反复喘息幼儿外周血CD4+CD25+Foxp3+调节性T淋巴细胞、IL-10及IgE水平的研究
颉雅苹?童志杰?樊慧峰?卢秉泰?陈容珊
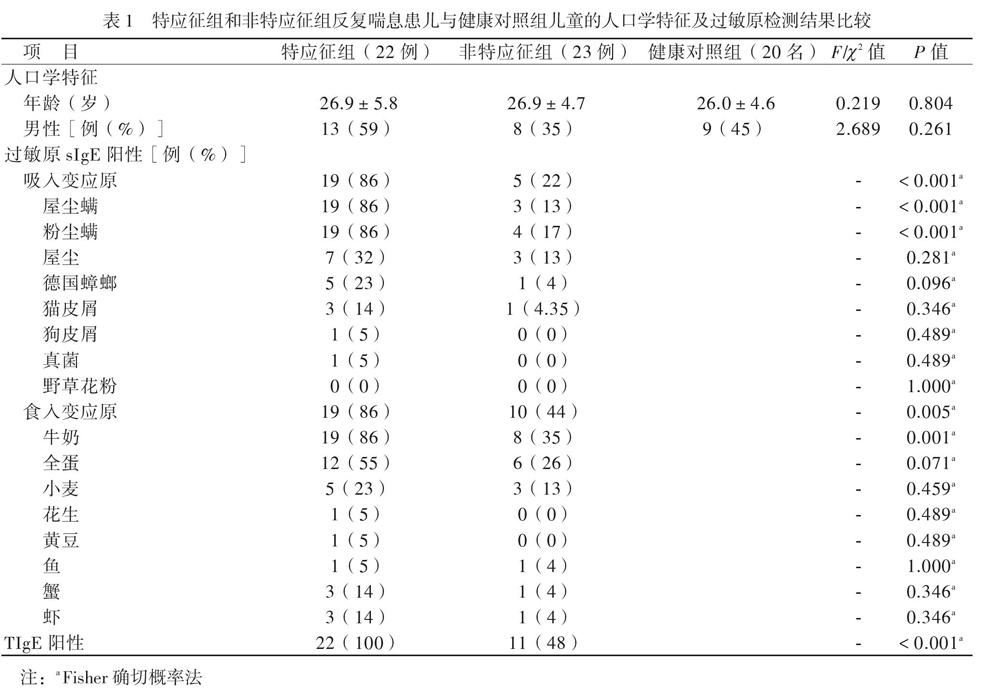
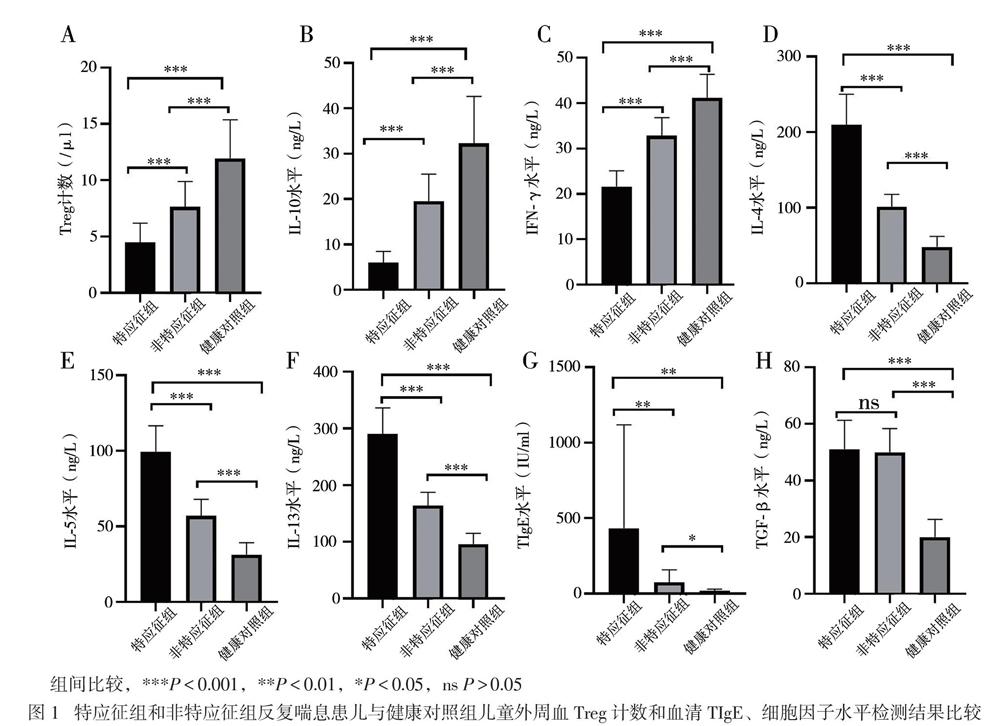
【摘要】目的 研究CD4+CD25+叉頭样转录因子3阳性(Foxp3+)调节性T淋巴细胞(Treg)及相关细胞因子、总IgE(TIgE)、特异性IgE(sIgE)在有或无特应征反复喘息幼儿外周血介导的免疫应答差异,为幼儿喘息性疾病的发展预测及治疗提供新思路。方法 选择反复喘息幼儿45例,分为特应征组22例和非特应征组23例,另选20名健康幼儿作为健康对照组,分别检测3组幼儿外周静脉血Treg计数和血清IL-10、IL-4、IL-5、IL-13、IFN-γ、TGF-β及TIgE、sIgE水平。结果 特应征组吸入及食入变应原sIgE阳性率高于非特应征组(P均< 0.05)。特应征组外周血Treg计数和血清IL-10、IFN-γ水平低于非特应征组,而非特应征组低于对照组(P均< 0.05);特应征组血清IL-4、IL-5、IL-13、TIgE水平高于非特应征组,而非特应征组高于对照组(P均< 0.05)。特应征组及非特应征组血清TGF-β水平比较差异无统计学意义(P > 0.05),均高于对照组(P均< 0.05)。反复喘息患儿外周血Treg计数与血清IL-10水平呈正相关(r = 0.875,P < 0.001)。结论 Treg、IL-10、IL-4、IL-5、IL-13、TGF-β及IFN-γ可能参与了幼儿喘息,在过敏性疾病中发挥免疫调节作用。反复喘息幼儿外周血Treg计数与血清IL-10水平呈正相关。Treg与IL-10未来可能作为幼儿反复喘息早期预测及治疗的新靶点。
【关键词】喘息;儿童;T淋巴细胞;白介素-10;免疫球蛋白E;免疫调节
Study of levels of CD4+ CD25+ Foxp3+ regulatory T cells, IL-10 and IgE in peripheral blood of children with repeated wheezing Xie Yaping, Tong Zhijie, Fan Huifeng, Lu Bingtai, Chen Rongshan. Department of Respiratory, Guangzhou Women and Childrens Medical Center, Guangzhou 510623, China
Corresponding author, Tong Zhijie, E-mail: tongzhijie1972@ 163. com
【Abstract】Objective To investigate the differences in the peripheral blood-mediated immune responses of CD4+CD25+Foxp3+ regulatory T cells (Treg) and related cytokines, total IgE (TIgE) and specific IgE (sIgE) in children with or without atopic physique and repeated wheezing, aiming to provide novel ideas for the development prediction and treatment of infantile wheezing diseases. Methods Forty-five infants with repeated wheezing were selected and divided into the atopic (n = 22) and non-atopic groups (n = 23), and 20 healthy children were allocated into the normal control group. The counts of Treg, the levels of IL-10, IL-4, IL-5, IL-13, IFN-γ, TGF-β and TIgE and sIgE in the peripheral blood were quantitatively detected. Results The positive rate of sIgE for inhalation and ingestion allergens in the atopic group was significantly higher than that in the non-atopic group (both P < 0.05). The peripheral blood Treg cell counts, IL-10 and IFN-γ levels in the atopic group were significantly lower compared with those in the non-atopic group, whereas the values in the non-atopic group were remarkably lower than those in the control group (all P < 0.05). The serum levels of IL-4, IL-5, IL-13 and TIgE in the atopic group were significantly higher than those in the non-atopic group, whereas the levels in the non-atopic group were considerably higher than those in the control group (all P < 0.05). The serum level TGF-β did not significantly differ between the atopic and non-atopic groups (P > 0.05), which were significantly higher than that in the control group (both P < 0.05). In children with repeated wheezing, the proportion of peripheral blood Treg cells was positively correlated with serum IL-10 levels (r = 0.875, P < 0.001). Conclusions Peripheral blood Treg cells, IL-10, IL-4, IL-5, IL-13, TGF-β and IFN-γ may be involved with the infantile wheezing, and play an immunomodulatory role in allergic diseases. The counts of peripheral blood Treg cells is positively correlated with the serum IL-10 levels in infants with repeated wheezing. Treg and IL-10 may serve as novel targets for early prediction and treatment of repeated wheezing in the future.
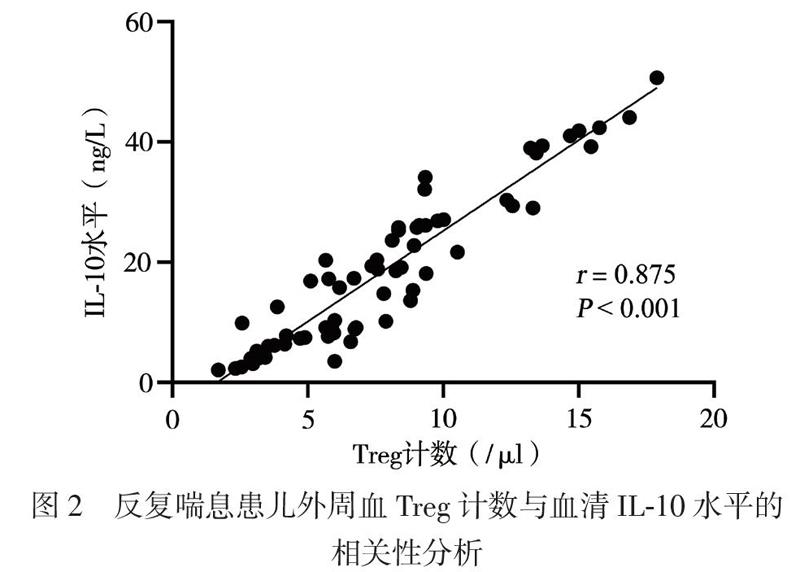
三、反复喘息患儿外周血Treg计数与血清IL-10水平的相关性分析
反复喘息患儿外周血Treg计数与血清IL-10水平呈正相关(r = 0.875,P < 0.001),见图2。
讨论
幼儿反复喘息是一种异质性疾病,与烟草暴露、母亲患哮喘、呼吸道病毒感染、特应征体质等因素有关[7-8]。临床观察显示,具有特应征的反复喘息幼儿较不具有特应征幼儿更易发展至哮喘,但其发病机制尚不明确,随着过敏性疾病发病率的增加,如何早期客观评价幼儿反复喘息的性质并评估其预后,已成为当前儿科领域的重要挑战。
现有研究显示,支气管哮喘是辅助性T淋巴细胞2型(Th2)介导的以气道高反应、可逆的气流受阻、气道嗜酸性粒细胞浸润、气道黏液高分泌及血清高IgE为特征的气道慢性炎症性疾病[9]。一般认为,哮喘患者体内存在Th1/Th2失衡,Th1细胞受到抑制,Th2细胞异常活化,Th1细胞分泌的细胞因子IFN-γ和Th2细胞分泌的细胞因子IL-4、IL-5、IL-13参与了过敏性哮喘[9]。本研究中,喘息患儿血清Th2细胞因子IL-4、IL-5、IL-13水平较健康对照组升高,特應征组高于非特应征组,而血清IFN-γ水平降低,表明IL-4、IL-5、IL-13、IFN-γ参与了幼儿喘息性疾病的免疫应答,反复喘息与哮喘同样具有Th1/Th2失衡,且在特应征组更明显。TGF-β是涉及哮喘肺部持续性炎症和气道重塑方面重要的炎症介质[10]。本研究中,反复喘息患儿血清TGF-β水平高于健康对照组,提示反复喘息患儿存在气道炎症及气道重塑,Lezmi等[11]报道学龄前严重喘息及哮喘儿童存在慢性气道炎症及气道重塑,但本研究中特应征组与非特应征组血清TGF-β水平接近,可能与患儿病情处于早期有关,还需延长时间继续观察。随着年龄的增长,反复喘息可能导致未来持续性哮喘的风险增加,故应尽早对喘息患儿进行积极干预,避免其进展至持续性哮喘。
T淋巴细胞参与了机体的免疫应答与耐受[12]。CD4+CD25+Foxp3+Treg是具有免疫调节功能的淋巴细胞亚群,在支气管哮喘发病机制中的作用受到广泛关注。Baatjes等[13]报道,哮喘患者外周血中的Treg较非哮喘正常人减少。本研究采用CD4+CD25+Foxp3+作为Treg计数检测标志物,与健康对照组相比,2组反复喘息患儿外周血Treg计数减少,特应征组明显低于非特应征组,提示反复喘息的幼儿与哮喘存在相似的免疫反应。特应征组血清TIgE高于非特应征组,提示存在Treg减少及高水平TIgE的特应征反复喘息幼儿可能将来发展至持续哮喘的可能性更大,需要对这类患儿提早干预。现有研究显示,IL-10具有免疫抑制和免疫刺激作用,可由Treg产生,在减轻过敏反应炎症方面发挥作用[14]。本研究中,与健康对照组相比,反复喘息幼儿IL-10水平降低,其中特应征组低于非特应征组,与Treg计数呈正相关,提示在过敏性疾病中,存在免疫不耐受,IL-10的降低可能促进了过敏性气道疾病,这与国外相关报道一致[15]。研究结果提示,Treg计数与IL-10水平的监测,有可能作为评估幼儿喘息未来是否进展至过敏性哮喘的客观指标。
儿童反复喘息的发生与变应原暴露有关[16]。在我国南方地区,尘螨是主要的吸入变应原[17]。我们发现,特应征组吸入变应原sIgE阳性率高于非特应征组,以屋尘螨/粉尘螨阳性占首位,尘螨水平为3级,高于非特应征组的2级水平,同时该组患儿的Treg计数减少及IL-10水平降低,提示可能存在吸入变应原不耐受,由于尘螨为常年性变应原,随着患儿年龄的增长,气道过敏性炎症可能进一步加重,从而导致肺功能受损,发展至哮喘,因此对这部分患儿应早期干预,注意环境控制,减少或避免吸入变应原。特应征组食入变应原sIgE阳性率高于非特应征组,但2组检测水平均不高(1 ~ 2级),否认食物过敏史,提示在临床工作中应结合病史评估检测结果,避免不必要的食物回避。本研究中,特应征组1例2岁患儿TIgE达3440 IU/ml,尘螨、蟑螂、真菌sIgE均阳性,其家居一楼,环境潮湿、发霉。真菌是强烈的致敏原,真菌致敏与持续性哮喘明显相关[18]。我们在临床工作中,对TIgE异常增高的喘息患儿应注意真菌检测。
综上所述,反复喘息的幼儿存在与哮喘相似的气道炎症,Treg计数减少与IL-10水平降低,TIgE及气道炎症因子水平增高,这在特应征患儿中尤其显著。Treg与IL-10在免疫耐受、降低过敏性炎症方面发挥重要作用,有可能作为预测幼儿喘息未来是否进展至过敏性哮喘的客观指标。有特应征体质的反复喘息幼儿吸入变应原阳性以屋尘螨/粉尘螨占首位,应提早干预,未来尘螨sIgE水平持续增高的该类患儿发展至持续性哮喘的风险更大。由于本研究为回顾性研究,且入组病例有限,今后将进一步设计前瞻性研究,以更进一步明确Treg与IL-10在儿童呼吸道过敏性疾病的起病、发展和转归中的免疫调节作用。
参 考 文 献
[1] Rodríguez-Martínez CE, Sossa-Brice?o MP, Castro-Rodriguez JA. Factors predicting persistence of early wheezing through childhood and adolescence: a systematic review of the literature. J Asthma Allergy, 2017, 10:83-98.
[2] Schmidt F, Hose AJ, Mueller-Rompa S, Brick T, H?m?l?inen AM, Peet A, Tillmann V, Niemel? O, Siljander H, Knip M, Weber J, von Mutius E, Ege MJ; DIABIMMUNE Study Group. Development of atopic sensitization in finnish and estonian children: a latent class analysis in a multicenter cohort. J Allergy Clin Immunol, 2019, 143(5):1904-1913.e9.
[3] 中华医学会儿科学分会呼吸学组; 《中华儿科杂志》编辑委员会. 儿童支气管哮喘诊断与防治指南,2016年版). 中华儿科杂志, 2016, 5(3):167-181.
[4] Lee E, Hong SJ. Phenotypes of allergic diseases in children and their application in clinical situations. Korean J Pediatr, 2019, 62(9):325-333.
[5] 向莉,赵京,鲍一笑,邵洁,刘传合,李孟荣,陈实,王成硕,申昆玲,陈育智. 儿童气道过敏性疾病螨特异性免疫治疗专家共识. 中华实用儿科临床杂志, 2018, 33(16):1215-1223.
[6] Scala G, Miceli Sopo S. When are serum specific IgE levels positive? J Allergy Clin Immunol, 2015, 135(1):291-292.
[7] Selby A, Munro A, Grimshaw KE, Cornelius V, Keil T, Grabenhenrich L, Clausen M, Dubakiene R, Fiocchi A, Kowalski ML, Papadopoulos NG, Reche M, Sigurdardottir ST, Sprikkelman AB, Xepapadaki P, Mills ENC, Beyer K, Roberts G. Prevalence estimates and risk factors for early childhood wheeze across Europe: the EuroPrevall birth cohort. Thorax, 2018, 73(11):1049-1061.
[8] Owora AH, Zhang Y. Childhood wheeze trajectory-specific risk factors: a systematic review and meta-analysis. Pediatr Allergy Immunol, 2020, 15:e13313.
[9] Tumes DJ, Papadopoulos M, Endo Y, Onodera A, Hirahara K, Nakayama T. Epigenetic regulation of T-helper cell differen-tiation, memory, and plasticity in allergic asthma. Immunol Rev, 2017 , 278(1):8-19.
[10] Michalik M, Wójcik-Pszczo?a K, Paw M, Wnuk D, Koczur-kiewicz P, Sanak M, P?kala E, Madeja Z. Fibroblast-to-myofibroblast transition in bronchial asthma. Cell Mol Life Sci, 2018, 75(21):3943-3961.
[11] Lezmi G, Gosset P, Deschildre A, Abou-Taam R, Mahut B, Beydon N, de Blic J. Airway remodeling in preschool children with severe recurrent wheeze. Am J Respir Crit Care Med, 2015, 192(2):164-171.
[12] Palomares O, Akdis M, Martín-Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev, 2017, 278(1):219-236.
[13] Baatjes AJ, Smith SG, Watson R, Howie K, Murphy D, Larché M, Denburg JA, Inman MD, O'Byrne PM. T regulatory cell phenotypes in peripheral blood and bronchoalveolar lavage from non-asthmatic and asthmatic subjects. Clin Exp Allergy, 2015, 45(11):1654-1662.
[14] Ouyang W, O'Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity, 2019, 50(4):871-891.
[15] Gern JE, Calatroni A, Jaffee KF, Lynn H, Dresen A, Cruikshank WW, Lederman HM, Sampson HA, Shreffler W, Bacharier LB, Gergen PJ, Gold DR, Kattan M, O'Connor GT, Sandel MT, Wood RA, Bloomberg GR. Patterns of immune development in urban preschoolers with recurrent wheeze and/or atopy. J Allergy Clin Immunol, 2017, 140(3):836-844.e7.
[16] Bacharier LB, Beigelman A, Calatroni A, Jackson DJ, Gergen PJ, O'Connor GT, Kattan M, Wood RA, Sandel MT, Lynch SV, Fujimura KE, Fadrosh DW, Santee CA, Boushey H, Visness CM, Gern JE; NIAID sponsored Inner-City Asthma Consortium. Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am J Respir Crit Care Med, 2019, 199(1):71-82.
[17] 陳晓倩,黎雅婷,钟韩荣,潘莉,杨丽芬,蔡亮鸣,王昭妮,陈壮桂. 佛山地区儿童过敏性疾病常见致敏过敏原分布——附7157例报告. 新医学, 2017, 48(9):638-643.
[18] Caillaud D, Leynaert B, Keirsbulck M, Nadif R; mould ANSES working group. Indoor mould exposure, asthma and rhinitis: findings from systematic reviews and recent longitudinal studies. Eur Respir Rev, 2018, 27(148):170137.
(收稿日期:2020-08-01)
(本文编辑:林燕薇)

