Four New Limonoids from the Barks of Toona ciliata
Pan-Pan Zhang ·Yun-Ge Bu ·Shang Xue ·Zhi-Rong Cui ·Peng-Fei Tang ·Jun Luo ·Ling-Yi Kong
Abstract Four new limonoids, toonayunnanaes F - I (1 - 4), and six known compounds (5 - 10) were isolated from the barks of Toona ciliata.Their structures were elucidated by thoroughly analyzing of NMR and HRMS data, and single-crystal X-ray diffraction of 1.The oxetane ring moiety in 1 was rare in limonoids and other natural products.Compound 1 showed nitric oxide (NO) inhibitory eff ect with an IC 50 38.45 ± 0.41 μM in lipopolysaccharide (LPS)-activated RAW 264.7 macrophages.
Keywords Meliaceae·Toona ciliate·Limonoids·NO inhibitory eff ects
1 Introduction
The genusToona, belonging to the family Meliaceae, contains 15 species mainly distributed in the regions of tropical Asia and Africa, of which four species and six varieties grow in China [1].Toona ciliataRoem.var.ciliatais a timber tree mainly found in the south of China, such as Yunnan, Sichuan, Guangdong, and Hainan provinces [2].Its bark has been used in Chinese folk medicine to treat dysentery, fever, and menstrual disorders [3].Our previous phytochemical investigations onT.ciliatavar.henryiandT.ciliatavar.yunnanensishave led to the isolation of a series of limonoids with potential biological activities such as cytotoxicity, anti-inflammatory, and anti-multidrug resistance (MDR) activities [4— 8].As a part of our continuous research for bioactive limonoids fromToonaplants, four new limonoids, toonayunnanaes F - I (1 - 4), and six known compounds (5 - 10) were isolated from the barks ofT.ciliata.Their structures were elucidated by thoroughly analyzing of NMR and HRMS data, and single-crystal X-ray diffraction of 1.The oxetane ring moiety in 1 was rare in limonoids and other natural products.Herein, we describe the isolation and structural elucidation of these limonoids as well as their inhibitory effects on NO production in LPS-induced RAW264.7 cells.
2 Results and Discussion
Toonayunnanae F (1), colorless crystals, possessed a molecular formula of C26H32O4according to the HRESIMS ion atm/z409.2376 [M + H] + (calcd.409.2373).The 1 H and 13 C NMR data of 1 (Table 1) showed characteristic resonances for aβ-substituted furan ring (δH7.35, 7.16, 6.20;δC111.2, 124.7, 139.4, 142.8), anα,β-unsaturated ketone (δH7.08 d,J= 10.4 Hz, 5.98 d,J= 10.4 Hz;δC125.7, 155.0, 204.3), and five singlet methyl groups (δH0.54, 1.05, 1.11, 1.29, 1.54).Those observations together with 26 carbon resonances and 11 indices of hydrogen defi ciency, suggested that 1 might be a ring-intact limonoid with anα,β-unsaturated carbonyl moiety in A ring [8— 10] (Fig.1).
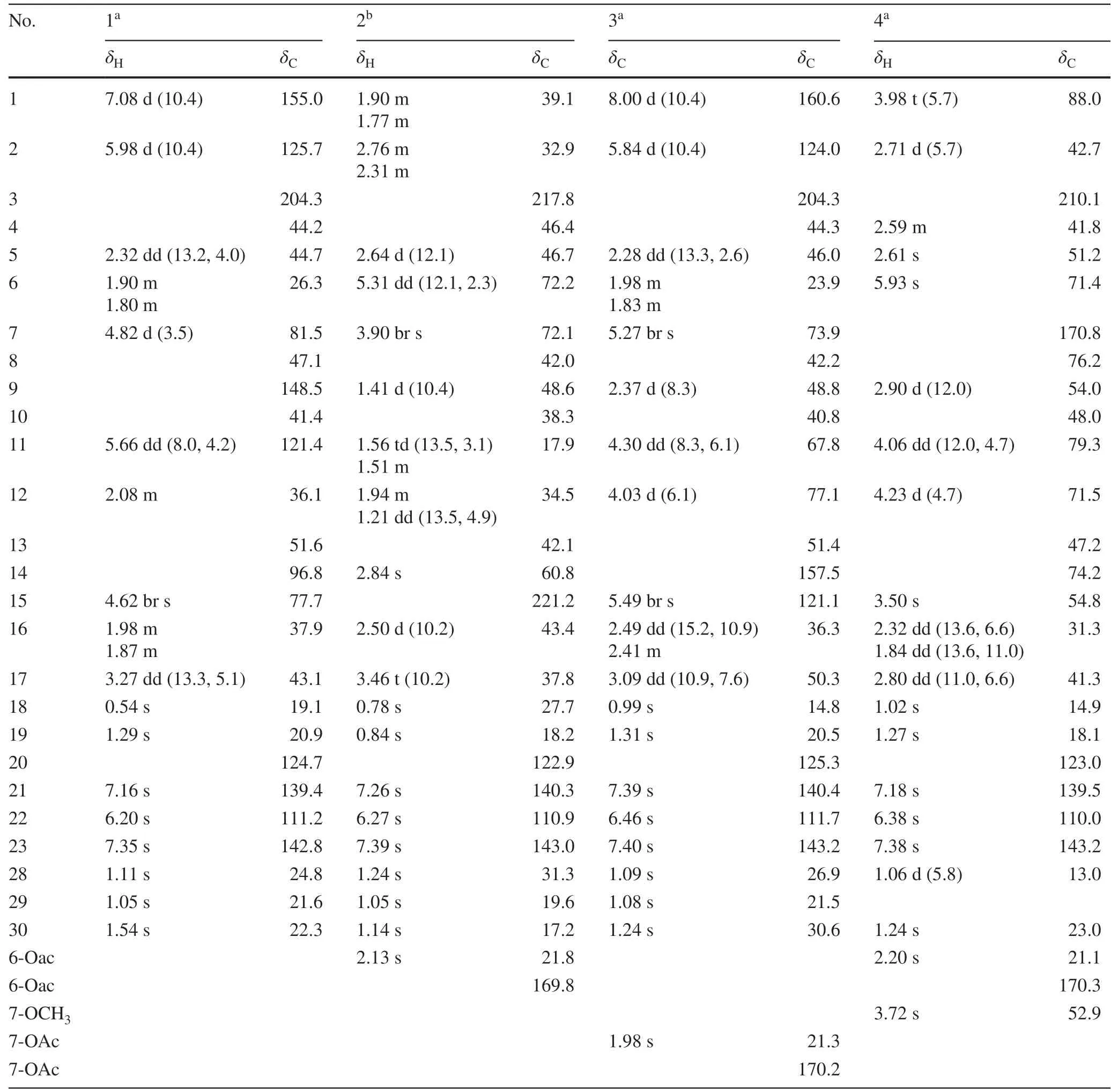
Table 1 1H and 13C NMR data of compounds 1 - 4 in CDCl 3 (δin ppm,J in Hz)
The double bond between C-9 and C-11 was confi rmed by the correlations of H3-19 (δH1.29) and H3-30 to C-9 (δC148.5); of H-11 (δH5.66) to C-12 (δC36.1); and of H2-12 (δH2.08) to C-9 and C-11 (δC121.4) in the HMBC spectrum.The HMBC correlations (Fig.2) from H-7 (δH4.82) to C-8 (δC47.1) and C-6 (δC 26.3); from H 3 -30 (δH 1.54) to C-7 (δC 81.5) and C-14 (δC 96.8); from H 3 -18 (δH 0.54) and H-15 (δH 4.62) to C-14; and from H 2 -16 and H-17 to C-15 (δC77.7) indicated that C-7, C-14, and C-15 were all oxygenated.The signifi cantly deshielded chemical shift of C-14 and the indices of hydrogen defi ciency suggested the presence of an oxetane ring between C-7 and C-14 [8,11,12] and a hydroxy group at C-15, similar to that in ciliatasecone S [8].Therefore, the 2D structure of 1 with a rare oxetane ring was constructed.The ROESY cross-peaks of H3-19/H3-29,H3-19/H3-30, and H3-30/H-7 (Fig.2) revealed theβ-orientations of Me-19, Me-29, Me-30, and H-7.Theα-orientations of H-5, Me-18, and Me-28 were determined by the ROESY cross-peaks of H-5/H3-28 and H-5/H3-18.The cross-peaks of H3-18/H-16 (δH1.98), H-16 (δH1.98)/H-15, and H-16 (δH1.87)/H-17 indicated theβ-orientation of 15-OH and theα-orientation of furan ring.A single-crystal X-ray crystallographic diffraction experiment with Cu Kα radiation of 1 (Fig.2) unambiguously determined theα-orientation of the oxetane ring moiety and the absolute confi guration as 5R, 7R, 8S, 10R, 13S, 14S, 15R, and 17S.The structure of compound 1 was thus established as shown.
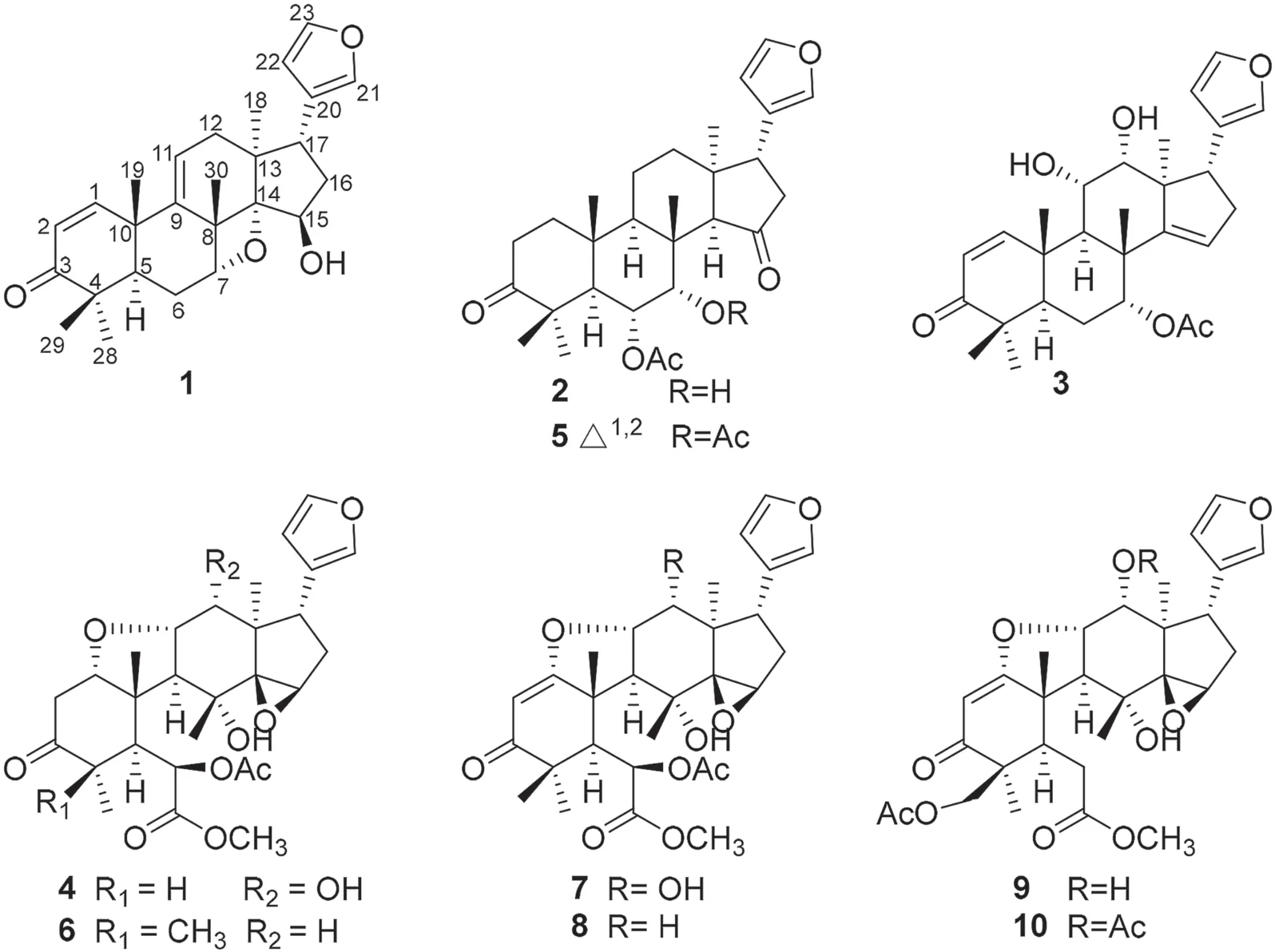
Fig.1 The structures of compounds 1 - 10
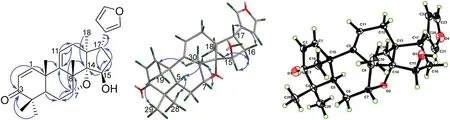
Fig.2 The key HMBC and ROESY correlations and X-ray crystallographic structure of compound 1
The molecular formula of toonayunnanae G (2) was established as C28H38O6based on the HRESIMS ion atm/z493.2551 [M + Na] + (calcd.493.2561) and its13C NMR data.Apart from the signals for an acetoxy substituent, the remaining 26 carbon resonances observed in the 1D NMR spectra indicated that 2 might also be a ring-intact limonoid [8— 10].Comparison of its NMR data (Table 1) with those of the known toonaciliatone F (5) [13] revealed that the main differences were the absence of a double bond and an acetoxy group.The chemical shift of C-3 (δC217.8) implied the presence of a keto carbonyl rather than anα,βunsaturated ketone moiety in ring A, which was confi rmed by the HMBC correlations of H2-1 (δH1.90, 1.77),H2-2 (δH2.76, 2.31),H3-28, and H3-29 to C-3.The HMBC correlations of H-6 (δH5.31) to C-5, C-7 (δC72.2), and an ester carbonyl carbon (δC169.8) and of H-7 (δH3.90) to C-6 (δC72.1), C-8, C-9, and C-30 (Fig.3) allowed the assignment of an acetoxyl at C-6 and a hydroxy group at C-7.The relative confi guration of 2 was deduced to be identical to that of 5 by a ROESY experiment.The ROESY cross-peaks of H3-19/H-6,H3-29/H-6,H3-30/H-6, H-6/H-7, and H-7/H3-30 revealed theα-orientations of 6-OAc and 7-OH.Therefore, the structure of 2 was assigned as depicted.
Toonayunnanae H (3) gave a molecular formula of C28H36O6as determined from HRESIMS ion atm/z469.2577 [M + H] + (calcd.469.2585).The1H and13C NMR spectroscopic data showed many similarities to those of the known 6α-acetoxyazadirone [13], except for the absence of an acetoxy group and the presence of a hydroxy group.The HMBC correlations of H-7 (δH5.27) to C-6, C-8, C-9, C-30, and an ester carbonyl carbon (δC170.2) indicated that an acetoxy group was located at C-7.The hydroxy groups at C-11 and C-12 were revealed by the HMBC correlations (Fig.3) of H-11 (δH4.30) to C-9 and C-12 (δC77.1) and of H-12 (δH4.03) to C-11 (δC67.8) and C-13.The ROESY cross-peaks of H3-30/H-7,H3-30/H-11, H-11/H-12, and H-12/H-17 (Fig.3) suggested theα-orientations of 7-OAc, 11-OH, and 12-OH.Therefore, the structure of 3 was determined as shown.
The clearly diagnostic signals (Table 1) for aβ-substituted furan moiety, five characteristic singlet methyl groups in the 1D NMR spectra, and the correlations of a methoxy group (δH3.72) with an ester carbonyl carbon (δC170.8) in the HMBC spectrum suggested that toonayunnanae I (4) was likely a B-secolimonoid with a C6-C7-methyl ester appendage [5,8,14— 16], similar to toonayunnanin G (6) [15].Comparison of the 1D NMR data (Table 1) of 4 with those of 6 revealed that the major difference was the presence of a doublet methyl signal (δH1.06, 3H, d,J= 5.8 Hz), which was ascribed to Me-28 at C-4 based on its HMBC correlations (Fig.3) with C-3 (δC210.1), C-4 (δC 41.8), and C-5 (δC 51.2).Therefore,4 possessed a B-seco-29-nor-limonoid skeleton similar to that of ciliatasecone N [8].The HMBC correlations of H-12 (δH4.23) to C-11 (δC79.3) and C-13 indicated that a hydroxy group was located at C-12.The relative confi guration of 4 was deduced to be identical to that of 6 by a ROESY experiment.Theα-orientations of 12-OH and Me-28 in 4 was inferred from the ROESY cross-peaks (Fig.3) of H-11/H-12, H-12/H-17, and H3-28/H-5.Accordingly, the structure of 4 was elucidated as shown.
By comparing their1H and13C NMR spectroscopic data with those reported in literatures, six known compounds were identifi ed as toonaciliatone F (5) [13], toonayunnanin G (6) [15], toonacilianin G (7) [16], toonaciliatin P (8) [17], toonaciliatin C (9) [18], and toonacilianin H (10) [16], respectively.
Additionally, the inhibitory eff ects on NO generation in LPS-activated RAW 264.7 macrophages of compounds 1 - 4 were evaluated at the concentrations of 50 μM and below.Compound 1 showed NO inhibitory eff ect with an IC5038.45 ± 0.41 μM.
3 Experimental
3.1 General Experimental Procedures
Optical rotations were measured on a JASCO P-1020 automatic digital polarimeter at room temperature.IR spectra were recorded on a Bruker Tensor 27 spectrometer using KBr pellets.UV spectra were recorded on a Shimadzu UV-2450 spectrophotometer (Shimadzu, Tokyo, Japan).High-resolution electrospray ionization mass spectrometry (HRESIMS) was obtained on an Agilent 6529B Q-TOF mass instrument using electrospray ionization.The 1D and 2D nuclear magnetic resonance (NMR) spectra were obtained on Bruker AVANCE III 500 MHz or Bruker AVIIIHD 600 MHz spectrometers in CDCl3with TMS as an internal standard.Analytical HPLC was conducted on an Agilent 1260 infi nity system equipped with a DAD-UV detector.Preparative HPLC was carried out using a Shimadzu LC-6A system (Shimadzu, Tokyo, Japan) equipped with a Shimpack RP-C18column (20 × 200 mm, i.d.10 μm, Shimadzu, Tokyo, Japan) with a flow rate of 10.0 mL/min, detected by a binary channel UV detector.Silica gel (200—300 mesh, Qingdao Haiyang Chemical Co.Ltd., China), MCI (Mitsubishi, Japan), and RP-C18silica (40—63 μm, FuJi, Japan) were used for column chromatography.
3.2 Plant Material
The air-dried bark ofToona ciliataRoem.var.ciliatawas collected in Baoshan, Yunnan Province, China, in August 2018.The plant material was identifi ed by professor Mian Zhang of the research Department of Pharmacognosy, China Pharmaceutical University.A voucher specimen (no.2018-TC) was deposited in the Department of Natural Medicinal Chemistry, China Pharmaceutical University.
3.3 Extraction and Isolation
The air-dried and powder bark ofT.ciliata(29 kg) was extracted with 95% EtOH three times (3 × 6.5 L) under reflux.The concentrated extract (3.1 kg) was suspended in H2O, and then partitioned with petroleum ether (PE) and dichloromethane (DCM), successively.The DCM extract (100.0 g) was subjected to a silica gel column (200 - 300 mesh) eluted with a PE-EtOAc mixture (100:0 - 100:1 - 100:2 - 100:3 - 100:5 - 10:1 - 5:1, v/v) in a step gradient to obtain eight major fractions (A - H).Fraction C (20.0 g) was loaded onto an ODS column eluted with a mixture of MeOH-H2O from 40 to 70% to afford four fractions (C1 - C4).Fraction C4 (3.3 g) was further applied to an ODS column (30% - 60% ACN-H2O) to give four subfractions (C4a - C4d).Fraction C4a (374 mg) was separated by preparative HPLC with 60% MeOH-H2O to yield 1 (4 mg) and 2 (11 mg).Similarly, fraction C4c (582 mg) aff orded 3 (5.3 mg),5 (14 mg), and 8 (10.3 mg) by preparative HPLC (50% ACN-H2O).Fraction D (15 g) was subjected to an MCI column (50% - 70% MeOH-H2O) to give three subfractions (D1 - D3).Fraction D2 (5.5 g) was separated by an ODS MPLC (40% - 60% ACN-H2O) to aff ord five subfractions (D2a - D2e), and fraction D2b (539 mg) was purifi ed by preparative HPLC with MeOH-H2O (60% MeOH-H2O) to give 6 (5.2 mg) and 9 (14 mg).Using the same purifi cation procedures, fraction D3 (3.3 g) was further fractionated by ODS column chromatography (40% - 60% MeOH-H2O), and the subfraction D3c (483 mg) was purifi ed by preparative HPLC with 45% ACN-H2O to give 4 (2.2 mg),7 (34.1 mg), and 10 (45 mg).
Toonayunnanae F (1): colorless crystals (MeOH-H2O);(c0.1, MeOH); UV (MeOH)λmax(logε) 218 (5.30) nm; IR (KBr)νmax3553, 2986, 2931, 2859, 1669, 1455, 1387, 1158, 1025 cm-1;1H and13C NMR data, see Table 1; HRESIMSm/z409.2376 [M + H] + (calcd.for C26H33O4, 409.2373).
Toonayunnanae G (2): white amorphous powder;(c0.1, MeOH); UV (MeOH)λmax(logε) 208 (3.95) nm; IR (KBr) νmax3409, 2964, 1724, 1384, 1247, 1028 cm -1 ; 1 H and 13 C NMR data, see Table 1; HRESIMSm/z493.2551 [M + Na] + (calcd.for C28H38NaO6, 493.2561).
Toonayunnanae H (3): white amorphous powder;+ 7.6 (c0.1, MeOH); UV (MeOH)λmax(logε) 208 (3.55) nm; IR (KBr)νmax3421, 2977, 1732, 1664, 1249, 1027 cm -1 ; 1 H and 13 C NMR data, see Table 1; HRESIMSm/z469.2577 [M + H] + (calcd.for C28H37O6, 469.2585).
Toonayunnanae I (4): white amorphous powder;- 30.2 (c0.1, MeOH); UV (MeOH)λmax(logε) 208 (4.02) nm; IR (KBr)νmax3466, 2954, 1747, 1717, 1226, 1026 cm-1;1H and13C NMR data, see Table 1; HRESIMSm/z550.2631 [M + NH4] + (calcd.for C28H40NO10, 550.2647).
3.4 Anti-inf lammatory Activities
The new compounds (1 - 4) were evaluated for their inhibitory eff ects on NO production in LPS-activated RAW 264.7 macrophages as described in the literature [19].Briefly, RAW 264.7 cells (6 × 10 6 cells/mL) were seeded in 96-well plates and treated with different concentrations of tested compounds for 1 h, and 1.0 μg/mL LPS solution was subsequently added to stimulate the cells for 18 h.NO level was evaluated by measuring the standard of accumulated nitrite in cell supernatants with the reagent of Griess.N-Monomethyl-L-arginine Monoacetate (L-NMMA) was used as a positive control (IC50= 42.38 ± 0.72 μM).
AcknowledgementsFinancial supports for this study were from the National Natural Science Foundation of China (31470416), the National New Drug Innovation Major Project (2018ZX09711-001-007), the National Key R&D Program of China (2018YFC1707105), and the "Double First-Class" University project (CPU2018GY08, China).
Compliance with Ethical Standards
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
 Natural Products and Bioprospecting2021年1期
Natural Products and Bioprospecting2021年1期
- Natural Products and Bioprospecting的其它文章
- In Memory of the Late Professor Jun Zhou (1932-2020)
- In Mourning and Memory of Late Professor Zhou Jun
- Natural Products in Cancer Therapy: Past, Present and Future
- On the Famous Traditional Chinese Medicine “Fu Zi”: Discovery, Research, and Development of Cardioactive Constituent Mesaconine
- The Outlook of the Development of Innovative Products from Biocompatible Natural Spider Silk in the Beauty Thread-Lifting Industry
- Insight into Medicinal Chemistry Behind Traditional Chinese Medicines: p-Hydroxybenzyl Alcohol-Derived Dimers and Trimers from Gastrodia elata
