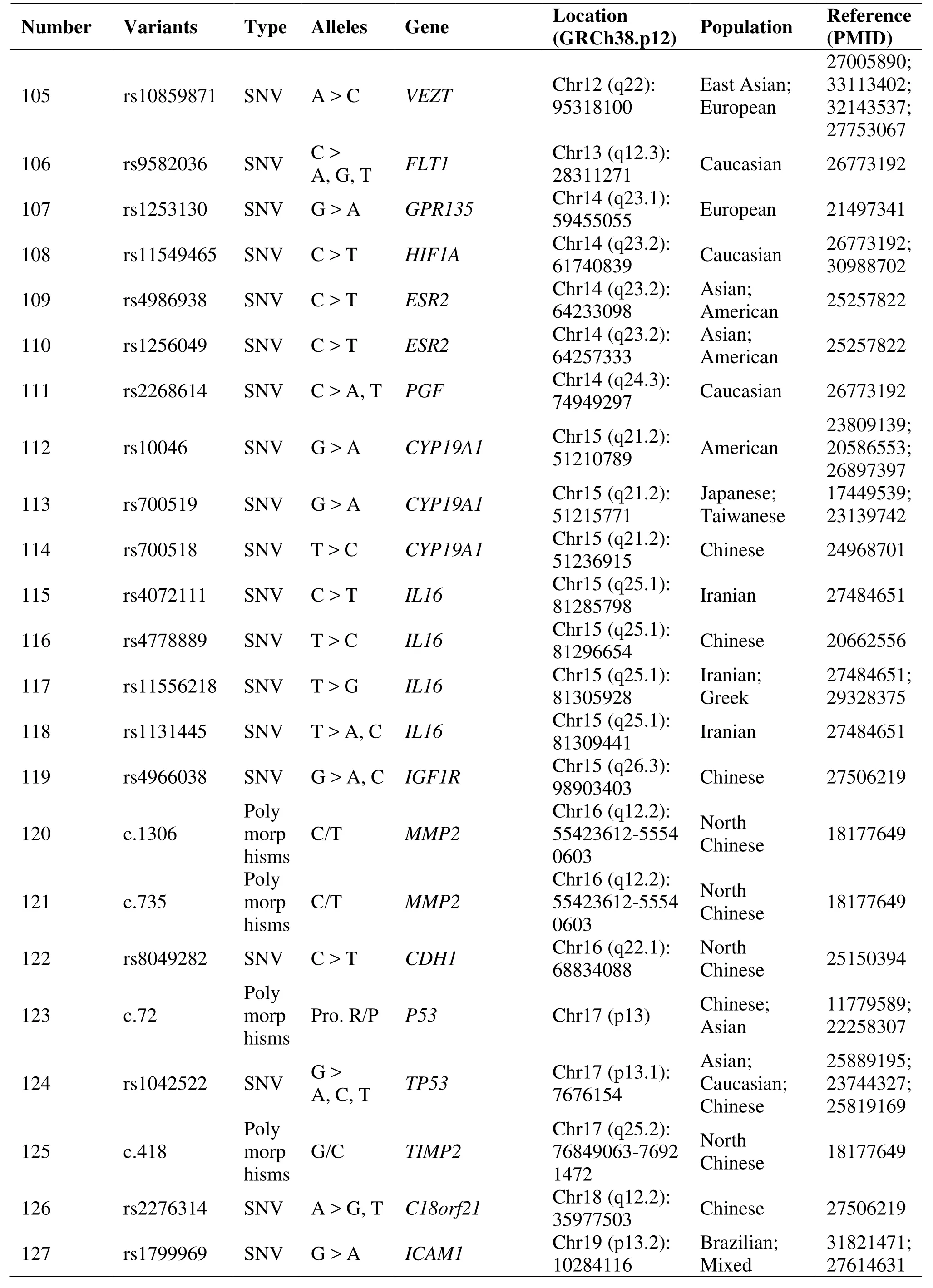
Table 1 Genetic polymorphisms related to the development of endometriosis (Continued)
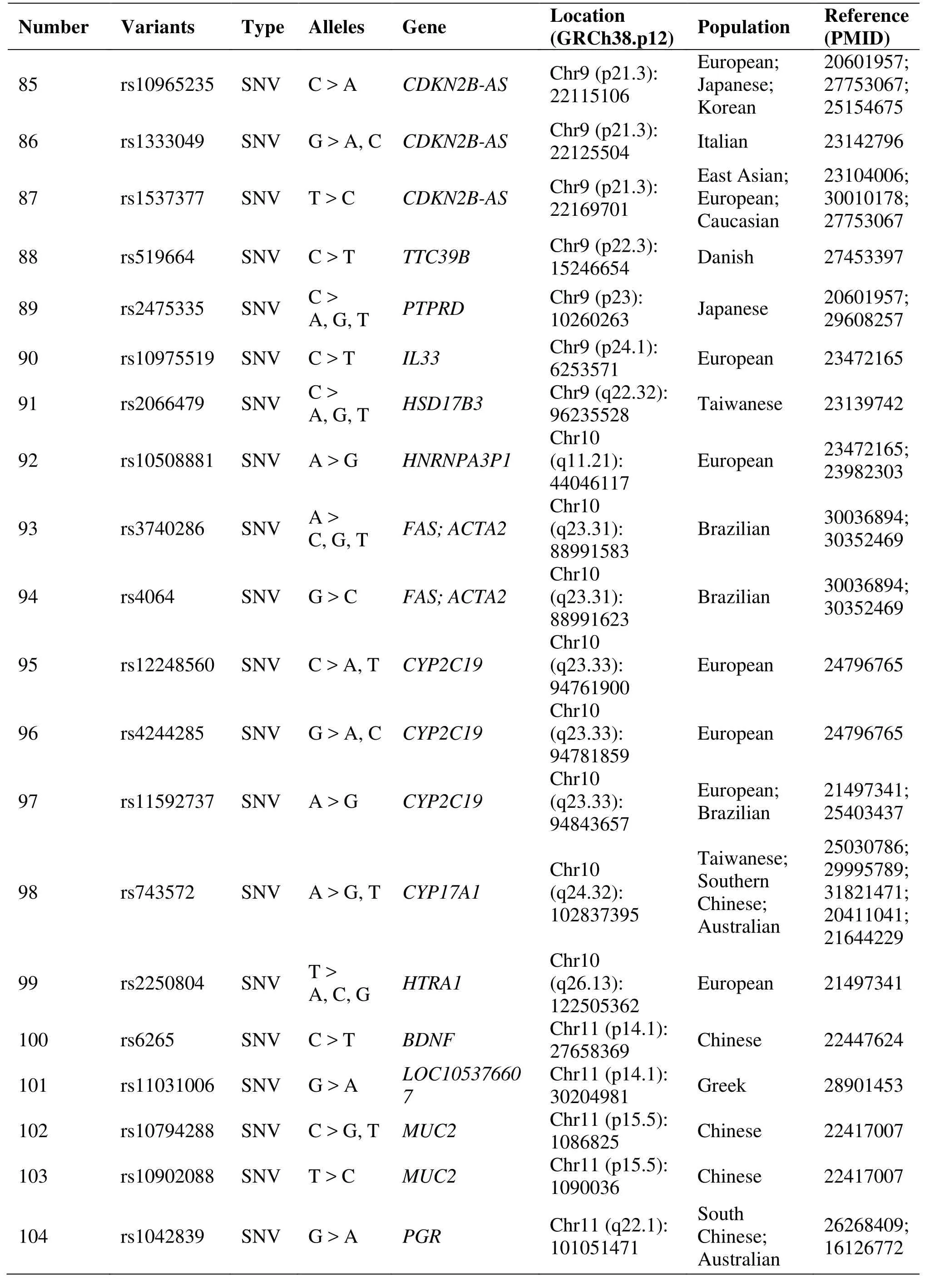
Table 1 Genetic polymorphisms related to the development of endometriosis (Continued)
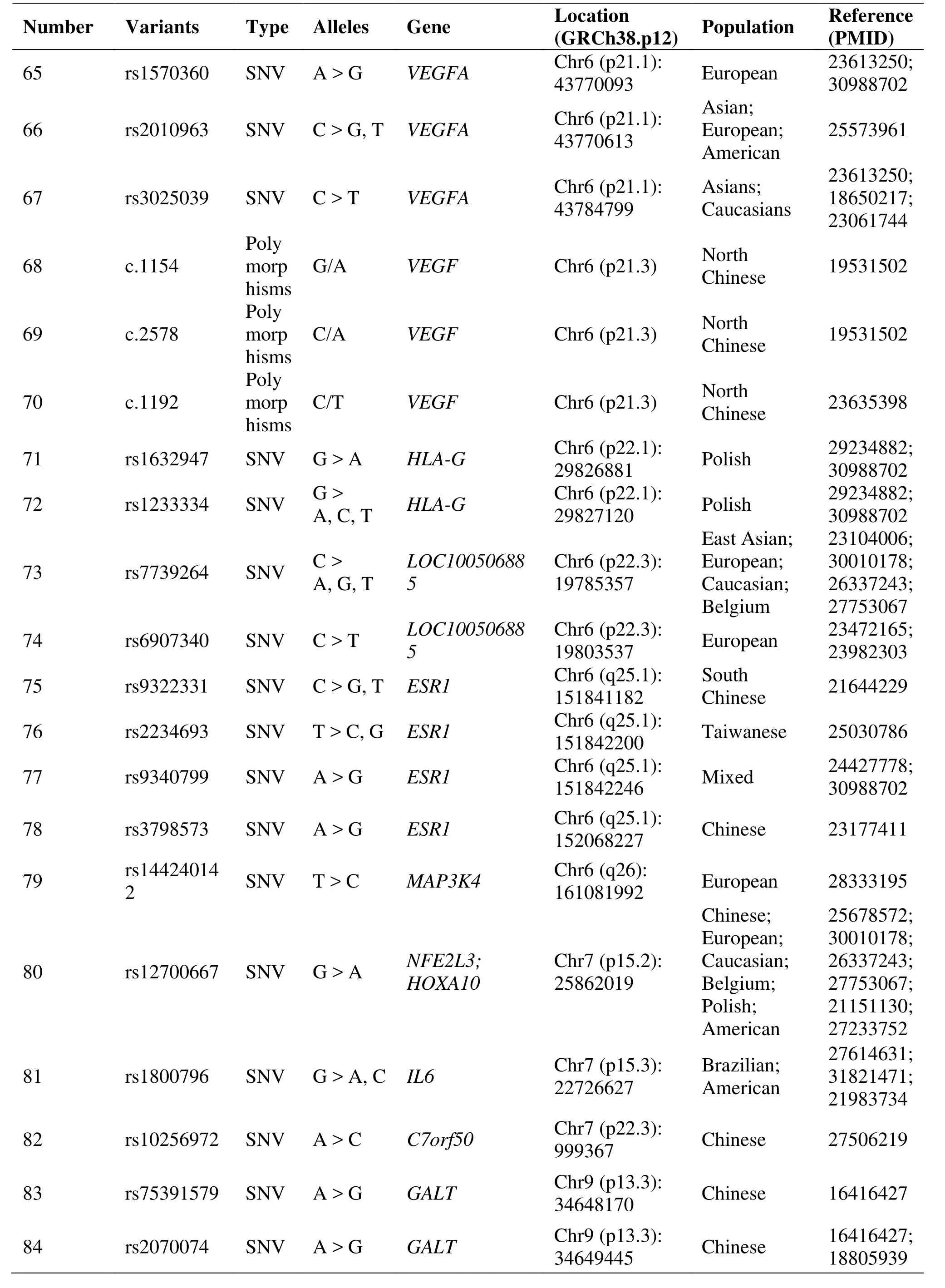
Table 1 Genetic polymorphisms related to the development of endometriosis (Continued)
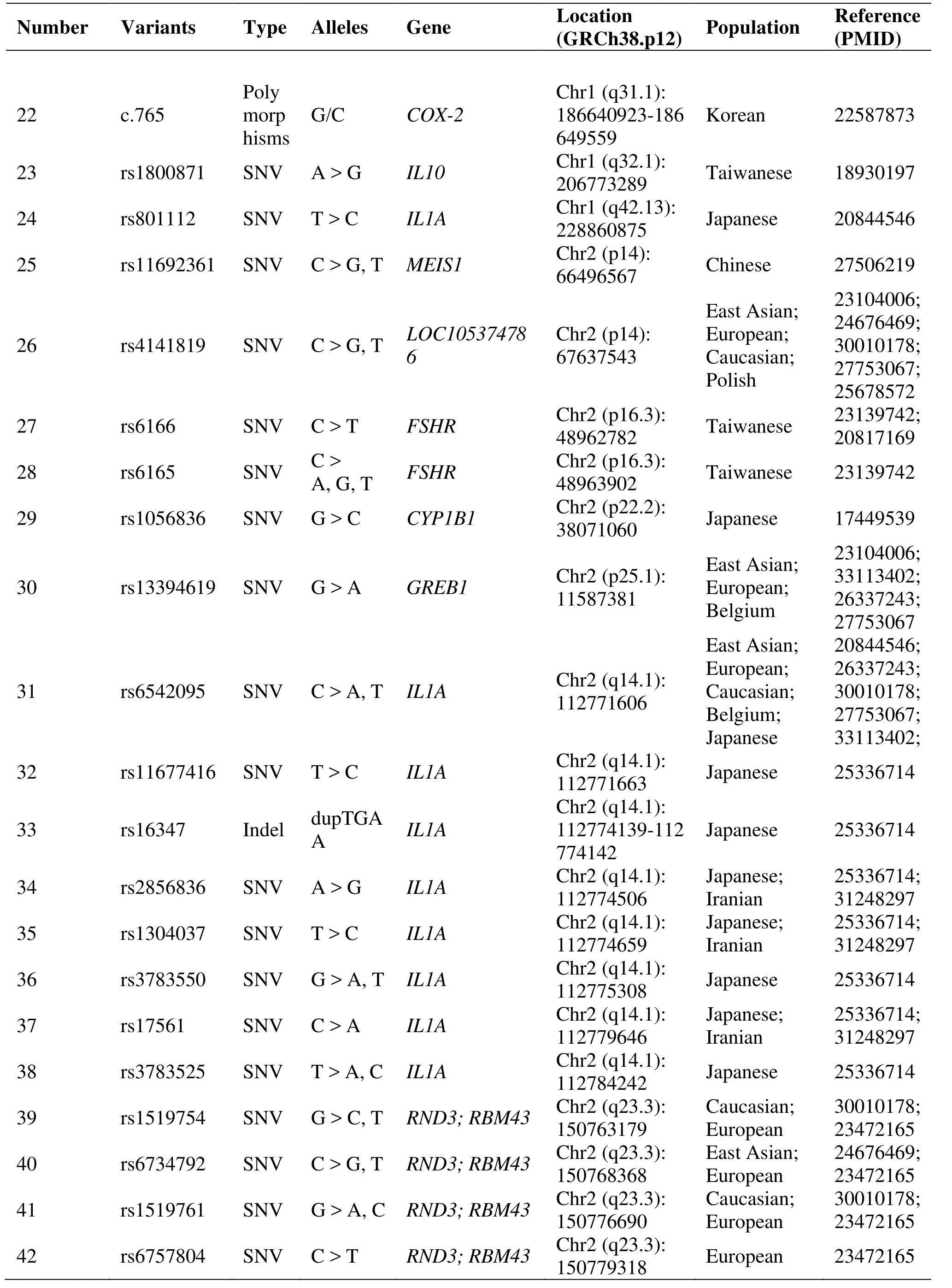
Table 1 Genetic polymorphisms related to the development of endometriosis (Continued)
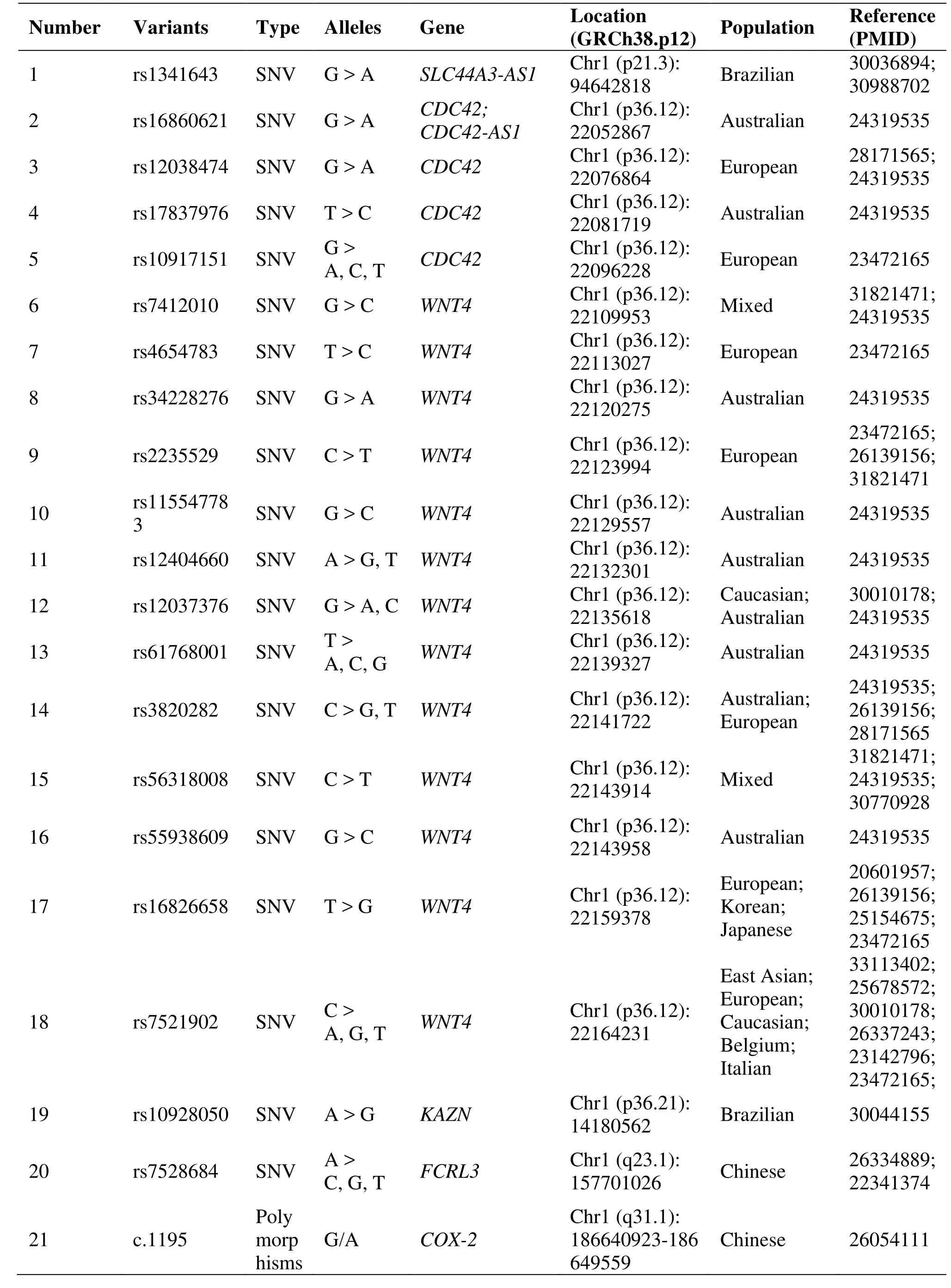
Table 1 Genetic polymorphisms related to the development of endometriosis
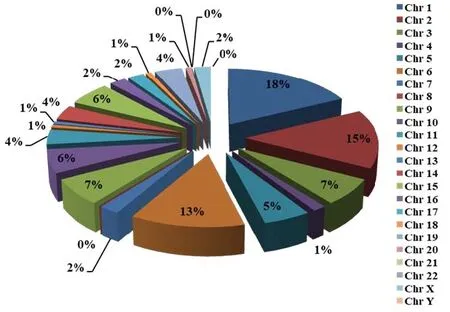
Figure 1 The percentage of genetic variations associated with endometriosis on chromosomes
Cell division cycle 42 and mitogen-activated protein kinase 4
Genome-wide association studies (GWAS) show strong evidence of association with EM for markers on the potential candidate gene cell division cycle 42 [22].Studies indicated that SNPs, such as rs16860621,rs17837976, rs3820282 and rs12038474, which located in cell division cycle 42 gene may be altered the gene expression and increased the risk of EM [22,23]. The GWAS analysis for EM revealed an intronic SNP (rs144240142) within mitogen-activated protein kinase 4. It may play a certain role in the occurrence of EM through the integrin pathway of Wnt signaling and p130Cas linkage to MAPK signaling [24].
Cyclin-dependent kinase inhibitor 2B-antisense RNA
Two studies have identified that the rs10965235,located in cyclin-dependent kinase inhibitor 2B-antisense RNA, has a strong association with EM in Japanese and Korean patients [25, 26]. The CDKN2BAS gene encoding the cyclin-dependent kinase inhibitor 2B antisense RNA and was implicated in regulating the expression of p15, p16 and p14 [25].Another study confirmed rs1333049 risk allele G was significantly higher than healthy women in EM Caucasian patients [27]. Genome-wide association meta-analysis identifies some new EM risk loci at 2p14 (rs4141819), 6p22.3 (rs7739264) and 9p21.3(rs1537377) [28]. As well as another study showed that cyclin-dependent kinase inhibitor 2B-antisense RNA 1(rs1537377) had nominally significant associations with EM [29].
Major histocompatibility complex class I G
Some reports have shown the expression of non-classical histocompatibility complex class I G(HLA-G) molecules on ectopic endometrium [30]. A research results support the role of polymorphisms of HLA-G and its receptors LILRB1 and LILRB2 in susceptibility to EM and its progression. This study indicated that the HLA-G rs1632947: GG genotype was associated with protection against the disease and its severe stages and HLA-G rs1233334: CT protected against progression; LILRB1 rs41308748: AA and LILRB2 rs383369: AG predisposed to the disease and its progression [31].
Interleukins
The rs6542095, located in IL1A, was confirmed association with EM in different populations, including East Asian, European, Caucasian, Belgium and Japanese [29, 32–35]. IL1A might be a functional candidate gene for EM [32]. The IL1A rs2856836 C allele may increase the risk of EM in Iranian women[36]. A functional promoter polymorphism rs1800871 in IL-10 gene influences susceptibility to EM. The T allele has a lower gene expression level than the C allele, suggesting inadequate suppression of inflammation leading to EM development [37]. The rs1800796, which is located in IL-6, polymorphism may be associated with EM in south Han Chinese women; however, it had not been confirmed in Brazilian and American women [38–40]. A study on Iranian women's susceptibility to EM may conclude that IL-16 gene polymorphisms (rs4072111 and rs1131445) are highly associated with increased risk of EM [41]. And a study suggests that the rs4778889 T/C polymorphism of the IL-16 gene may be associated with risk of EM in the Chinese population, especially in patients with pain phenotype [42]. A significant association with EM in Greek women was the GG and GT genotype of rs11556218 [43].
Wingless-type MMTV integration site family member 4
The expression of WNT4 gene was down regulated in ectopic lesions [44]. SNPs (rs16826658, rs2235529,rs12404660, rs3820282, rs55938609, rs56318008, et al)loci in WNT4 gene were found to be significantly associated with EM. They were confirmed in different populations such as European, Australian, Caucasian,Korean, et al [22, 23, 33, 39]. A significant association was detected with the AC genotype of rs7521902 in patients with stage III and IV disease [45].
Cytochrome P450 family
A case-control study among infertile Japanese women assessed whether cytochrome P450 (CYP) gene polymorphisms modulate the effect of dioxins and polychlorinated biphenyls in EM risk, and concluded that CYP1A1 and CYP1B1 polymorphisms may modify the environmental exposure to organochlorine and advanced the risk of EM [46]. CYP2C19 gene polymorphism (rs11592737) is associated with EM in Brazilian women [47]. And functional variants in CYP2C19 may contribute to EM susceptibility in both familial and sporadic cases [48]. Evidences from a case-control study showed that CYP17A1 rs743572 polymorphism might contribute to EM susceptibility[49].
Mucin family members
The relationship between Mucin gene polymorphisms and EM showed that MUC4 polymorphisms(rs2291653, 2291654, rs375068067, rs882605,rs2688513 and rs2246901) were associated with the risk for EM in Korean and Taiwanese women [50, 51].MUC2 polymorphisms, especially rs10794288 and rs10902088, are associated with EM and endometriosis-related infertility in Taiwanese Han women [52].
Estrogen receptor
A study demonstrated an association between Estrogen receptor 1 (ESR) rs9340799 polymorphism and infertile women with EM [53]. Association of an ESR1 gene polymorphism rs3798573 A/G was associated with risk of EM in Chinese Han women [54]. Many reports that ESR polymorphism may be associated with EM; however, rs9322331 (ESR1 gene 397T/C) and ESR2 gene rs4986938 and rs1256049 polymorphisms have not been confirmed [55, 56].
Vascular endothelial growth factor and kinase insert domain receptor
Results from the meta-analysis suggest that the rs3025039 C/T polymorphism of the vascular endothelial growth factor (VEGF) gene increases the risk of endometriosis; however, the rs699947 A/C and rs1570360 G/A polymorphisms might be protective factors for endometriosis [57]. A meta-analysis supports that rs3025039 polymorphism is capable of causing endometriosis susceptibility [58]. Two studies concluded that the VEGF 460/1154/2578/1192 variants were associated with EM in North Chinese women [59,60]. Notably, the 1154 A and 2578 A alleles may be protective against the development of EM in North Chinese women [59]. The involvement of kinase insert domain receptor in endometriosis risk highlights the importance of the VEGF pathway in the pathogenesis of the disease [61].
Other gene variants
Although association of Forkhead box P3 and Fc receptor-like 3 gene polymorphism was not confirmed in Chinese women with EM, other populations might dig out new variants of this gene in the future [62, 63].A research concluded that X-ray repair complementing defective repair in Chinese hamster cells 4 codon 247*A and X-ray repair complementing defective repair in Chinese hamster cells 4 promoter 1394*T related genotypes and alleles might be associated with endometriosis susceptibilities and pathogenesis [64].
Gene polymorphisms in TNF receptor superfamily member 6 (rs3740286 and rs4064) are involved in EM development in Brazilian women, but not those in caspase 8 apoptosis-related cysteine peptidase(rs13416436 and rs2037815) [65]. A study confirmed the Kazrin periplakin interacting protein gene(rs10928050) and laminin alpha 5 gene (rs2427284)polymorphisms were associated with EM-related infertility [66].
Two polymorphisms (c.765 and c.1195) of Cyclooxygenase-2 were related to the high-risk of EM occurring [67, 68]. The pooling-based GWAS for EM identified some variants, including meis homeobox 1,chromosome 7 open reading frame 50, insulin-like growth factor 1 receptor, et al. in Chinese Han women[69]. And the most common galactose-1-phosphate uridylyl transferase mutations (rs75391579 and rs2070074) are not likely to be associated with an increased risk of EM among Chinese Women [70].
For the polymorphism of follicle stimulating hormone receptor gene analysis demonstrated that it was associated with decreasing risk of EM [71]. A research showed that the fibroblast growth factor polymorphism might be associated with a risk of developing EM in north Chinese women [72]. The TIMP metallopeptidase inhibitor 2 418 CC homozygote may be a protective factor against the development of endometriosis in North Chinese women [73]. Matrix metallopeptidase 2 1306 C/T and 735C/T and TIMP metallopeptidase inhibitor 2 418G/C genes were related to the risk of EM [73].Protein 53 codon 72 genotypes are associated with increased risk of EM in Asians, but Protein 53 homozygotes have a lower risk for EM [74, 75].
GWAS also linked these gene variants were associated with EM, such as growth regulation by estrogen in breast cancer 1 (rs13394619), rho family GTPase 3 (rs1519754, rs6734792, rs1519761, and rs6757804), peroxisome proliferator-activated receptor gamma (rs1801282), et al [23, 28, 33, 35]. Other variants confirmed by GWAS are listed in Table 1.
Conclusion
Despite recent advances in research on the etiology,pathogenesis, and genetic factors for EM, the field continues to be limited by requiring a more extensive number of variants to achieve diagnosis, prevention and treatment. However, these genetic variants factors can provide new insights into the etiology of the disease, which could lead to significant advances in identifying potential screening DNA markers and treatment targets. Due to differences in geography,ethnicity, and populations, it is worth noting that genetic variants may vary. To enrich the population gene profile, we should research more variants and their functions on EM in the future.
 Precision Medicine Research2021年1期
Precision Medicine Research2021年1期
- Precision Medicine Research的其它文章
- Auricular acupoint application combined with Fujing prescription treatment for patients with premature ovarian failure
- Research progress of non-specific neck pain in traditional Chinese medicine and western medicine
- A network pharmacology analysis on Jiawei Buhuanjin Zhengqi powder against COVID-19 in children
