海洋环境中有机胺的迁移转化研究进展*
胡清静 崔正国 孟 赫 白 莹 宋若晗 陆长坤 王宏胜 曲克明①
海洋环境中有机胺的迁移转化研究进展*
胡清静1,2崔正国1,2孟 赫3白 莹1,2宋若晗1,4陆长坤1,4王宏胜1曲克明1,2①
(1. 中国水产科学研究院黄海水产研究所 农业农村部海洋渔业可持续发展重点实验室 青岛 266071; 2. 青岛海洋科学与技术试点国家实验室海洋渔业科学与食物产出过程功能实验室 青岛 266071; 3. 山东省青岛生态环境监测中心 青岛 266003;4. 天津农学院水产学院 天津 300384)
大气中的有机胺具有潜在的气候效应,这是当今国际的研究热点之一。海洋是大气中有机胺的重要来源,但由于海水中有机胺检测难度较大,导致海洋环境中有机胺的产生机制尚不清楚。本文概述了海洋生物体内有机胺前体物的浓度特征及其对环境中有机胺的影响,阐述了沉积物、海水及大气中有机胺的浓度特征,分析了海洋大气气溶胶中有机胺的形成途径,并指出海水中有机胺检测的难点及现阶段亟待解决的科学问题。为深入认识海洋环境中有机胺的迁移转化规律及其潜在气候效应提供科学依据。
有机胺;海洋生物;盐度;形成途径;检测方法
大气中的有机胺可促进新粒子生成及颗粒物增长,进而增加云凝结核数浓度,通过改变辐射强迫对气候变化产生潜在重要的影响(Almeida, 2013; Chen, 2016; Sellegri, 2016; Yao, 2018)。大气中有150余种有机胺,其中,三甲胺(TMA)、二甲胺(DMA)和一甲胺(MMA)是最常见且含量最高的有机胺(Ge, 2011; Yu, 2014)。海洋是这3种有机胺的重要来源,每年向大气中释放80 Gg N (Ge, 2011)。有机胺还是海洋生物很重要的氮源和能量源,例如,TMA可被约占海洋水体中总细菌20%的玫瑰杆菌()降解为NH4+,同时释放三磷酸腺苷(ATP),ATP可为该细菌提供能量,NH4+则可被其他生物利用(Lidbury, 2015);在海洋沉积物中,TMA是温室气体甲烷(CH4)的重要前体物之一,在含盐环境中,35%~90%的CH4是由TMA降解而来的(Oremland, 1982; King, 1983)。但当有机胺浓度过高时,也会对生物造成危害,如在海水养殖环境中,TMA和DMA是鱼类和藻类腐烂所释放气体(鱼腥味)的重要组成部分(Chung, 2009),也是致癌物–亚硝基胺的前体物质。高浓度的TMA和DMA会抑制大分子物质(如DNA、RNA和蛋白质)的合成,对动物晶胚有致畸作用(Guest, 1992)。因此,认识海洋环境中有机胺的形成机制、浓度变化特征等对海洋生物的健康、海洋氮循环乃至全球气候变化具有重要的意义。
1 海洋环境中有机胺的源与汇
有机胺主要来源于细菌或酶对甜菜碱(GBT)、氧化三甲胺(TMAO)、胆碱(CHO)等季铵化合物的降解(图1)(Welsh, 2000; Carpenter, 2012; Lidbury, 2014; Lidbury, 2015; Lidbury, 2015a、2015b; Cree, 2015)。在海洋环境中,为了应对高盐度(NaCl)的压力,海洋植物、动物和微生物需要合成或吸收GBT、TMAO来调节细胞渗透压(Carpenter, 2012; Oren, 2015; Cree, 2015; Sun, 2019)。所以,季铵化合物在海洋生物中的含量远远大于淡水生物。季铵化合物被玫瑰杆菌等细菌降解后会释放出有机胺(图1) (Carpenter, 2012; Lidbury, 2015; Lidbury, 2017)。以上大部分有机胺的形成过程主要发生在碎屑颗粒、浮游动物肠道及沉积物等厌氧环境中(Carpenter, 2012; Cree, 2015)。因此,有机胺在海洋沉积物和水体中广泛存在。海洋沉积物或水体中有机胺的汇主要有以下3种方式:一部分通过海气交换从海水释放到大气中;一部分被硅藻和鞭毛藻等浮游植物直接吸收(Cree, 2015);剩余部分被降解后最终转化为NH4+、CH4和CO2。因此,季铵化合物及有机胺是海洋环境中很重要的碳源、氮源、能量源(Chen, 2012; Lidbury, 2015; Lidbury, 2015a; Cree, 2015; Taubert, 2017;Mausz, 2019; Sun, 2019)。
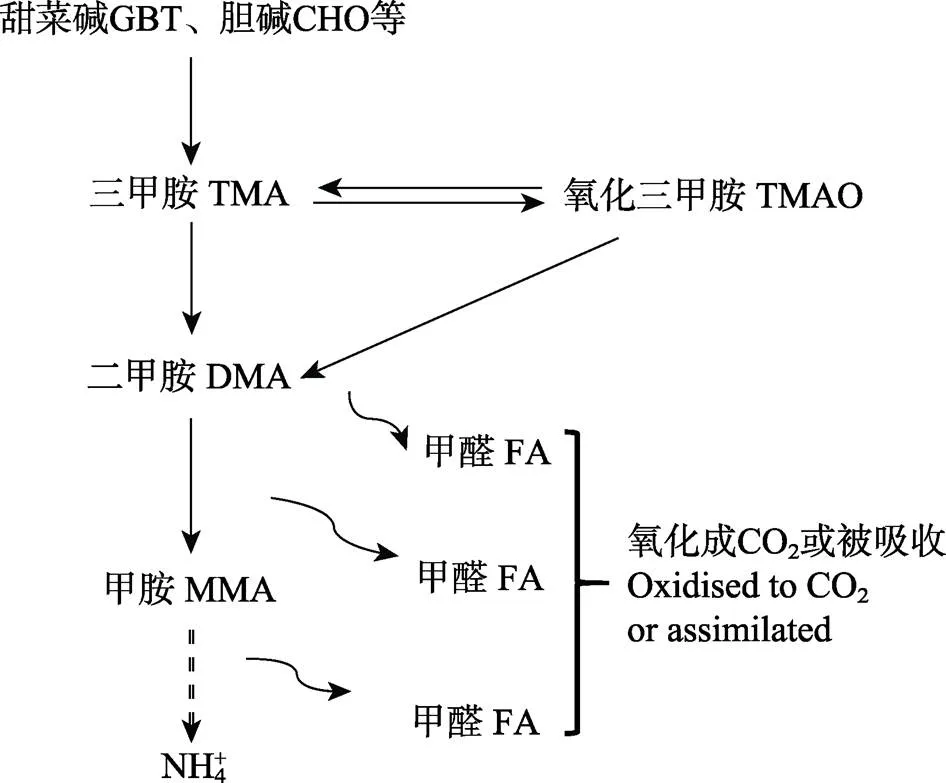
图1 海洋环境中有机胺的产生与降解过程
2 海洋生物体内有机胺前体物的浓度分布特征
国内外很多研究发现,海洋植物生物量的增加会显著促进水体或大气中有机胺浓度的增加(Gibb, 1999a; Facchini, 2008; Müller, 2009; Hu, 2015、2018; Yu, 2016; Dall’Osto, 2017),这主要是因为GBT和TMAO等季铵化合物广泛存在于海洋大型植物和浮游植物等体内,GBT可占藻类干重的2% (Blunden, 1992)。这些GBT通过细胞破裂和摄食等方式释放到环境中,在含螺旋藻(sp.)的沉积物中,GBT浓度可达100 μmol/gdw (King, 1988)。另外,de Vooys等(2002)研究发现,贝类体内也含有大量的GBT,在地中海贝类体内GBT的浓度高达30.0 g/kg。
20世纪30年代,Beatty(1938)在腐烂的鱼中第1次发现了TMA,因为在鱼类及软体动物中广泛存在有机胺的另一种重要前体物TMAO (Seibel, 2002; Summers, 2017)。TMAO占海洋生物(鱼类和甲壳类等)组织干重的7% (de Vooys, 2002)。在海洋鱼类体内,TMAO可应对盐度、温度和浮力的变化,还可增加蛋白质的稳定性(Summers, 2017)。例如,广盐性鲨鱼从淡水转入海水中,体内TMAO浓度显著增加(Pillans, 2005)。广盐性软骨鱼随着盐度的减小,TMAO的浓度显著降低(Sulikowski, 2003)。Chung等(2009)通过对香港89种(共266条)海水鱼、海水和淡水两栖鱼类、淡水鱼体内的TMAO分析发现,9种淡水鱼中只有3种可检测出TMAO,8种海水和淡水两栖鱼类中有6种可检测出TMAO,而72种海水鱼均可检测出TMAO,海水鱼体内TMAO的浓度范围为(0.12~3.5) g/kg,平均浓度为(1.4±0.75) g/kg。在山东青岛阿根廷鱿鱼()的TMAO含量为8.8 g/kg,硬骨鱼TMAO的含量范围为(0.35~2.3) g/kg,甲壳类TMAO的含量>1.7 g/kg,贝类的TMAO含量低于0.5 g/kg。因此,在不同种类动物体内TMAO的含量存在差异,例如,头足类>甲壳类>硬骨鱼类>贝类 (姜城子等, 2014)。海洋动物会通过分泌、排泄或被降解向环境中释放TMAO (Sun, 2019)。通过宏基因组研究发现,海洋环境中含有TMAO还原酶(把TMAO降解为TMA),这说明TMAO降解产生的TMA也可能是海洋环境中TMA等有机胺的一个重要来源(Sun, 2019)。因此,海洋动物越多,海洋环境中有机胺的浓度就会越高(Sørensen, 1987)。虽然,海洋环境中有大量鱼类等动物(陆尧等, 2019; 戴芳群等, 2020),但现阶段关于海洋动物对海水中有机胺影响的报道较少,特别是关于海洋动物种类、数量与海水或大气中有机胺浓度的定性或定量关系鲜有报道。

图2 海洋植物和动物对环境中有机胺的影响示意图
3 海洋环境中有机胺的浓度特征
海洋生物体内的TMAO、GBT和CHO等被降解后会影响环境中有机胺的浓度(图2)。Mausz等(2019)和Sun等(2019)对海水及沉积物空隙水中有机胺的浓度做了详细总结,一般情况下,1 L海水中有机胺的浓度为纳摩尔级,其浓度较低,所以检测难度较大,1 L沉积物的孔隙水中有机胺的浓度较1 L海水中高1~3个数量级(微摩尔级)。例如,在英国默尔塞河口沉积物的孔隙水中MMA、DMA和TMA的最高浓度分别是319、9和50 μmol/L (Mausz, 2019)。由于沉积物中的有机质可吸附有机胺,所以,沉积物中有机胺的浓度也较高(Wang, 1990; Wang, 1994)。例如,在泰晤士河口沉积物中3种有机胺的浓度之和可达26 μmol/g (Fitzsimons, 2006)。
一般情况下,海洋大气中颗粒态有机胺盐的浓度是皮克至纳克级(Cree, 2015; van Pinxteren, 2019)。例如,阿拉伯海大气PM1.0(空气动力学直径≤1.0 μm的颗粒物)中TMA+和DMA+的浓度范围为(0.02~0.9)和(1.0~17.1) ng/m3(Gibb, 1999b);van Pinxteren等(2019) 2011~2013年在热带大西洋的佛得角岛进行观测,发现PM1.0中MMA+和DMA+的浓度范围为(0~0.6)和(2.2~13.0) ng/m3;地中海东部克里特岛大气PM1.0中DMA+的平均浓度为(9.0±36.1) ng/m3,但是TMA+浓度低于检测限(Violaki, 2010);北大西洋(爱尔兰西海岸)大气PM8.0中DMA+的浓度<(1.0~ 24.0) ng/m3(Facchini, 2008);我国近海大气PM10(或PM0.056~10)中DMA+和TMA+的平均浓度范围为(0~49.5)和(6.5~44.9) ng/m3(Yu, 2016; Xie, 2018; Hu, 2018; Zhou, 2019),而PM0.1中DMA+和TMA+的浓度范围分别是(0.0~5.4)和(2.7~ 19.5) ng/m3(Yu, 2016)。这说明我国近海大气中有机胺盐的浓度比世界上大部分其他海域高,特别是2012年5月PM11中有机胺盐浓度甚至比世界其他海域高1~3个数量级(Hu, 2015),如此高浓度有机胺可能具有很重要的气候效应,但其形成原因有待近一步确认。
4 海洋大气气溶胶中有机胺盐的形成途径
目前,国际上对海洋大气气溶胶中有机胺盐的形成途径存在争议。国际主流观点认为,海水中有机胺通过海气交换进入大气中,一部分以气态形式存在,另一部分气体再通过大气化学反应形成二次有机胺盐(即来自二次源)(Gibb, 1999b; Facchini, 2008; Müller, 2009; Myriokefalitakis, 2010; Köllner, 2017; Willis, 2017)。例如,Müller等(2009)研究发现,海洋大气气溶胶中高浓度的有机胺盐主要分布在(0.14~0.42) µm粒径段上,Facchini等(2008)研究发现,海洋大气气溶胶中高浓度的有机胺盐主要分布在(0.25~0.50) µm粒径段上,并且与二次生成的NH4+、SO42–和甲基磺酸盐(MSA–)的粒径分布相似,认为该大气气溶胶中有机胺盐是二次生成的。通过对2012~2016年从我国近海到西北太平洋10多个航次分析发现,大气气溶胶中的DMA+和TMA+主要分布在(0.20±0.10)、(0.40±0.10)和(0.80±0.20) µm等的粒径段上,推测这些有机胺盐也是来自二次源(Hu, 2015; Yu, 2016; Xie, 2018)。
但是,Frossard等(2014)在开阔大洋的一次源有机气溶胶中检测出了有机胺官能团;Gorzelska等(1990)推测,在大风浪天气条件下,富含有机胺的海洋表层物质可能会随着海盐气溶胶被传输到大气中,从而导致大气气溶胶中有机胺盐浓度升高;Hu等(2018)在2014年春季对西北太平洋大气气溶胶中有机胺盐进行分析发现,该区域有机胺盐的平均浓度比近海高1个数量级,TMA+浓度最高(4.4 nmol/m3)的样品是在风速最高时(14 m/s)被检测到,并且绝大部分样品中TMA+的粒径分布与最高浓度样品的相似,即:大气气溶胶中TMA+的浓度随粒径的减小逐渐升高(Hu, 2018)。Hu等(2017)研究发现,当风速>5.4 m/s时,西北太平洋水体中的有机质和细菌可能会通过海洋飞沫气溶胶被传输到大气中,并且海洋飞沫气溶胶中有机质的丰度会随粒径的减少而增加(Gantt, 2013; Quinn, 2014、2015)。另外,国际上很多专家通过室内海洋飞沫气溶胶模拟实验,也发现海水中有机质主要富集在亚微米大气气溶胶上(Quinn, 2015; Hultin, 2010; Rastelli, 2017)。因此,Hu等(2018)推测,在高风速条件下,海水中的TMA+随海洋飞沫被直接传输到大气中(即来自一次源)。Dall’Osto等(2019)研究显示,大气气溶胶中一次源有机胺盐占总有机胺盐的11%~25%。总体来说,现阶段关于一次源有机胺盐对总有机胺盐占比的报道仍然较少。
5 海水中有机胺的检测方法
现阶段,国际上关于海洋生物体内有机胺前体物浓度、大气中有机胺浓度的报道相对较多,但由于海水中有机胺浓度较低(nmol/L级),且MMA、DMA和TMA具有易溶于水、极性强和易挥发等特点,一直以来海水中有机胺的检测具有较大的挑战(Carpenter, 2012; Cree, 2015; Sun, 2019),从而也限制了对海洋环境中有机胺迁移转化规律的认识。目前,海水中有机胺的检测主要分为3个关键的阶段:预浓缩阶段、分离阶段和检测阶段。预浓缩所用的前处理装置主要有:固相微萃取(SPME)、顶空固相微萃取(HS-SPME)和吹扫捕集装置(P&T)等。分离和检测阶段主要是由离子色谱(IC)或气相色谱仪(GC)等组合不同检测器完成(Cree, 2015)。
采用IC可检测大气中有机胺的浓度(Hu, 2015、2018; Yu, 2016; Xie, 2018),但由于有机胺分析柱承载不了海水中高浓度的Na+,导致海水中有机胺不能直接在IC上测试(Ferreira, 2017)。Ferreira等(2017)通过向P&T装置中加入NaOH,把含盐水体中有机胺挥发出来,再进行浓缩,最后进入IC测试。但P&T-IC方法对有机胺检测的灵敏度较低,且在吹扫捕集的捕集阱内会存在严重残留,且捕集阱内装填的吸附剂种类不同其吸附性能差异也较大(中华人民共和国国家标准HJ 1042-2019),也不适合对海水中低浓度有机胺的浓缩。Gibb等(1995)采用流动注入气体扩散(FIGD)装置与IC结合可很好地测试海水中的有机胺。但由于FIGD比较复杂,近20年来该方法未得到广泛地应用(Cree, 2015)。
GC对有机胺检测的灵敏度较高(Huang, 2014)。国内外很多专家采用GC结合不同检测器来测定大气、水体、孔隙水和沉积物中的有机胺,如气相色谱仪‒质谱仪(GC-MS)、气相色谱仪‒火焰检测器(GC-FID)和气相色谱仪‒氮磷检测器(GC-NPD)(Cree, 2015)。采用该仪器检测大气中有机胺的方法与水体的大体相似。基本原理是把大气采样膜或沉积物中的有机胺萃取到水体中,然后,通过衍生或把有机胺挥发、再浓缩后进行测试。但GC-MS只能测DMA+或TMA+中的1种(Liu, 2017; Zhuang, 2017、2018),很难实现DMA+和TMA+的同步检测(Cree,2018)。GC-FID和GC-NPD可同步检测以上2种有机胺。与GC-FID相比,GC-NPD对海水中有机胺的灵敏度会更高(Cree, 2015)。Cree等(2018)研究显示,可采用SPME-GC-NPD检测海水中的TMA+、DMA+和MMA+,检测限可达(0.4~2.9) nmol/L。虽然,该方法也存在一些不足之处,如该前处理方法耗时较长、分析较低浓度样品时可能会存在实验误差等(Sun, 2019),但相比较而言,目前,该方法是国际上检测海水中有机胺比较有效的方法之一。
6 存在问题及展望
综上所述,虽然目前国内外对海洋生物体内有机胺前体物的浓度、降解这些前体物的优势细菌及大气中有机胺的潜在气候效应等方面开展了一定的研究,但海洋环境中有机胺的迁移转化规律还不清楚,还有许多问题需要解决,具体体现在以下几点:
(1)海洋生物对环境中有机胺的贡献。系统分析不同种类浮游植物、动物体内有机胺前体物的浓度分布特征;阐明这些前体物在食物链上的传递规律;鉴定天然海域中把这些前体物降解为有机胺的优势细菌;通过计算海洋生物量与环境中有机胺浓度的比值,估算海洋生物对环境中有机胺的贡献。
(2)海水中有机胺向大气中传输的影响机制。通过利用在线离子色谱获取高时空分辨率的气态和颗粒态有机胺数据,解析温度、相对湿度和风速等气象因子对它们从海水向大气中传输的影响。
(3)我国近海高浓度有机胺的来源解析。我国近海不仅藻华和绿潮等灾害频发,同时,还进行着大规模的海水养殖。通过现场观测,进一步解析我国近海大量的浮游植物和养殖动物对我国近海大气中高浓度有机胺的贡献。
(4)海水中有机胺检测方法的建立。目前,GC-NPD是一种有效分离3种有机胺的检测方法,但采用SPME浓缩海水中有机胺的方法还存在一定的不足,未来通过研发更好的海水中有机胺的提取方法,再结合GC-NPD实现海水中有机胺的检测。从而进一步阐明有机胺在海洋生物、水体和大气中的迁移转化规律,以提升对海洋大气中有机胺潜在气候效应的认识。
Almeida J, Schobesberger S, Kürten A,. Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere. Nature, 2013, 502(7471): 359–363
Beatty SA. Studies of fish spoilage:Ⅱ. The origin of trimethylamine produced during the spoilage of cod muscle press juice. Journal of the Fisheries Research Board of Canada, 1938, 4a(2): 63–68
Blunden G, Smith BE, Irons MW,Betaines and tertiary sulphonium compounds from 62 species of marine algae. Biochemical Systematics and Ecology, 1992, 20(4): 373– 388
Carpenter LJ, Archer SD, Beale R. Ocean-atmosphere trace gas exchange. Chemical Society Reviews, 2012, 41(19): 6473–6506
Chen HH, Varner ME, Gerber RB,. Reactions of methanesulfonic acid with amines and ammonia as a source of new particles in air. Journal of Physical Chemistry B, 2016, 120(8): 1526–1536
Chen Y. Comparative genomics of methylated amine utilization by marineclade bacteria and development of functional gene markers (). Environmental Microbiology, 2012, 14(9): 2308–2322
Chung SWC, Chan BTP. Trimethylamine oxide, dimethylamine, trimethylamine and formaldehyde levels in main traded fish species in Hong Kong. Food Additives and Contaminants Part B Surveillance, 2009, 2(1): 44–51
Cree C. Distributions of glycine betaine and the methylamines in coastal waters: Analytical developments and a seasonal study. Doctoral Dissertation of Plymouth University, 2015
Cree CHL, Airs R, Archer SD,. Measurement of methylamines in seawater using solid phase microextraction and gas chromatography. Limnology and Oceanography: Methods, 2018, 16(7): 411420
Dai FQ, Zhu L, Chen YL. Variations of fishery resource structure in the Yellow Sea and East China Sea. Progress in Fishery Sciences, 2020, 41(1): 1–10 [戴芳群, 朱玲, 陈云龙. 黄、东海渔业资源群落结构变化研究. 渔业科学进展, 2020, 41(1): 1–10]
Dall'Osto M, Airs RL, Beale R,. Simultaneous detection of alkylamines in the surface ocean and atmosphere of the Antarctic sympagic environment. ACSEarth and Space Chemistry, 2019, 3(5): 854–862
Dall'Osto M, Ovadnevaite J, Paglione M,. Antarctic sea ice region as a source of biogenic organic nitrogen in aerosols. Scientific Reports, 2017, 7(1): 6047
de Vooys CGN, Geenevasen JAJ. Biosynthesis and role in osmoregulation of glycine-betaine in the Mediterranean musselLMK. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 2002, 132(2): 409–414
de Vooys CGN. Occurrence and role of a Quaternary base, trimethylamine oxide, in two cockle species,and. Journal of Sea Research, 2002, 47(1): 69–73
Facchini MC, Decesari S, Rinaldi M,. Important source of marine secondary organic aerosol from biogenic amines. Environmental Science and Technology, 2008, 42(24): 9116–9121
Ferreira FN, Afonso JC, Pontes FVM,. Ultrasound-assisted purge-and-trap extraction for simultaneous determination of low-molecular weight amines and ammonium in high salinity waters by ion chromatography. Microchemical Journal, 2017, 133: 658662
Fitzsimons MF, Millward GE, Revitt DM,. Desorption kinetics of ammonium and methylamines from estuarine sediments: Consequences for the cycling of nitrogen. Marine Chemistry, 2006, 101(1–2): 12–26
Frossard AA, Russell LM, Burrows SM,. Sources and composition of submicron organic mass in marine aerosol particles. Journal of Geophysical Research Atmospheres, 2014, 119(22): 12977–13003
Gantt B, Meskhidze N. The physical and chemical characteristics of marine primary organic aerosol: A review. Atmospheric Chemistry and Physics, 2013, 13(8): 3979–3996
Ge X, Wexler AS, Clegg SL. Atmospheric amines-Part I. A review. Atmospheric Environment, 2011, 45(3): 524–546
Gibb SW, Mantoura RFC, Liss PS,. Distributions and biogeochemistries of methylamines and ammonium in the Arabian Sea. Deep Sea Research Part Ⅱ Topical Studies in Oceanography, 1999a, 46(3–4): 593–615
Gibb SW, Mantoura RFC, Liss PS. Analysis of ammonia and methylamines in natural waters by flow injection gas diffusion coupled to ion chromatography. Analytica Chimica Acta, 1995, 316(3): 291–304
Gibb SW, Mantoura RFC, Liss PS. Ocean-atmosphere exchange and atmospheric speciation of ammonia and methylamines in the region of the NW Arabian Sea. Global Biogeochemical Cycles, 1999b, 13(1): 161–178
Gorzelska K, Galloway JN. Amine nitrogen in the atmospheric environment over the North Atlantic Ocean. Global Biogeochemical Cycles, 1990, 4(3): 309–333
Guest I, Varma DR. Teratogenic and macromolecular synthesis inhibitory effects of trimethylamine on mouse embryos in culture. Journal of Toxicology and Environmental Health, 1992, 36(1): 27–41
Hu QJ, Qu KM, Gao HW,. Large increases in primary trimethylaminium and secondary dimethylaminium in atmospheric particles associated with cyclonic eddies in the Northwest Pacific Ocean. Journal of Geophysical Research: Atmospheres, 2018, 123(21): 12133–12146
Hu QJ, Yu PR, Zhu YJ,. Concentration, size distribution and formation of trimethylaminium and dimethylaminium ions in atmospheric particles over marginal seas of China. Journal of the Atmospheric Sciences, 2015, 72: 3487–3498
Hu W, Murata K, Fukuyama S,. Concentration and viability of airborne bacteria over the Kuroshio extension region in the northwestern Pacific Ocean: Data from three cruises. Journal of Geophysical Research: Atmospheres, 2017, 122(23): 12892–12905
Huang RJ, Li WB, Wang YR,. Determination of alkylamines in atmospheric aerosol particles: A comparison of gas chromatography-mass spectrometry and ion chromatography approaches. Atmospheric Measurement Techniques, 2014, 7(7): 2027–2035.
Hultin KAH, Nilsson ED, Krejci R,. In situ laboratory sea spray production during the marine aerosol production 2006 cruise on the northeastern Atlantic Ocean. Journal of Geophysical Research: Atmospheres, 2010, 115, D06201
Jiang CZ, Cui J, Zhou MM,. Determination of trimethylamine-N-oxide content in common aquatic products in Qingdao. Journal of Food Safety and Quality, 2014(1): 41–46 [姜城子, 崔洁, 周苗苗, 等. 青岛地区部分水产品中氧化三甲胺含量的测定. 食品安全质量检测学报. 2014(1): 41–46]
King GM, Klug MJ, Lovley DR. Metabolism of acetate, methanol, and methylated amines in intertidal sediments of Lowes Cove, Maine. Applied and Environmental Microbiology, 1983, 45(6): 1848–1853
King GM. Methanogenesis from methylated amines in a hypersaline algal mat. Applied and Environmental Microbiology, 1988, 54(1): 130–136
Köllner F, Schneider J, Willis MD,. Particulate trimethylamine in the summertime Canadian high Arctic lower troposphere. Atmospheric Chemistry and Physics, 2017, 17(22): 13747–13766
Lidbury I, Kimberley G, Scanlan DJ,Comparative genomics and mutagenesis analyses of choline metabolism in the marine. Environmental Microbiology, 2015a, 17(12): 5048–5062
Lidbury I, Mausz MA, Scanlan DJ,. Identification of dimethylamine monooxygenase in marine bacteria reveals a metabolic bottleneck in the methylated amine degradation pathway. ISME Journal, 2017, 11(7): 1592–1601
Lidbury I, Murrell JC, Chen Y. Trimethylamine N-oxide metabolism by abundant marine heterotrophic bacteria. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(7): 2710–2715
Lidbury I. Microbial methylated amine metabolism in marine surface waters. Doctoral Dissertation of University of Warwick, 2015
Lidbury I, Murrell JC, Chen Y. Trimethylamine and trimethylamine N-oxide are supplementary energy sources for a marine heterotrophic bacterium: Implications for marine carbon and nitrogen cycling. ISME Journal, 2015b, 9(3): 760–769
Liu FX, Bi XH, Zhang GH,. Concentration, size distribution and dry deposition of amines in atmospheric particles of urban Guangzhou, China. Atmospheric Environment, 2017, 171: 279–288
Lu Y, Chen XJ, Wang JT,. Dynamics of suitable habitat ofin the Northwest Pacific Ocean. Progress in Fishery Sciences, 2019, 40(5): 19–25 [陆尧, 陈新军, 汪金涛, 等. 西北太平洋柔鱼适宜栖息地动态变化研究. 渔业科学进展. 2019, 40(5): 19–25]
Mausz MA, Chen Y. Microbiology and ecology of methylated amine metabolism in marine ecosystems. Current Issues in Molecular Biology, 2019, 33: 133–148
Müller C, Iinuma Y, Karstensen J,. Seasonal variation of aliphatic amines in marine sub-micrometer particles at the Cape Verde islands. Atmospheric Chemistry and Physics, 2009, 9(4): 9587–9597
Myriokefalitakis S, Vignati E, Tsigaridis K,. Global modeling of the oceanic source of organic aerosols. Advances in Meteorology, 2010(4): 1–16
Oremland RS, Marsh LM, Polcin S. Methane production and simultaneous sulphate reduction in anoxic, salt marsh sediments. Nature, 1982, 296(5853): 143–145
Oren A. Cyanobacteria in hypersaline environments: Biodiversity and physiological properties. Biodiversity and Conservation, 2015, 24(4): 781–798
Pillans RD, Good JP, Anderson WG,. Freshwater to seawater acclimation of juvenile bull sharks (): Plasma osmolytes and Na+/K+-ATPase activity in gill, rectal gland, kidney and intestine. Journal of Comparative Physiology B, 2005, 175(1): 37–44
van Pinxteren M, Fomba KW, van Pinxteren D,. Aliphatic amines at the Cape Verde Atmospheric Observatory: Abundance, origins and sea-air fluxes. Atmospheric Environment, 2019, 203: 183–195
Quinn PK, Bates TS, Schulz KS. Contribution of sea surface carbon pool to organic matter enrichment in sea spray aerosol. Nature Geoscience, 2014, 7(3): 228–232
Quinn PK, Collins DB, Grassian VH,. Chemistry and related properties of freshly emitted sea spray aerosol. Chemical Reviews, 2015, 115(10): 4383–4399
Rastelli E, Corinaldesi C, Dell’Anno A,. Transfer of labile organic matter and microbes from the ocean surface to the marine aerosol: An experimental approach. Scientific Reports, 2017, 7(1): 11475
Seibel BA, Walsh PJ. Trimethylamine oxide accumulation in marine animals: Relationship to acylglycerol storage. Journal of Experimental Biology, 2002, 205(3): 297–306
Sellegri K, Pey J, Rose C,. Evidence of atmospheric nanoparticle formation from emissions of marine microorganisms. Geophysical Research Letters, 2016, 43(12): 6596–6603
Sørensen J, Glob E. Infuence of benthic fauna on trimethylamine concentrations in coastal marine sediments. Marine Ecology Progress Series, 1987, 39: 15–21
Sulikowski JA, Treberg JR, Howell WH. Fluid regulation and physiological adjustments in the winter skate,, following exposure to reduced environmental salinities. Environmental Biology of Fishes, 2003, 66(4): 339–348
Summers G, Wibisono RD, Hedderley DI,. Trimethylamine oxide content and spoilage potential of New Zealand commercial fish species. New Zealand Journal of Marine and Freshwater Research, 2017, 51(3): 393–405
Sun J, Mausz MA, Chen Y,. Microbial trimethylamine metabolism in marine environments. Environmental Microbiology, 2019, 21(2): 513–520
Taubert M, Grob C, Howat AM,. Methylamine as a nitrogen source for microorganisms from a coastal marine environment. Environmental Microbiology, 2017, 19(6): 2246–2257
Violaki K, Mihalopoulos N. Water-soluble organic nitrogen (WSON) in size-segregated atmospheric particles over the Eastern Mediterranean. Atmospheric Environment, 2010, 44(35): 4339–4345
Wang XC, Lee C. Sources and distribution of aliphatic amines in salt marsh sediment. Organic Geochemistry, 1994, 22(6): 1005–1021
Wang XC, Lee C. The distribution and adsorption behavior of aliphatic amines in marine and lacustrine sediments. Geochimica et Cosmochimica Acta, 1990, 54(10): 2759– 2774
Welsh DT. Ecological significance of compatible solute accumulation by micro-organisms: From single cells to global climate. FEMS Microbiology Reviews, 2000, 24(3): 263–290
Willis MD, Köllner F, Burkart J,. Evidence for marine biogenic influence on summertime Arctic aerosol. Geophysical Research Letters, 2017, 44(12): 6460–6470
Xie H, Feng LM, Hu QJ,. Concentration and size distribution of water-extracted dimethylaminium and trimethylaminium in atmospheric particles during nine campaigns-Implications for sources, phase states and formation pathways. Science of the Total Environment, 2018, 631–632: 130–141
Yao L, Garmash O, Bianchi F,. Atmospheric new particle formation from sulfuric acid and amines in a Chinese megacity. Science, 2018, 361(6399): 278–281
Yu F, Luo G. Modeling of gaseous methylamines in the global atmosphere: Impacts of oxidation and aerosol uptake. Atmospheric Chemistey and Physics, 2014, 14(22): 12455– 12464
Yu PR, Hu QJ, Kai L,. Characteristics of dimethylaminium and trimethylaminium in atmospheric particles ranging from supermicron to nanometer sizes over eutrophic marginal seas of China and oligotrophic open oceans. Science of the Total Environment, 2016, 572: 813–824
Zhou SQ, Li HW, Yang TJ,. Characteristics and sources of aerosol aminiums over the eastern coast of China: Insights from the integrated observations in a coastal city, adjacent island and surrounding marginal seas. Atmospheric Chemistry and Physics, 2019, 19(16): 10447–10467
Zhuang GC, Heuer VB, Lazar CS,. Relative importance of methylotrophic methanogenesis in sediments of the Western Mediterranean Sea. Geochimica et Cosmochimica Acta, 2018, 224: 171–186
Zhuang GC, Lin YS, Bowles MW,. Distribution and isotopic composition of trimethylamine, dimethylsulfide and dimethylsulfoniopropionate in marine sediments. Marine Chemistry, 2017, 196: 35–46
Advancements in the Transport and Transformation of Amines in the Marine Environment
HU Qingjing1,2, CUI Zhengguo1,2, MENG He3, BAI Ying1,2, SONG Ruohan1,4, LU Changkun1,4, WANG Hongsheng1, QU Keming1,2①
(1. Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Sustainable Development of Marine Fisheries, Ministry of Agriculture and Rural Affairs, Qingdao 266071; 2. Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266071; 3. Qingdao Eco-Environment Monitoring Center of Shandong Province, Qingdao 266003; 4. Department of Fishery Science, Tianjin Agricultural University, Tianjin 300384)
In the atmosphere, amines play potentially important roles in climatechange, which is a hot spot of the current international research. The ocean is an important source of amines in the atmosphere; however, the mechanism of the formation of amines in the environment has not been elucidated due to the difficulty of detecting amines in seawater. This article outlines the concentration characteristics of amine precursors in marine organisms and their impact on amines in the marine environment; summarizes the concentration characteristics of amines in sediments, seawater, and the atmosphere; analyzes the formation pathway of amines in marine atmospheric particles; and identifies the difficulties in the detection of amines in seawater and the related problems that need urgent attention. This study provides insights into the transport and transformation of amines in the marine environment and the resulting climatic effects on the marine atmosphere.
Amines; Marine organisms; Salinity; Formation pathways; Detection methods
QU Keming, E-mail: qukm@ysfri.ac.cn
P76
A
2095-9869(2021)02-0184-08
10.19663/j.issn2095-9869.20200509002
http://www.yykxjz.cn/
胡清静, 崔正国, 孟赫, 白莹, 宋若晗, 陆长坤, 王宏胜, 曲克明. 海洋环境中有机胺的迁移转化研究进展. 渔业科学进展, 2021, 42(2): 184–191
Hu QJ, Cui ZG, Meng H, Bai Y, Song RH, Lu CK, Wang HS, Qu KM. Advancements in the transport and transformation of amines in the marine environment. Progress in Fishery Sciences, 2021, 42(2): 184–191
* 国家自然科学基金青年基金(41606097)、中国水产科学研究院黄海水产研究所基本科研业务费(20603022020006;2020TD12)和国家重点研发计划项目(2019YFD0900500; 2019YFD0901401)共同资助 [This work was supported by Youth Program of National Natural Science Foundation of China (41606097), Central Public-Interest Scientific Institution Basal Research Fund, YSFRI, CAFS (20603022020006; 2020TD12), and National Key Research and Development Program of China (2019YFD0900500; 2019YFD0901400)]. 胡清静,E-mail: huqj@ysfri.ac.cn
曲克明,研究员,E-mail: qukm@ysfri.ac.cn
2020-05-09,
2020-07-03
(编辑 马璀艳)

