Lagerindicine, a New Pyrrole Alkaloid Isolated from the Flowers of Lagerstroemia indica Linnaeus
Yi Chen ·Song-Wei Li ·Fang-Zhou Yin ·Min Yang ·Xia-Juan Huan ·Ze-Hong Miao ·Xiao-Ming Wang ·Yue-Wei Guo ,4
Abstract A phytochemical investigation of the EtOH extract of the flowers of Lagerstroemia indica L.led to the isolation and characterization of a new pyrrole alkaloid, named lagerindicine (1), along with four known compounds (2— 5).Their structures were elucidated by the detailed spectroscopic analysis and comparison with literature data, whereas the structure, in particularly, the absolute confi guration (AC) of 1, was firmly determined by total synthesis.All the isolates were evaluated for their cytotoxic eff ects against human colon cancer cell (HCT-116), and compound 3 exhibited weak cytotoxicity with IC 50 value of 28.4 μM.
Keywords Lagerstroemia indica Linnaeus·Pyrrole alkaloid·Total synthesis·Stereochemistry·Cytotoxicity
1 Introduction
Terrestrial plants of Lythracea is a large family, widely used in architecture, landscaping, food and drug applications.The plants of genusLagerstroemiacomprise more than 15 species, which are either small trees or shrubs with colourful flowers distributed in warm temperate regions of China [1].Earlier chemical investigation of the different species of Lythracea can trace back to decades, and a lot of structurally diverse and complex compounds, such as alkaloids, sesqui-, triterpenoids, steroids and flavonoids [2] were reported.Especially, alkaloids have attracted much attention of natural product chemists and pharmacologists due to their extensive biological activities, ranging from antifungal activities to cytotoxicity.The earlier phytochemical studies on the plants of theLagerstroemia indicainitiated in 1962.Ferris et al.had reported a series of phenylquinolizidine alkaloids from the aerial of title species [3— 8].Recently, several new biphenylquinolizidine alkaloids were also isolated from the aerial parts ofL.indicaby Bae et al.[9,10].It is interesting to note that the chemical constituents of the flowers ofL.indicaare not reported till now.
In our continuous eff orts towards the searching for novel bioactive secondary metabolites from marine and terrestrial sources [11— 14], we have recently collected the fresh flowers ofL.indicaL.from Hunan Province, China, and carried out a chemical investigation on the EtOH extract of the flowers.As a result, a new pyrrole alkaloid, namely lagerindicine (1), and four known compounds (2— 5) were isolated and characterized.Herein, we reported the isolation, structural elucidation, especially total synthesis of 1, and in vitro biological evaluation of these isolates (Fig.1).
2 Results and Discussion
Then-BuOH and EtOAc soluble portion of the EtOH extract was repeatedly column chromatographed over silica gel, Sephadex LH-20, and RP-HPLC to yield pure compounds 1,2, and 3— 5, respectively.The structures of the known compounds 2— 5 were rapidly identifi ed as pterolactam [15], betulinic acid [16], oleanolic acid [17] and 24-methylenecycloartanol [18], respectively, by the comparison of their spectral data and optical rotations with those reported in the literature.
Compound 1, named lagerindicine, was isolated as a colorless oil.Its molecular formula was determined to be C7H13O3N by the13C NMR data and HR-ESIMS positive ion atm/z182.0782 [M + Na] + (calcd.for C7H13NO3Na, 182.0788), indicating two degrees of unsaturation.Consistent with this data, analysis of the13C NMR and the HSQC spectra revealed the presence of 7 carbon signals, comprising one sp3methyl carbon (oxygenated atδC53.7), four sp3methylene carbons (one oxygenated atδC60.5), one sp3methine carbon (oxygenated atδC92.8), and one carbonyl carbon resonating atδC178.2 (Table 1).The 1 H NMR data showed resonance of one methoxyl singlet atδH3.31 (3H, s, —OMe).The four protons resonating atδH3.67 (2H, each t,J= 5.8 Hz, H-2a’ and H-2b’),δH3.55 (1H, dt,J= 14.0, 5.8 Hz, H-1a’), andδH3.27 (1H, dt,J= 14.0, 5.8 Hz, H-1b’), along with the corresponding carbons atδC60.5 (C-2′) and 44.3 (C-1′), indicated the presence of hydroxyethyl group.Furthermore, the four protons resonating atδH2.50 (1H, dt,J= 17.3, 8.6 Hz, H-3a),δH 2.32 (1H, ddd,J= 17.3, 9.9, 2.8 Hz, H-3b),δH2.21 (1H, dddd,J= 13.8, 9.9, 8.6, 6.1 Hz, H-4a), andδH2.02 (1H, dddd,J= 13.8, 8.6, 2.8, 1.2 Hz, H-4b), together with the coupled oxymethine proton atδH5.08 (1H, dd,J= 1.2, 6.1 Hz, H-5), suggested the direct connection from C-3 to C-5 through C-4.The above observations were further supported by the 1 H- 1 H COSY correlations (Fig.2).In HMBC spectrum, the correlation between —OMe to C-5 (δC92.7) suggested the methoxyl locating at C-5.All the above NMR data closely resembled those of the co-occurring compound 2, pterolactam [(S)-5-methoxypyrrolidin-2-one], a known alkaloid previously isolated from the aerial parts ofChrysanthemum coronariumL.(family Compositae) [15].In fact,1 differs from 2 only ascribing to the substituent at N-1.Due to the replacement of the hydrogen atom of N-1 in 2 by the hydroxyethyl group in 1, the 13 C NMR chemical shifts of the vicinal carbons at C-2 and C-5 of 1, respect to those of 2, were upfi eld and downfi eld shifted (Table 1), respectively, according to the 44 mass units differences between them.The hydroxyethyl linked at N-1 in 1 was further confi rmed by the observed key HMBC correlations from H2-1′ to C-2 and C-5 (Fig.2).Thus, the planar structure of compound 1 was unambiguously determined as depicted in Fig.2.Lagerindicine isN-hydroxyethyl derivative of 2.

Fig.2 1 H- 1 H COSY and the key HMBC correlations of compound 1
Since there is only one chiral center (C-5) in compound 1, the final step to complete the full structure of 1 is to determine its absolute confi guration.Since the AC of cooccurring related compound 2 is 5S[15], it is obvious that 1 should share the sameSabsolute confi guration at C-5 based on the biogenetic consideration.
This assignment was supported by observing the same optical rotation signs of both compounds 1 and 2 { 1:+ 13 (c0.05 MeOH); 2:+ 12 (c0.2 MeOH)}.However, considering the different substitution pattern at N-1, which may cause unexpected influence on the optical properties of 1 and 2, we decided to synthesize 1 so as to confi rm the deduced absolute stereochemistry of 1.As shown in Scheme 1, a total synthesis of both (+)- and (-)-enantiomers of 1 was carried out.Thus, the key intermediate 2-pyrrol-1-ylethan-1-ol (7) was prepared starting from the commercially available 2,5-dimethoxytetrahydrofuran (6) [19].The photooxidation of pyrrole 7 in the presence of Rose Bengal (RB, sensitizer) and methanol as polar solvent under irradiation with a 250 W long arc mercury lamp aff orded (±)-5-methoxylactam (8) [20], which, in turn,was further separated by the chiral-phase HPLC to generate the expected (+)-5-methoxylactam { 8a,+ 131 (c0.1 MeOH)} and (-)-5-methoxylactam { 8b,- 121 (c0.1 MeOH)}, respectively.Furthermore, as the presence of aα, β-unsaturated lactone chromophore in structures 8a and 8b, the absolute confi guration of this pair of optically pure enantiomers could be further determined to be 5Sand 5R, respectively, by the observed opposite Cotton eff ects due to the n—π*andπ-π*transitions (Fig.3) [21], in combination with the results of TDDFT-ECD calculation (Fig.4), respectively.Having confi rmed the AC at C-5, the subsequent reduction of the olefi n of 8a and 8b with PtO2in EtOH yielded the target molecule (+)- 1 and its enantiomer (-)- 1, respectively.Finally, overall comparison of the physical and chemical data of synthetic (+)- 1 with those of natural product 1 confi rmed the 5Sabsolute stereochemistry of natural 1.
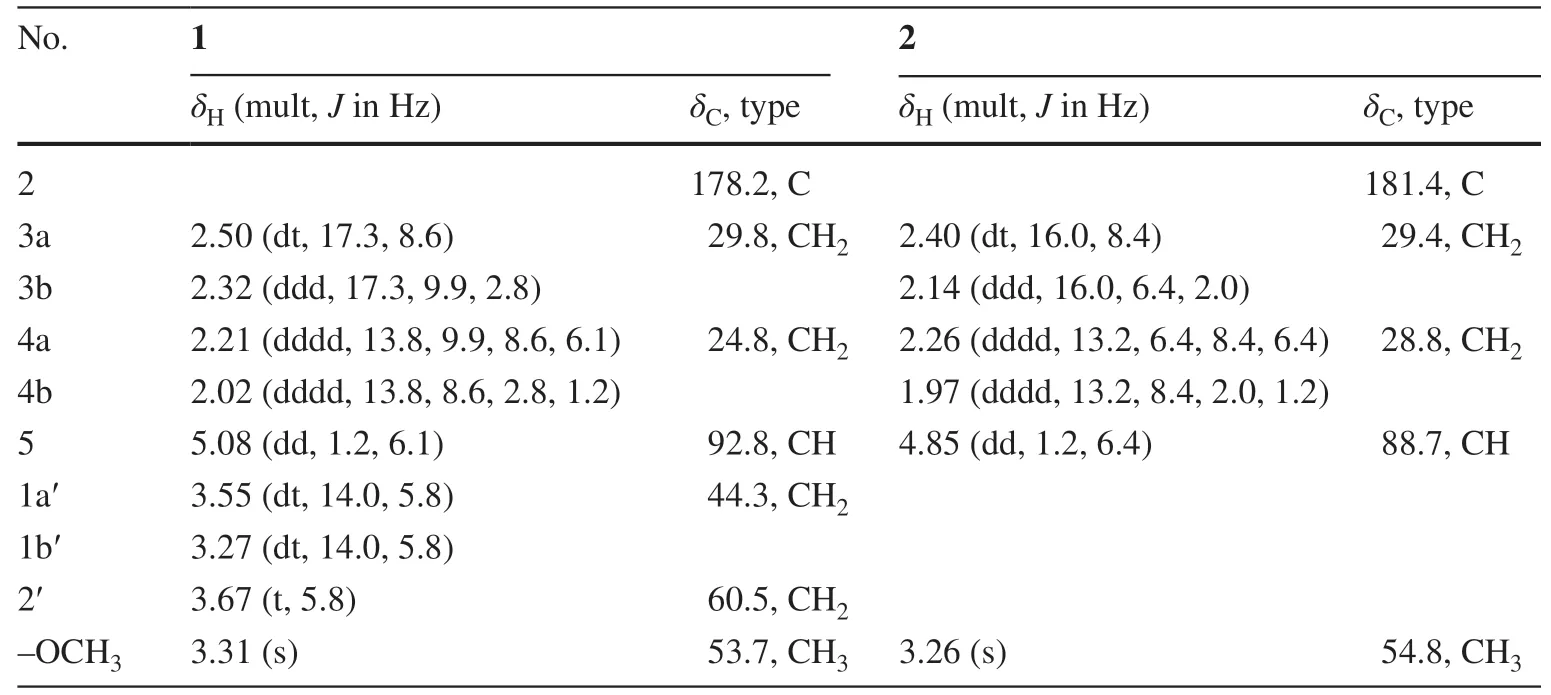
Table 1 1 H and 13C NMR data of compound 1 a and 2 b
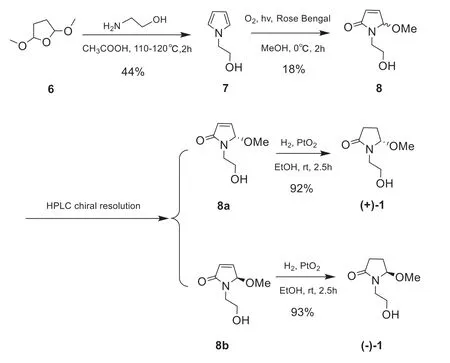
Scheme 1 Total synthetic route of (+)- 1 and (-)- 1
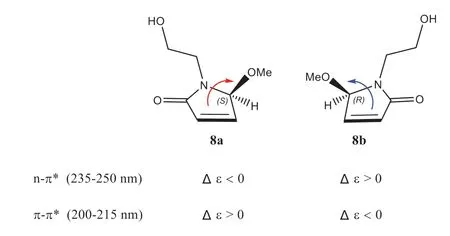
Fig.3 Correlation of the Cotton eff ects with absolute confi guration

Fig.4 The TDDFT-ECD calculation results of 8a and 8b
Considering that the promising biological activities of pyrrole alkaloids [22], we have performed in vitro biological evaluation of all the isolated compounds on human colon cancer cell line (HCT-116).The cytotoxic effects were evaluated against HCT-116 with TAK-243 (MLN7243) as the positive control.As shown in Table 2, only compound 3 showed weak cytotoxicity with the IC50value of 28.4μM.Furthermore, other pharmaceutical screens, such as antimicrobial, anti-inflammatory, and insecticidal assays are undergoing.
In conclusion, this is the first phytochemical investigation on the flowers ofL.indicaL.Interestingly, the previously reported metabolites from the different parts of the title plant, such as phenyl quinolizidine alkaloids, flavonoids, and flavone glycosides, etc.were not found in the present work.Moreover, it is noteworthy that pyrrole alkaloids 1 and 2 were isolated from the plants belonging to family Lythracea for the first time.The real origin of 1 is a matter worth to discuss since having used EtOH in the extraction process.To rule out the possibility that 1 may be an artifi cial product, we have tried to treat compound 2 by dissolving it in EtOH and stirred overnight at room temperature, but failed to detect the presence of 1.
Further study should be conducted to extend the screening range to other bioassays such as anti-inflammatory, antimicrobe etc.on alkaloids 1 and 2 since the supply issue of these intriguing compounds has been solved by synthetic method.
3 Experimental
3.1 General Experimental Procedures
Optical rotations were measured on a Perkin-Elmer 241MC polarimeter (PerkinElmer, Fremont, CA, USA).ECD spectrawere recorded on a Jasco J-810 spectropolarimeter (JASCO, Japan) at ambient temperature using chromatographic grade CH3OH and acetonitrile as solvent.IR spectra were recorded on a Nicolet Magna FT-IR 750 spectrometer (Thermo Scientifi c, Waltham, MA, USA); peaks are reported in cm —1.The NMR spectra were measured at 300 K on a Bruker DRX-400 (400 MHz for1H and 100 MHz for13C) spectrometer (Bruker Biospin AG, Fӓllanden, Germany), Chemical shifts (δ) are reported in parts per million (ppm) in CD3OD and coupling constants (J) in Hz, with the residual CH3OH (δH= 3.31 andδC= 49.0) as internal standard.LR-ESIMS and HR-ESIMS were carried out on a Bruker Daltonics Esquire 3000 plus instrument (Bruker Daltonics K.K., Kanagawa, Japan) and a Waters Q-TOF Ultima mass spectrometer (Waters, MA, USA), respectively.The semi-preparative HPLC was performed on an Agilent 1260 series liquid chromatography equipped with a DAD G1315D detector at 210 and 254 nm, and a semi-preparative ODS-HG-5 column [5 μm, 250 × 9.4 mm] was also employed.Commercial silica gel (Qingdao Haiyang Chemical Co., Ltd., Qingdao, China, 200—300 mesh, 300—400 mesh) was used for column chromatography, Sephadex LH-20 gel (Amersham Biosciences) was used for column chromatography and precoated silica gel plates (Yan Tai Zi Fu Chemical Group Co., Yantai, China, G60 F-254) were used for analytical TLC.All solvents used for extraction and isolation were of analytical grade.

Table 2 The inhibitory eff ect of the isolated compounds on the proliferation of HCT-116
3.2 Plant Material
The flowers ofL.indicaL.were collected from the experimental forest farm of Hunan Academy of Forestry, Hunan province, China, in July 2019.The plant was identifi ed by Dr Xiao-Ming Wang (Hunan Academy of Forestry, Hunan, China).A voucher specimen (No.19-HN-1) was deposited at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences.
3.3 Extraction and Isolation
The fresh flowers (10 kg) were picked off on the morning, and then dried at room temperature.The dried flowers were crushed and extracted exhaustively three times with 95% EtOH (3 × 5 L), each time for 24 h.After evaporation of the solvent under vacuum, the concentrate was suspended in H2O (2 L), acidifi ed to pH 4~5 with H2SO4, extracted three times with EtOAc (3 × 2 L).The EtOAc extract (59.9 g) was obtained after removing the solvents under vacuum.The aqueous layer was then basifi ed with Na2CO3to pH 9~10 and extracted three times with CHCl3(3 × 2 L), after filtration, the organic solvents were removed under vacuum to give the CHCl3extract (0.5 g).The aqueous layer was further extracted three times withn-BuOH (3 × 2 L).After filtration, the organic solvents were removed under vacuum to give then-BuOH extract (5.0 g).Then-BuOH extract was successively separated by silica column chromatography (CC) (column: 30 × 6 cm, eluting with petroleum ether: Acetone = 100:0 → 0:100), Sephadex LH-20 CC (column: 150 × 4 cm, eluting with dichloromethane: methanol = 1:1), and reversed-phase HPLC (CH3CN:H2O = 5:95) to afford compound 1 (3.6 mg,tR= 16.0 min) and compound 2 (4.1 mg,tR= 14.6 min).The EtOAc extract was repeatedly separated via silica CC (column: 30 × 6 cm, eluting with petroleum ether: diethyl ether = 100:0 → 0:100), and Sephadex LH-20 CC (column: 150 × 4 cm, eluting with petroleum ether: dichloromethane: methanol = 2:1:1) to obtain compound 3 (4.8 mg), compound 4 (38.0 mg) and compound 5 (10.3 mg).
3.3.1 Lagerindicine (1)
3.3.2 Pterolactam (2)
White amorphous powder (MeOH);+ 12 (c0.2 MeOH); Lit [15]:+ 10 (c0.1, MeOH).1H NMR and13C NMR data see Table 1.
3.4 Synthesis Procedures
3.4.1 2-Pyrrol-1-ylethan-1-ol (7)
Ethanolamine (6.6 mL, 0.1 mmol) was added to glacial acetic acid (12 mL) at 0 °C, and kept the temperature at 15—25 °C.Then, 2,5- dimethoxytetrahydrofuran (6) (3.4 mL, 0.02 mmol) was added and the reaction was stirred at 110 - 120 °C.After 2 h at that temperature, the residual liquid in the reaction vessel was cooled to rt and water was added.The usual work-up (DCM, brine, saturated aqueous Na2CO3) aff orded a residue that was dissolved in MeOH (6.7 mL).NaOH (20 wt %, 3.3 mL) was added, and the solution was stirred at rt for 1 h, then poured into brine, and extracted with DCM.The solvent was removed under reduced pressure to aff ord 7 (1.2 g, 44%) as a yellow oil.The spectroscopic data of 7 are as follows:1H NMR (400 MHz,CDCl3)δH6.70 (t, 2H), 6.18 (t, 2H), 4.00 (t, 2H), 3.81 (t, 2H) ppm.
3.4.2 1-(2-Hydroxyethyl)-5-methoxy-1H-pyrrol-2(5H)-one (8)
A solution of the compound 7 (1.8 mmol) in methanol (50 mL) containing a catalytic amount of sensitizer Rose Bengal (RB) was placed in a flask, and oxygen was gently bubbled through it.The solution was cooled to 0 ℃ and irradiated with a 250 W long arc mercury lamp for 2 h.The photooxidations gave complex mixtures of oxygenated products, and compound 8 (49.9 mg, 18%) were purifi ed by flash column chromatography using silica gel (petroleum ether/acetone = 5:1 → 1:1 v/v).8a {20.1 mg,(c0.1 MeOH),tR= 7.5 min} and 8b {20.3 mg,(c0.1 MeOH),tR= 10.0 min} were obtained through the chiral-phase HPLC resolution of 8 (CHIRAPAK IC, Lot No.IC00CE-QH040) (20% isopropanol, flow rate: 2 mL/min).The spectroscopic data of 8 are as follows:1H NMR (400 MHz,CDCl3)δH6.90 (dd, 1H,J= 1.6, 6.1 Hz), 6.23 (dd, 1H,J= 0.7, 6.1 Hz), 5.47 (br s, 1H), 3.73 (m, 2H), 3.56 (m, 1H), 3.41 (m, 1H), 3.12 (s, 3H) ppm;13C NMR (100 MHz,CDCl3)δC170.7, 144.1, 130.3, 89.3, 61.3, 51.0, 43.1 ppm
3.4.3 (±)-Lagerindicine (1)
Compound 8a (9.6 mg, 0.067 mmol) and 8b (11.5 mg, 0.071 mmol) in 5 mL of EtOH were reduced with H2at room temperature, using the catalytic amount of PtO2(2.0 mg) as catalyst, respectively.At the end of 2.5 h, the mixtures were filtered.Upon removed of the EtOH, compounds (+)- 1 {8.9 mg,+ 6.8 (c0.1 MeOH), 92%} and (-)- 1 {10.9 mg,-12.7 (c0.1 MeOH), 93%) were obtained, respectively.The NMR spectra of (+)- 1 and (-)- 1 see Figure S19—22.
3.5 Cytotoxicity Assays
The cytotoxicity of the isolated compounds was evaluated against the HCT-116 by using the sulforhodamine B (SRB) method, according to the protocols described in previous literature [23].The cytotoxicity of the compounds was expressed as the half-maximal inhibition (IC50, μM).TAK-243 (MLN7243) was used as a positive control.
AcknowledgementsThis research work was financially supported by the National Natural Science Foundation of China (NSFC) (Nos.81991521, 41676073), the National Key Research and Development Program of China (No.2018YFC0310903), the Drug Innovation Major Project (No.2018ZX09711-001-001-009), and the SKLDR/ SIMM Project (No.SIMM1903ZZ-04).Changsha Engineering Technology Research Center of Woody flower (kq1907081).
Compliance with Ethical Standards
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
 Natural Products and Bioprospecting2021年1期
Natural Products and Bioprospecting2021年1期
- Natural Products and Bioprospecting的其它文章
- In Memory of the Late Professor Jun Zhou (1932-2020)
- In Mourning and Memory of Late Professor Zhou Jun
- Natural Products in Cancer Therapy: Past, Present and Future
- On the Famous Traditional Chinese Medicine “Fu Zi”: Discovery, Research, and Development of Cardioactive Constituent Mesaconine
- The Outlook of the Development of Innovative Products from Biocompatible Natural Spider Silk in the Beauty Thread-Lifting Industry
- Insight into Medicinal Chemistry Behind Traditional Chinese Medicines: p-Hydroxybenzyl Alcohol-Derived Dimers and Trimers from Gastrodia elata
