Contribution to the taxonomy of the genus Lycodon H.Boie in Fitzinger,1827 (Reptilia:Squamata:Colubridae) in China,with description of two new species and resurrection and elevation of Dinodon septentrionale chapaense Angel,Bourret,1933
Kai Wang ,Zhong-Bin Yu ,Gernot Vogel ,Jing Che
1 State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming,Yunnan 650223, China
2 Sam Noble Oklahoma Museum of Natural History, Department of Biology, University of Oklahoma, Norman, Oklahoma 73072, USA
3 Kunming College of Life Science, University of the Chinese Academy of Sciences, Kunming, Yunnan 650204, China
4 Society for Southeast Asian Herpetology, Heidelberg D-69115, Germany
ABSTRACT While considerable progress has been made in the taxonomic studies of the genus Lycodon in Asia,questions remain to be clarified regarding the taxonomy of certain groups, particularly those containing species in China.Not only do many regions in China remain overlooked by herpetologists, resulting in the possibility of undiscovered new species,but the surveyed areas also have suspicious records of recognized congeners that require taxonomic confirmations.Combining both morphological and genetic data,we tackle these outstanding issues in the taxonomy of Lycodon in China.In particular,we discover two new species of Lycodon: one from the previously neglected hot-dry valley in the northern Hengduan Mountain Region close to Tibet,and another recluse and cryptic species from the L.fasciatus complex in the downtown park of a major city in southern Sichuan Province. Additionally, we clarify the distribution of L. septentrionalis in China and resurrect and elevate its junior synonym subspecies,Dinodon septentrionale chapaense,as a full,valid species,and we synonymize the recently described L.namdongensis to the resurrected L.chapaensis comb.nov..Lycodon chapaensis comb.nov.thus represents a new national record of reptilian fauna of China.Lastly,based on literature review,we also correct some of the erroneous records of L.fasciatus and L.ruhstrati in China,point out remaining taxonomic issues of the genus for future research,and update the dichotomous key and distribution of the 20 species of Lycodon currently recorded from China.
Keywords:Erroneous records;Guangdong;Hunan; Misidentification; Serpentes; Sichuan;Wolf Snake; Yunnan
INTRODUCTION
After major generic revisions (Guo et al.,2013; Siler et al.,2013),Wolf Snakes of the genusLycodonFitzinger,1826 represent one of the most diverse snake radiations in Asia,including 66 recognized species to date (Ganesh et al.,2020;Uetz et al.,2020).Majority of the currently recognized taxa are known inhabiting tropical to subtropical forests at a mid-to-low elevation (Luu et al.,2018; Vogel & Brachtel,2008; Vogel et al.,2009; Wang et al.,2020a),where species of the genus are known to feed heavily on reptiles,particularly on lizards(Zhang & Wang,2014; Zhao et al.,1998).
In China,17 species have been recorded (Janssen et al.,2020; Wang et al.,2020b),includingL.aulicus(Linnaeus,1758),L.cathayaWang,Qi,Lyu,Zeng,Wang,2020,L.fasciatus(Anderson, 1879),L. flavozonatus(Pope, 1928),L.futsingensis(Pope,1928),L.gongshanVogel,Luo,2011,L.laoensisGünther,1864,L.liuchengchaoiZhang,Jiang,Vogel,Rao, 2011,L. multizonatus(Zhao, Jiang, 1981),L.meridionalis(Bourret,1935),L.pictusJanssen,Pham,Ngo,Le,Nguyen,Ziegler,2019,L.ruhstrati(Fischer,1886),L.rosozonatus(Hu,Zhao,1972),L.rufozonatusCantor,1842,L.septentrionalis(Günther,1875),L.subcinctusBoie,1827,andL.synaptorVogel,David,2010.In the Hengduan Mountain Region (HMR) in Southwest China alone,seven recognized species have been recorded from Yunnan and Sichuan Provinces,namelyL.fasciatus,L.gongshan,L.liuchengchaoi,L.ruhstrati,L.multizonatus,L.septentrionalis,andL.synaptor(Chen et al.,2018a,2018b; Guo et al.,2007; Vogel & David,2010; Vogel & Luo,2011; Yang & Rao,2008; Zhang et al.,2011b; Zhao,2004; Zhao & Yang,1997).Of these seven species found in the HMR,onlyL.multizonatusis from the high-elevation regions in the northeast (Lei et al.,2014).As most parts of the HMR have not been surveyed in details for herpetological diversity,and given previous studies already suggested that the northern parts of the HMR actually harbor a surprising number of undocumented reptilian diversity (Peng et al.,2014b; Wang et al.,2021),it is likely that the diversity ofLycodonin the northern HMR is also underestimated.
In relation to the overlooked diversity,many recognized species have outstanding taxonomic issues.Species currently recorded from HMR are known by having wide distribution ranges that expand across distinct zoogeographic regions(Zhao & Adler,1993),particularlyL.fasciatus,L.ruhstrati,andL.septentrionalis(Zhao,2006; Zhao et al.,1998).As studies have suggested that cryptic diversity and misidentification of recognized congeners explain some of the existing suspicious records (Vogel & David,2010; Vogel & Luo,2011; Vogel et al.,2009),the current remaining records of these species across China and Southeast Asia warrant further confirmations.
In this study,we combined both morphological and genetic data to shed lights into the current taxonomy ofLycodonin China.As results,we discover two new species ofLycodon:one species from northern HMR that has never been documented before,and another one from the previously identified population ofL.fasciatusin Panzhihua,Sichuan.Additionally,we found that the previously identified “L.septentrionalis” in Yunnan Province represent the same lineage as the recently described speciesL.namdongensisfrom northern Vietnam, and this lineage matches the diagnosis of an existing synonym,Dinodon septentrionale chapaenseAngel,Bourret,1933 (=Lycodon septentrionalis chapaensisafter generic revision),which we resurrect and elevate to full species status.We provide an expanded description of the poorly knownL.chapaensiscomb.nov.based on additional specimens from China.Furthermore,we confirm that the questionable records of “L.fasciatus” from Hunan and Guangdong represent misidentifications overL.liuchengchaoi,and records of “L.ruhstrati” in Yunnan represent clear misidentifications overL.chapaensiscomb.nov.andL.gongshan.Lastly,we provide an updated dichotomous key and distribution to the recognized species in China and discuss some remaining taxonomic issues for future studies.
MATERIALS AND METHODS
Taxonomic sampling
A total of 13 specimens and a non-vouchered genetic tissue of the genusLycodonwere collected from Southwest China between 2016 and 2020 (Figure 1; Table 1; Appendix I,II).Liver or muscle tissues were taken after the specimens were euthanized,and the voucher specimens were fixed in 10%buffered formalin in the field,transferred to 70% ethanol after 48h for permanent storage,and deposited at the Zoological Museum of Kunming Institute of Zoology,Chinese Academy of Sciences (KIZ).
Specimens of recognized congeners were examined in museum collections, including Natural History Museum(BMNH),California Academy of Sciences (CAS),Chengdu Institute of Biology,Chinese Academy of Sciences (CIB),Field Museum of Natural History (FMNH), KIZ, and Henan University (HENU) (Appendix II).For species that we could not examine in person,data were obtained from literature (Angel& Bourret,1933; Boulenger,1893; Janssen et al.,2019; Luu et al.,2019; Peng et al.,2014a,2015,2017; Vogel et al.,2009; Wang et al., 2020a; Zhang, 2019). Additional abbreviations of voucher collections included herpetological collection of Dr.Guo Peng at Yibin University (GP),Muséum National d’Histore Naturelle (MNHN),and Vietnam National University of Forestry (VNUF).Photos of the holotype ofDinodon septentrionale chapaensewere obtained from the website of MNHN (https://science.mnhn.fr/institution/mnhn/collection/ra/item/1 933.11?listIndex=25&listCount=253).

Figure 1 Distributions of focal members of the genus Lycodon in Southwest China and nearby countries for this paper
Morphological data
With the exception of total length,snout–vent length,and tail length,which were taken using a string and a ruler to the nearest 1mm,measurements were taken using a digital caliper to the nearest 0.1mm.Morphometric and pholidosis characters and their measurement/counting methods followed Wang et al.(2020a) and include:eye diameter (ED),head length (HL),head width (HW),snout–vent length (SVL),TaL(Tail length),total length (ToL); supralabial count (SL),infralabial count (IL),chin shield count (CS),preocular count(PrO),postocular count (PtO),loreal count (LoR),loreal entering orbit (LoR-E),temporal count (TMP),preventral scale count (PrV),ventral scale count (VEN),subcaudal count (SC),dorsal scale rows at one head length posterior to the neck(DSRH),dorsal scale rows at midbody (DSRM),dorsal scale rows at one head length anterior to the vent (DSRV),number of maxillary teeth (MT),body scale texture (BST; smooth vs.keeled),numbers of light bands on the dorsum (NDB; which excludes the collar-band on head),and numbers of light bands on the tail (NTB).All paired head pholidosis characters were given in the left/right order.Maxillary teeth formula are recorded as A-B-C format,where from left to right each letter represents the number of teeth in that specific tooth group from anterior end to posterior end of maxillary bone,and “-”indicates the presence of a gap.Hemipenis morphology was described based on Dowling & Savage (1960),and the color description followed Köhler (2012) for maximum comparability.
For SL,scale count was given in “A-B-C” format,where A is the number of anterior SL that do not enter the orbit,B is number of SL that enter the orbit,and C is the number of remaining SL that are posterior to and do not contact the orbit.For IL,scale counts were given in “A(B)” format,where A is the total number of IL,and B is the number of IL that are in contact with the anterior chin shield.For TMP,scale count was given in “A+B” format,where A and B are the number of anterior and posterior temporal scales,respectively.For posterior temporal scale count, paraparietal scale was included.
Additionally,the following morphological characters were also recorded:vertical eye diameter (VED),measured linearly between superior most and inferior most points of eye;distance between head and first body cross-band (DHB),measured between the posterior meeting point of parietal and the anterior edge of first dorsal cross-band along the vertebral line; position of first body cross-band (PBB),recorded as the number of the anterior most ventral scale at which the first body cross-band is located; paraparietal scale count (PPT),defined as the number of enlarged scales bordering the partial
scales on each side,excluding the anterior temporal and frontal scales; nuchal scale (NS),defined as the total number of small nuchal scales bordering the posterior end of parietal;presence or absence of collar-band of occipital head (NCB),were also recorded.

Table 1 Samples and their Genbank accession Nos.in the present study
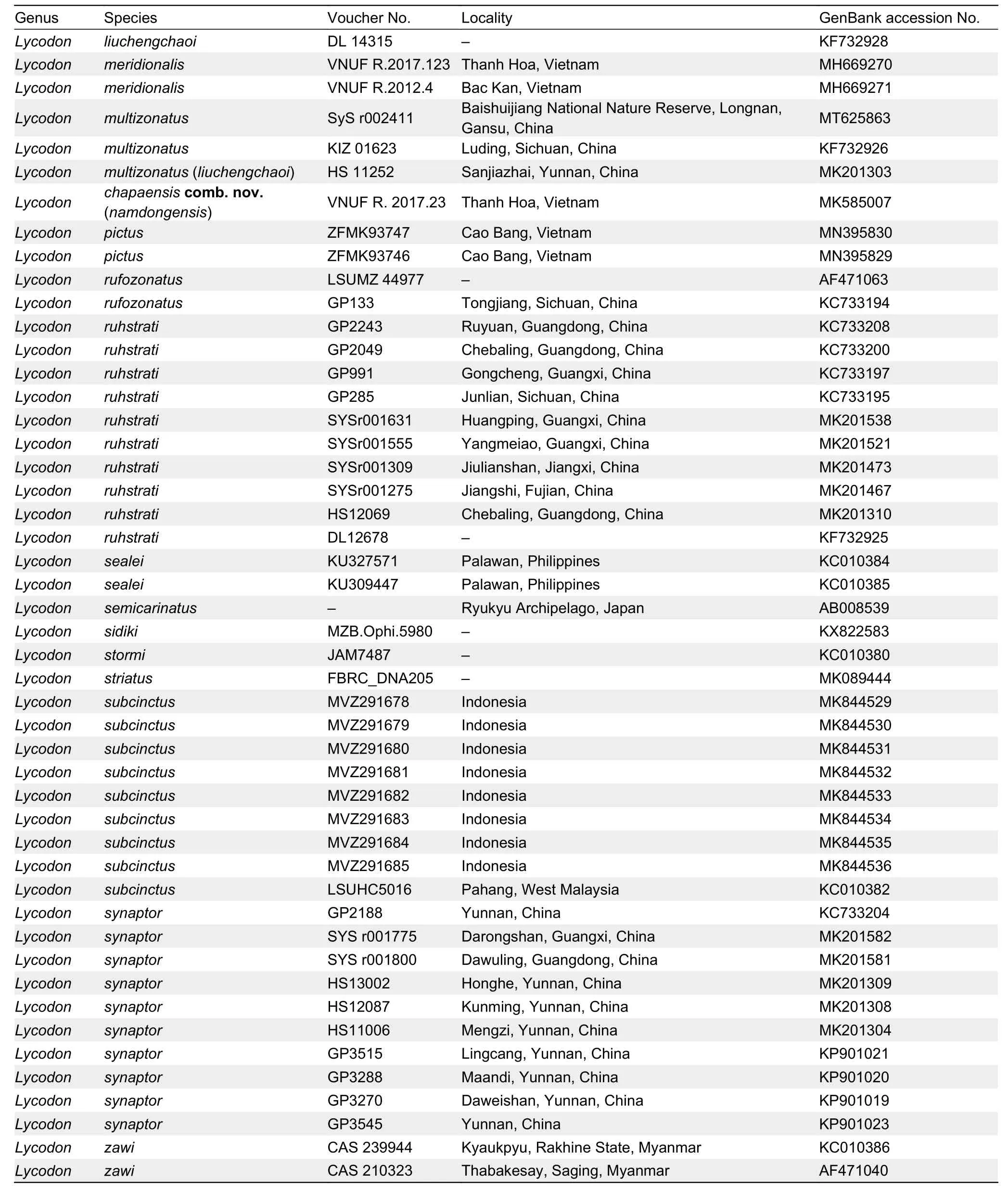
Continued
Genetic data
The genomic DNA was extracted from liver or muscle tissues with a standard three-step phenol-chloroform extraction method (Sambrook et al.,1989).The fragment of the mitochondrial cytochrome b (cytb) gene was targeted using published primers (Burbrink et al.,2000),and PCR and sequencing protocols followed Wang et al.(2020a).Data were filtered and trimmed manually using Geneious v.10.0,and the final sequence for alignment contains 1 117 bp,and all newly generated sequences were deposited in GenBank (accession No.MW353736– 353749; Table 1).
In addition, available sequences of congeners were downloaded from Genbank (Table 1).Boiga cynodonandDasypeltis atrawere selected as outgroups following previous phylogenetic studies (Lei et al.,2014; Siler et al.,2013).Sequences were edited and aligned using Geneious v.10.0.
Both maximum likelihood analyses (ML) and Bayesian inferences (BI) were conducted on the final cytbalignment.Partitioned Bayesian analyses were conducted using MRBAYES v.3.2.7a (Ronquist et al.,2012) on CIPRES (Miller et al.,2010).Sequence data was partitioned by three codon positions,and the best model of nucleotide substitution was selected for each partition by the Akaike Information Criterion(AIC),implemented in JMODELTEST2 v.2.1.10 (Darriba et al.,2012),which was GTR+Γ for all three partitions.Two independent Markov chain Monte Carlo analyses were run,each with four Metropolis-coupled chains.Bayesian analyses were run for 90 million generations,with parameters and topologies sampled every 1 000 generations.Stationarity and convergence were assessed with TRACER v.1.6.0 (Rambaut et al.,2013),and the first 20% of samples were discarded as burn-in.
Partitioned Maximum Likelihood analyses were performed using RAxML-VI-HPC v.8.2.10 (Stamatakis,2014) using the same partition strategy as for the Bayesian analyses.The most complex model (GTR+Γ) was applied for all the partitions,with 1 000 replicate ML inferences run.Each inference was initiated with a random starting tree,and nodal support was assessed with 1 000 bootstrap pseudoreplicates.Nodes having ML bootstrap values of 70 and above and BI posterior probabilities of 0.95 and above were considered well supported.Pairwise uncorrected genetic distances were calculated using PAUP v.4.0 b10 (Swofford,2002).
RESULTS
Molecular results
ML and BI yield overall similar topology,although some nodes have different level of supports (strongly supported in one but not in the other) (Figure 2).Overall,with addition of most available Indian and Southeast Asian taxa (i.e.,L.alcalai,L.chrysoprateros,L.dumerilii,L.jara,andL.zawi),our phylogeny shows similar topology as to recent studies for the well-supported nodes (Luu et al.,2019; Wang et al.,2020a)(Figure 2).Although the genusLycodonis still recovered as monophyletic,the current dataset could not resolve higher relationships among major clades (polytomy in BI and/or low bootstrap support <60 in ML).
The samples of “L.septentrionalis” from China and holotype ofL.namdongensistogether from a monophyletic clade(Clade A,0.93/93),with two distinct,genetically diverged groups recovered within this clade:first group includes the sample from southern Tibet,which is close to and in the same zoogeographic region of the type locality (i.e.,Khasi Hills in East Himalaya) ofL.septentrionalis; and the second group(Clade B, 1.00/100) contains the specimens of “L.septentrionalis” from western and southern Yunnan and the holotype ofL.namdongensis,withL.namdongensisnested within the Yunnan “L.septentrionalis” (Figure 2).The Yunnan populations of “L.septentrionalis” show minimal divergence from the holotype ofL.namdongensis(uncorrected genetic distance 0%–1.8%),but they have considerable divergences from the Tibetan population of trueL. septentrionalis(5.7%–7.4%) (Table 2).
The putative new species from northern HMR is recovered sister toL.multizonatuswith strong supports (Clade D,1.00/98),and it shows a considerable genetic divergence fromL.multizonatus(3.6%–4.0%).The previously reported sample of “L.liuchengchaoi” from Yunnan,China is nested withinL.multizonatus(1.00/99).Samples that are currently identified asL.fasciatusare polyphyletic,consisting of three major groups:the first well-supported group (1.00/100) contains samples trueL.fasciatusfrom Myanmar,southern Yunnan and western Yunnan,which is within the close proximity of the type locality of the species,and this group forms a strongly supported clade withL.butleri,L.gongshan,L.cavernicolus,andL.sidiki(Clade C; 1.00/100),although relationships within Clade C remain unresolved (Figure 2); the second group includes samples of “L.fasciatus” from Guangdong,which are nested withinL.liuchengchaoi(Clade E,1.00/100); and the third group includes the sample of the putative new species from Panzhihua,which forms a monophyletic group withL.synaptor(Clade G,1.00/100).These three groups are genetically diverged:the Guangdong samples of “L.fasciatus”are nearly identical toL.liuchengchaoi(≤0.2%) and show substantial genetic divergence from the trueL.fasciatusfrom Myanmar and Yunnan (9.4%–11.7%); the Panzhihua sample is also substantially diverged from the trueL.fasciatus(13.3%–13.6%),and it is also substantially diverged from its sister speciesL.synaptor(8.6%–9.5%; Table 2).
Morphological results and taxonomic conclusion
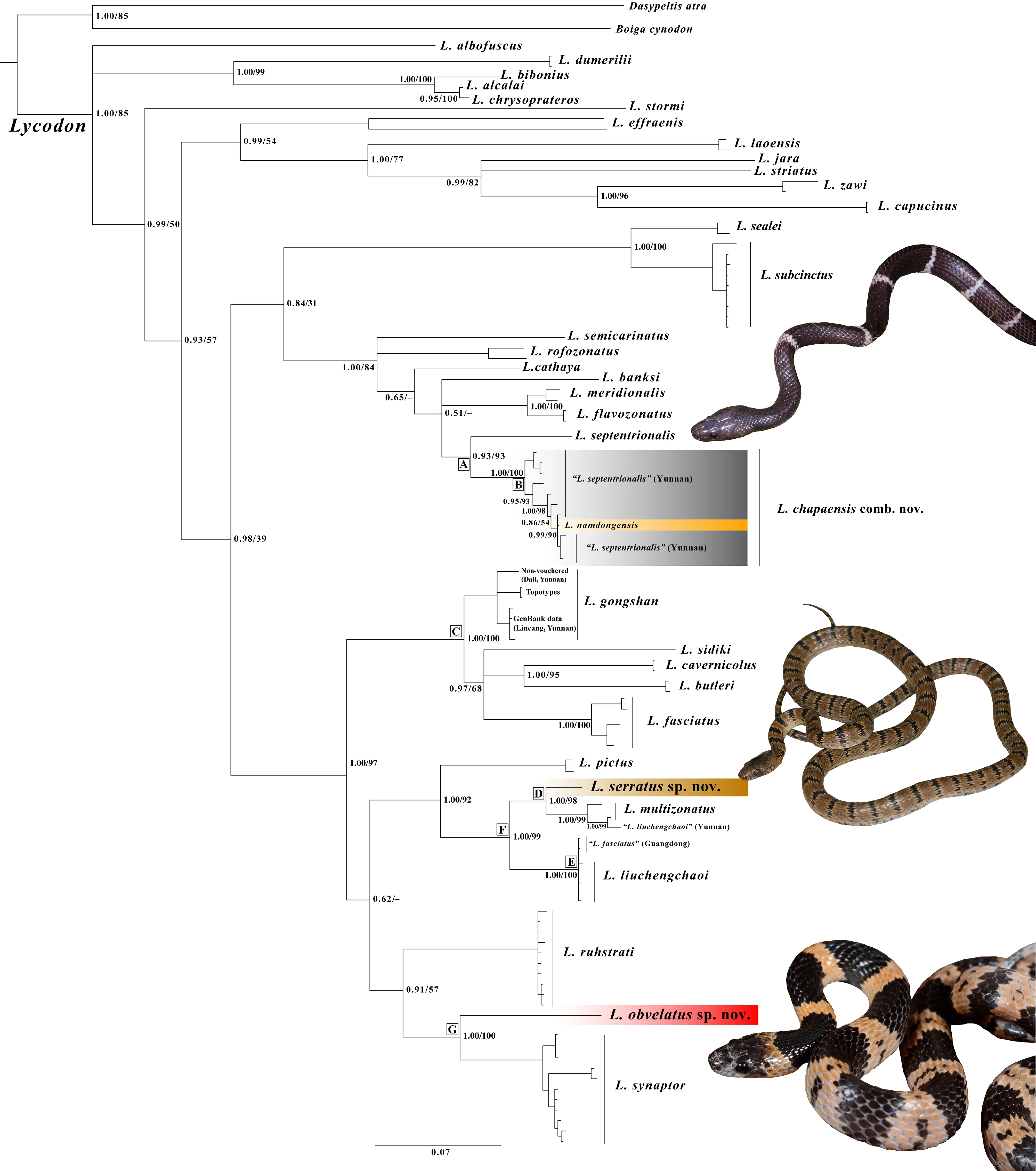
Figure 2 Phylogenetic trees of the genus Lycodon inferred by Bayesian analyses (BI) based on 1 117 bp of mitochondrial gene cyt b
All examined specimens of the currently identified “L.septentrionalis” from Yunnan have overlapping body sizes and tail ratios,same head pholidosis characters,similar dorsal pholidosis characters, and same body coloration and ornamentation with respect to the holotype ofL.namdongensisand the holotype ofDinodon septentrionale chapaense(Figures 3,5; Table 3).On the other hand,specimens ofL.septentrionalisfrom southern Tibet,which is close to its type locality,differs from the above Yunnan and Vietnam populations by having multiple rows of keeled dorsal scales (vs.smooth or only posterior vertebral row feebly keeled) and different number of maxillary teeth (8 vs.11 or 12).Such morphological differentiation suggests that the Yunnan population of “L.septentrionalis”,the holotype ofL.namdongensis, and the holotype ofD. septentrionale chapaenserepresent the same lineage,which is different from trueL.septentrionalis.
The specimen of the putative new species from the northernHMR is morphologically most similar toL.multizonatus(i.e.,coloration),but it shows morphological differentiations from the latter and all remaining recognized species,including different head shape,more IL,more DSRH,smooth DST,and distinct ornamentation patterns (details see comparison section in the taxonomic account below; Figures 4,5;Table 4).
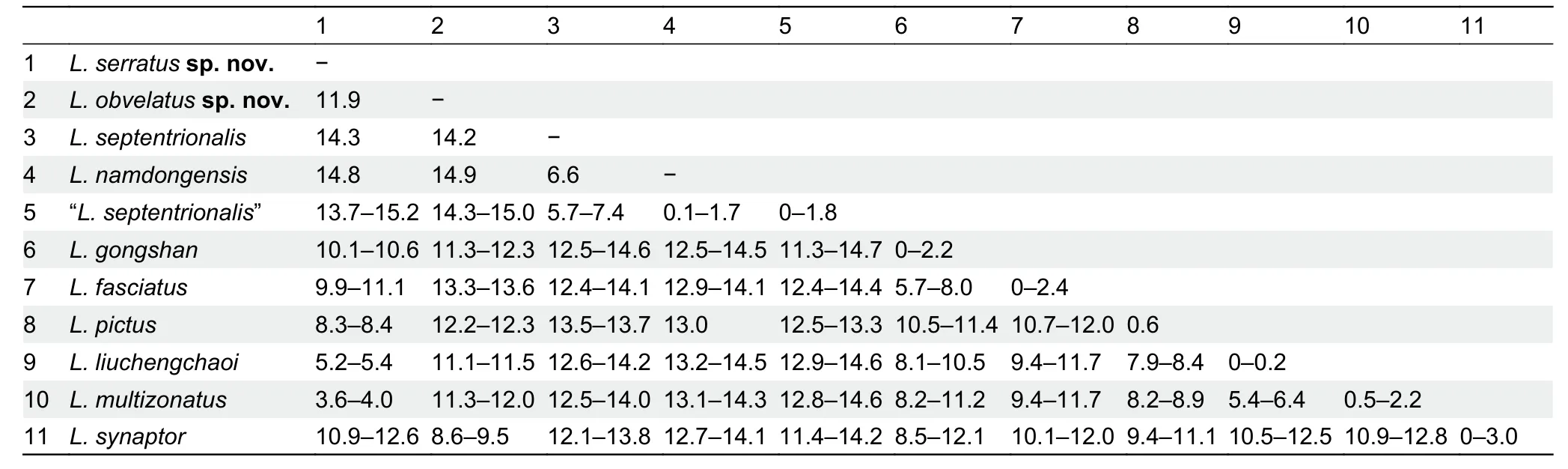
Table 2 Uncorrected genetic distance (%) based on 1 117 bp fragment of cyt b among selected members of the genus Lycodon
The specimen of “L.fasciatus” from Panzhihua in southern Sichuan Province is morphologically similar to the trueL .fasciatus,but it can be differentiated from the trueL.fasciatusreadily by having a smaller body size,smooth dorsal scales,fewer infralabials,and a distinct collar-band on neck in adult(details see comparison section in the taxonomic account below; Figures 4,5; Table 4).Additionally,the Panzhihua specimen differs from the type ofDinodon yunnanensis,which is currently considered as a junior synonym ofL.fasciatusbut was believed to be valid (Vogel & David,2010; details see comparisons in the taxonomic account below).
In conclusion,theLycodonspecimens from northern HMR and from Panzhihua represent two distinct evolutionary lineages that cannot be assigned to any recognized species.Hence we describe them as two new species.Additionally,populations of “L.septentrionalis” from Yunnan Province represent the same lineage asL.namdongensisandDinodon septentrionale chapaensefrom northern Vietnam,which are distinct and diverged from the trueL.septentrionalisfrom the East Himalaya both morphologically and genetically.We resurrectD.septentrionale chapaenseand elevate it as a full species,L.chapaensiscomb.nov.,and synonymizeL.namdongensisas its junior synonym.The distribution ofL.chapaensiscomb.nov.in Yunnan hence represents a new national record of reptilian fauna of China.
Taxonomic account
Lycodon chapaensiscomb.nov.(Angel,Bourret,1933)(Figures 3C–G,5F,G)
Proposed Chinese common name:沙坝白环蛇 (Pinyin:Sha Ba Bai Huan She)
Proposed English common name:Chapa Wolf Snake
Chresonyms:Dinodon septentrionale chapaenseAngel &Bourret,1933
Dinodon septentrionalisSmith,1943 (in part); He& Zhou,2000; Zhang et al.,2002
Dinodon septentrionale:Zhao & Yang,1997; Zhao et al.,1998; He & Zhou,2002; Zhao,2006; Yang & Rao,2008
Lycodon septentrionalis:Siler et al.,2013; Guo et al.,2013;Cai et al.,2015 (in part); Jiang et al.,2016; Wang et al.,2020bLycodoncf.septentrionalisYang et al.,2019
Lycodon namdongensisLuu et al.,2019
Holotype:MNHN-RA-1 933.001 1,adult female,from 20 km SW of Lao-Kay (=Lao Cai),Tonkin,Vietnam.Collected by Bourret R.on 01 July 1931.
Additional referred specimens:VNUF R.2017.23 (holotype ofL.namdongensis) from Nam Dong Nature Reserve,Thanh Hoa Province,Vietnam; KIZ 06753,female from Mengzi,Honghe Prefecture,Yunnan,China; KIZ 35113,male from Dulongjiang, Gongshan Prefecture, Yunnan, China; KIZ 035594,male from Yongping,Dali,Yunnan,China; KIZ 027593,male from Tengchong,Baoshan,Yunnan,China; KIZ 038282,male from Fugong,Nujiang Prefecture,Yunnan,China; and KIZ 035045,subadult female from Lushui,Gongshan Prefecture,Yunnan,China.
Diagnosis:Lycodon chapaensiscomb.nov.differs from congeners by a combination of the following characters:(1)body size large,ToL 691–1 114 mm; (2) tail length moderate,TaL 17.1%–20.5% ToL; (3) dorsal scale rows 17-17-15,mostly smooth,except the posterior vertebral row,which very feebly keeled; (4) VEN 200–225; (5) SC 74–84; (6) cloacal plate entire; (7) loreal short,not entering orbit; (8) SL 7 or 8,2-3-3,3-2-3,or 2-2-3; (9) IL 8–10,first 4 or 5 in contact with anterior chin shield; (10) preocular single,in contact with supraocular and prefrontal; (11) postocular 2; (12) temporal 2+2 or 2+3; (13) paraparietal much enlarged,single; (14)maxillary teeth 11 or 12,forming four distinct groups separated by three gaps (3-1-1-6 or 5-1-1-5),fourth and fifth tooth largest,about 2.5 times larger than first; first gap twice as wide as between the first two teeth; second gap largest,about four times as wide as between the first two teeth; third gap in same width as in first gap; (15) hemipenis single,not forked at tip,bulbous shaped,with medium sized spines on distal end of stem,and spinose and calyculate with spinulate ridges on bulb,apical nude; (16) dorsal Jet Black (Color 300)or dark Indigo (Color 190) in life,with 23–37 white crossbands on dorsum,11–16 on tail; (17) cross-bands with rather clearly defined edges,not serrated or only slightly serrated,single scale width dorsally,widen ventrolaterally; and (18)ventral white,with black transverse bands or irregular speckles.

Figure 3 Comparisons between true Lycodon septentrionalis (A,B); L.namdongensis (C),and Yunnan specimen of “D.septentrionalis”(D–G)

Figure 4 The dorsolateral view (1) and ventral view (2) of the holotype of Lycodon serratus sp.nov.(KIZ 038335) (A) and L.obvelatus sp.nov.in life (KIZ 040146) (B) (Photos by Wen-Jie Dong and Kai Wang)
Comparisons:Lycodon chapaensiscomb.nov.differs from trueL.septentrionalisby having smooth or only feebly keeled vertebral scale row on posterior body (vs.much more distinctively keeled on medial 3–5 rows),more maxillary teeth(11 or 12 vs.8),and different maxillary teeth formula (3-1-1-6 or 5-1-1-5,forth and fifth teeth largest,second gap widest,as four times of distance as in between first two teeth vs.4-2-2,last two teeth largest,two gaps about same length,as twice as in between first two teeth).
Additionally,L.chapaensiscomb.nov.further differs fromL.butleri,L.cavernicolus,L.davisonii,L.dumerilii,L.fasciatus,L. gibsonae,L. gongshan,L. gracilis,L.liuchengchaoi,L.multizonatus,L.nympha,L.orientalis,L.philippinus,L.pictus,L.sealei,L.sidiki,L.subcinctus,L.submaculatus,andL.tristrigatusby having loreal not entering orbit (vs.entering); fromL.albofuscus,L.aulicus,L.capucinus,L.flavicollis,L.flavomaculatus,L.hypsirhinoides,L.jara,L.kundui,L.laoensis,L.mackinnoni,L.meridionalis,L.stratus,L.tessellatus,L.tiwartii,andL.zawiby having a single cloacal plate (vs.divided); fromL.alcalai,L.banksi,L.cathaya,L.bibonius,L.cardamomensis,L.carinatus,L.chrysoprateros,L.davidi,L.effraenis,L.fausti,L.ferroni,L.flavozonatus,L.futsingensis,L.gammiei,L.kundui,L.muelleri,L.multifasciatus,L.rosozonatus,L.rufozonatus,L.ruhstrati,L.semicarinatus,L.solivagus,L.stormi,L.synaptor,L.travancoricus,L.zoosvictoriaeby having a larger maximum body size (ToL >1 000 mm vs.<1 000 mm); and fromL.paucifasciatusby having lower number of dorsal scale rows at midbody (17 vs.19); and fromL.ophiophagusby having a shorter tail (TaL 17.1%–18.4% vs.20.1%–22.8%).
Description of L.chapaensis comb.nov.based on holotype and referred materials:LargeLycodon,maximum ToL 1 114 mm; tail moderate,TaL 17.1%–20.5% ToL; head oval,rather wide,moderately distinct from neck; eye large,oval in shape,not laterally compressed.Rostral large,broader than height,pentagonal,visible from above; nasal divided,anterior half bordering rostral,first supralabial,and internasal,posterior half bordering first and second supralabials,loreal,internasal, and prefrontal; internasal paired, roughly rectangular,wider than long or subequal to,much smaller than prefrontals; prefrontal paired,hexagonal,bordering preocular,supraocular,and frontal posteriorly; loreal rather short,longer than wide,bean-shaped or spear-shaped,separated from orbit by preocular and third supralabial; preocular single,taller than wide; supralabials 8 (rarely 7),third to fifth or third and fourth entering orbit; postocular 2,superior one larger; anterior temporal 2,superior one longer and narrower,inferior one shorter and wider; posterior temporal 2 or 3 (including paraparietal); frontal pentagonal, spear-like tip pointing posteriorly; supraocular elongated; parietal paired,inlaying spear tip of frontal anteriorly,bordering supraocular and superior postocular anteriorly,paraparietal and 1–4 nuchal scales posteriorly; paraparietal single,much enlarged and elongated.Infralabials 8–10,anterior most pair enclosing mental and meeting medioposteriorly; 5 or 6 infralabials bordering chin shields,first to fouth or fifth bordering anterior chin shield,fifth or sixth bordering posterior chin shield,respectively; anterior chin shield much longer,forming Vshape,inlaying tip of first pair of infralabials anteriorly;posterior chin shields slightly smaller,not separated from each other by distinct mental groove.Dorsal body scales smooth,except vertebral row that only feebly keeled toward very posterior portion in some individuals; dorsal scale rows 17 at one head-length posterior to neck,17 at midbody,15 onehead length anterior to vent.Preventral 1 or 2; ventrals 200–225,angulate; cloacal plate entire; subcaudal paired,74–85 excluding tail tip.DHB 4.5%–7.9% SVL,PBB at 15th–22th ventral scale.

Figure 5 Comparisons of (1) dorsal overview,(2) ventral overview,(3) lateral head,(4) dorsal head,(5) ventral head,and (6) dorsum close-up among Lycodon serratus sp.nov.(KIZ 038335; holotype) (A),L.obvelatus sp.nov.(KIZ 040146; holotype) (B),L.multizonatus(KIZ 01623; topotype) (C),L.fasciatus (KIZ 74II0262) (D),L.gongshan (KIZ 730 034; holotype) (E),“L.septentrionalis” (KIZ 035594; from Dali,Yunnan,China) (F),L.chapaensis comb.nov.(MNHN-RA-1 933.001 1,holotype; from Chapa,Tonkin,Vietnam) (G),and L.septentrionalis (CIB 117 521; from Medog,Tibet,China) (H) (Photos of the holotype of L.chapaensis comb.nov.are obtained from the website of Muséum National d’Histore Naturelle,remaining photos by Zhong-Bin Yu and Jin-Long Ren)
Maxillary teeth 11 or 12,forming four distinct groups separated by three gaps.First three or five teeth in first group,gradually enlarged; single tooth in second and third group,respectively,both significantly enlarged (about 2.5 times of second tooth); remaining six or four teeth in last group,gradually decrease in size,eventually about the same size as first or second tooth.First gap twice as wide as regular width between first two teeth; second gap largest,about four times wider as regular; last gap about same as in first gap.Most teeth curved posteriorly towards tip,except first two or three.
Hemipenis morphology based of KIZ 027593:hemipenissingle,bulbous shape,with single sulcus spermaticus; rather short,reaching only fifth caudal scale from cloaca when everted,length unknown at retracted state; proximal 1/4 length with some shallow transverse flounces; middle 1/4 densely covered with medium sized spines; distal bulbous structure large,about 1/2 of total length,spinose toward basal end,gradually transition to calyculate with spinulate ridges toward 2/3 of bulbous,and eventually back to flounced toward very tip; apical nude (Figure 3).
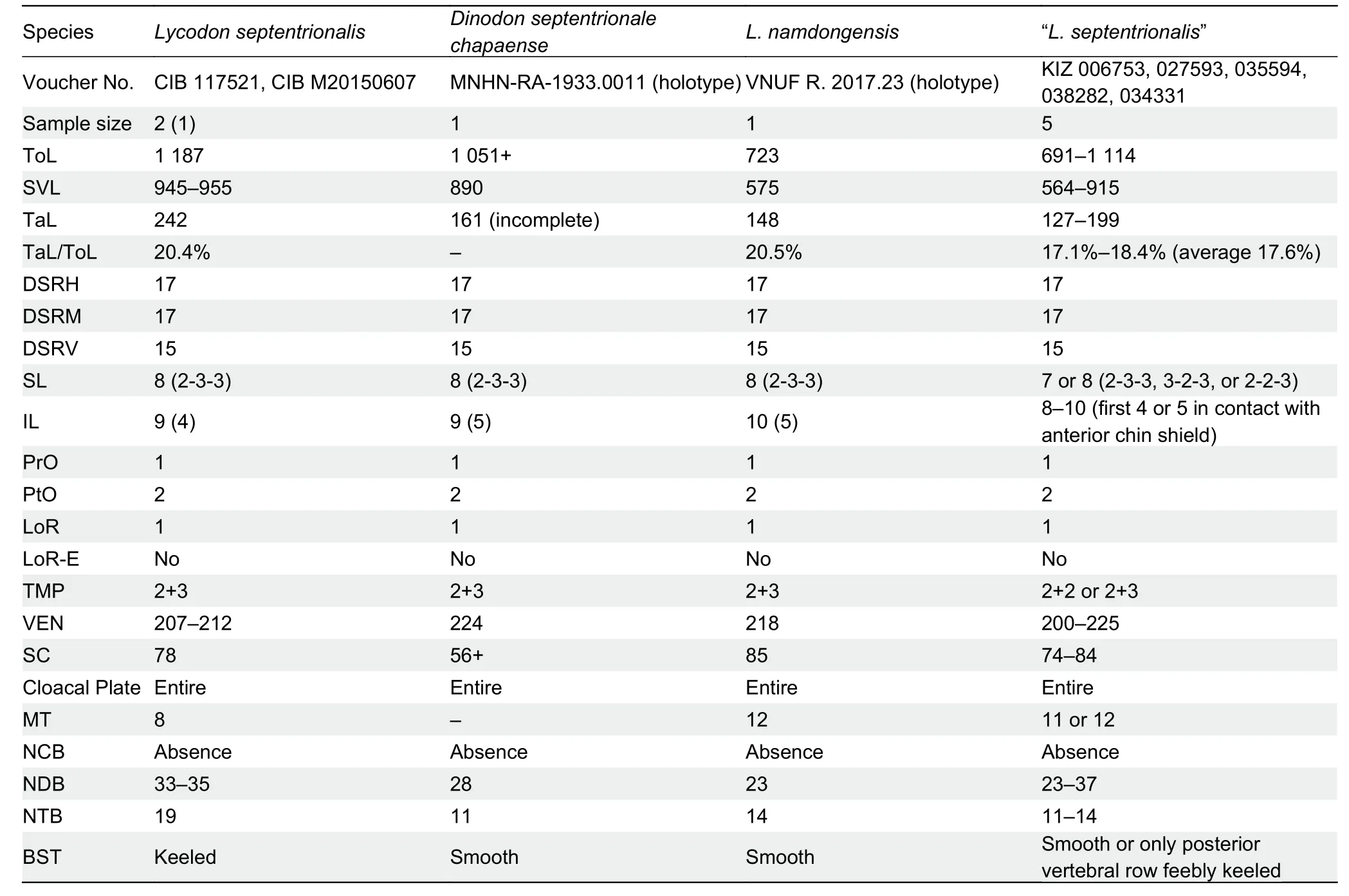
Table 3 Comparisons between holotype of Dinodon septentrionale chapaense,holotype of Lycodon namdongensis,true L.septentrionalis from Tibet,and “L.septentrionalis” from Yunnan Province
Coloration:The dorsal surfaces of the head and body are Jet Black (Color 300) or sometimes dark Indigo (Color 190).A single white collar-band is present on the occipital region of juveniles,but not in adults.White cross-bands are single-scale broad dorsally and widen into triangular shape ventrolaterally.A total of 23–37 cross-bands are present on the body and 11–16 on the tail.The ventral surface of the head and body is white,with some Medium Neutral Gray (Color 298) patches on the anterior infralabials and the gular region.The ventral surface of the body is white to Light Buff (Color 2),with Dark Neutral Gray (299) to Jet Black (Color 300) cross-bands,transverse groups of speckles,or random speckles.Ventral surface of the tail is nearly completely Dark Neutral Gray (299)to Jet Black (Color 300),with white to Light Buff (Color 2)cross-bands,transverse groups of speckles,or random speckles.
Natural history:Lycodon chapaensiscomb.nov.inhabits subtropical and tropical evergreen and sometime mixed forests (i.e.,with planted coniferous trees in Dali,Yunnan) at mid to low elevation (from 616 m at Nam Fong Nature Reserve,Quan Son District,Thanh Hoa Province,Vietnam,to 2 030 m at Dahaoping,Tengchong,Yunnan,China).The species is nocturnal,where all individuals were found at night actively foraging when collected in China.Unlike other congeners that feed heavily on reptiles,L.chapaensiscomb.nov.have been reported to feed mostly on rodents,and sometimes frogs (Yang & Rao,2008; Zhao & Yang,1997).Yang & Rao (2008) stated the specimens from Yunnan are often found in areas near agriculture fields where rodents are abundant,and individuals from Lushui in western Yunnan were observed actively hunting for rodents in village houses.
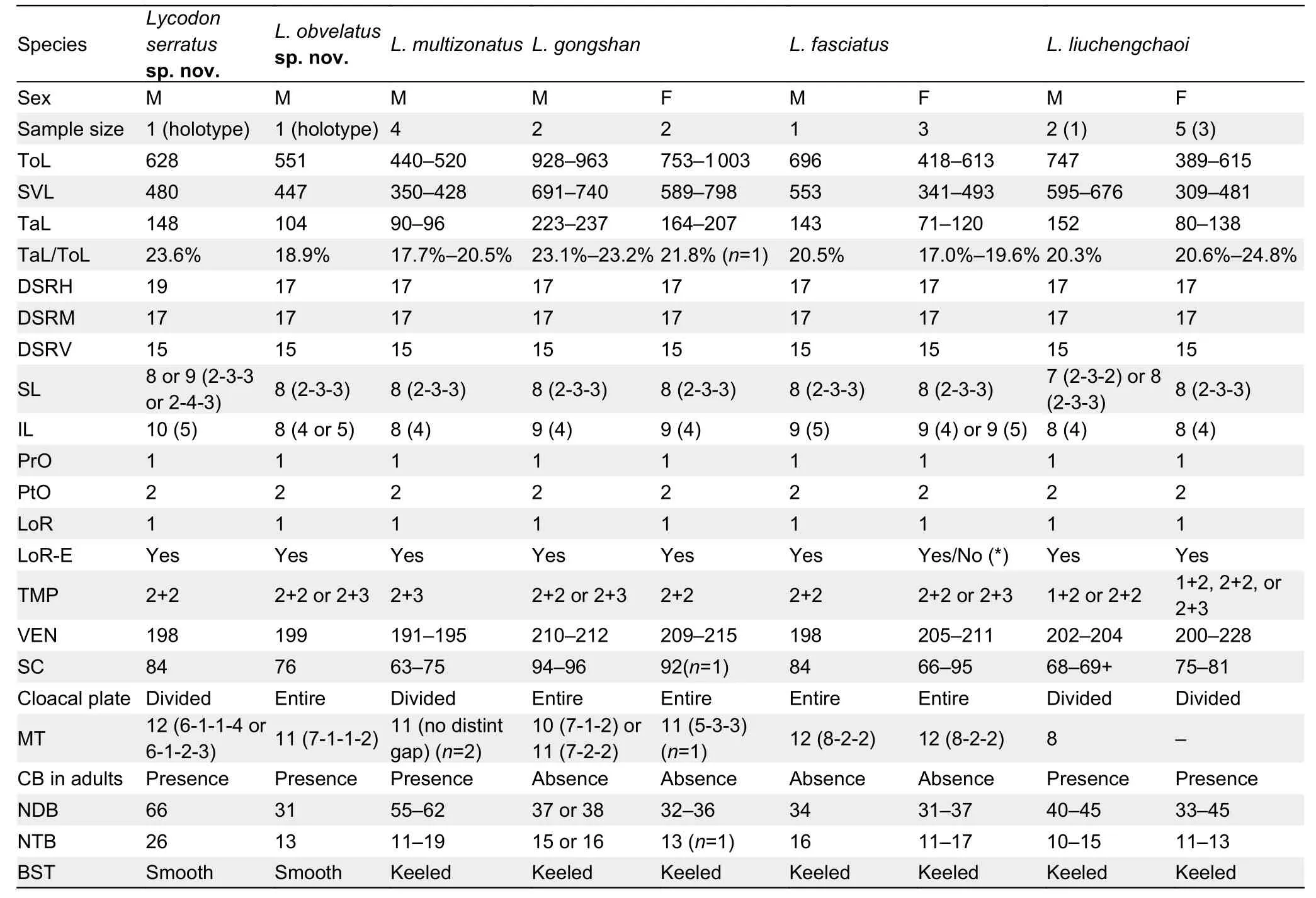
Table 4 Comparison of key morphological characters between the holotypes of Lycodon serratus sp.nov.,L.obvelatus sp.nov.,and morphologically similar congeners that are also found in the Hengduan Mountain Region (i.e.,L.gongshan,L.fasciatus,L.multizonatus,and L.liuchengchaoi)
CurrentlyL.chapaensiscomb.nov.has been recorded from western (Gaoligong Mountain Range,including Baoshan,Dali,Dehong Prefecture,and Nujiang Prefecture),south central (Puer and Lincang Prefectures),and southeastern(Honghe and Wenshan Prefectures) Yunnan Province in China and Lao Cai and Thanh Hoa Provinces in northern Vietnam (Luu et al., 2019). Based on the reported questionable records of “L.septentrionalis” in eastern Myanmar,northern Laos,and northern Thailand,it is likely that the species is also found in these countries as well (see Discussion below).
Lycodon serratussp.nov.(Figures 4A,5A; Table 2)
ZooBank LSID:355B3EDA-546E-417B-9E16-7BC92789DE81 Proposed Chinese common name:“锯纹白环蛇” (Pinyin:Ju Wen Bai Huan She)Proposed English common name:Serrate-banded Wolf Snake
Holotype:KIZ 038335,adult male,collected by Zhong-Bin Yu and Wen-Jie Dong on 25 July 2020 from the Jinsha River Valley near Geyading Village,Deqin County,northwest Yunnan Province,China (N28.7720º,E99.1128º,WGS84,elevation 2 200 m a.s.l.).
Etymology:The Latin species nameserratusmeans“serrated”,which describes the diagnostic narrow,serrated black cross-band of the new species.
Diagnosis:Lycodon serratussp.nov.can be diagnosed from recognized congeners by a combination of the following morphological characters:(1) body size moderate,slender,ToL 628 mm,SVL 480 mm; (2) tail long,TAL 23.6% ToL; (3)head flat,distinct from neck,snout narrow; (4) eye large,vertical ellipse in shape; (5) dorsal body scales smooth,19 rows at one-head-length behind the neck,17 rows at midbody,and 15 rows at one-head-length before vent; (6) ventral scale count 198; (7) subcaudal scale count 84; (8) cloacal plate divided; (9) supralabials 8 or 9,2-3-3 or 2-4-3; (10) infralabials 10(5); (11) preocular single,postocilar 2; (12) loreal scale entering orbit; (13) enlarged paraparietal 2,bordered by 7 additional nuchal scales other than posterior upper temporal and parietal; (14) maxillary teeth 12 in four groups (6-1-1-4 or 6-1-2-3),sixth and seventh much larger,first and second gap about same size,twice as distance between first two teeth;(15) dorsal surface dirty Tawny Olive (Color 17) with narrow,strongly serrated Jet Black (Color 300) cross-bands,two-scale broad middorsally at anterior 1/7 of body,single-scale broad for the remaining ones; (16) single collar-band on neck,66 cross-bands on dorsum,26 on tail; (17) first dorsal cross-band at 10th ventral scale; (18) anterior 1/3 of ventral surface uniform white,remaining 2/3 of ventral body and whole tail speckled with Dark Neutral Gray (Color 299).
Comparisons:Lycodon serratussp.nov.is morphologically most similar and closely related toL.multizonatus,where both species have divided cloacal plate,large eyes,and similar number of black bands across the body.However,the new species can be differentiated fromL.multizonatusby having more DSRH (19 vs.17),more IL (9 or 10 vs.8),more IL-aCS(5 vs.4),a flatter head that is distinct from the neck (vs.robust and indistinct),a narrower snout (vs.wide),much narrower black bands on the middle to posterior body (mostly single scale broad,rarely two vs.≥3 scales),more black bands on the tail (26 vs.11–19),and a distinct ventral ornamentation patterns (irregular speckles vs.regularly paired black spots or complete black bands) (Figure 5).
For remaining species that are found in the close proximity in the Hengduan Mountain Region (L.fasciatus,L.gongshan,L.liuchengchaoi,L.ruhstrati,L.chapaensiscomb.nov.,andL.synaptor),L.serratussp.nov.differs from all by having more DSRH (19 vs.17),smooth dorsal scales (vs.feebly or distinctively keeled medially),a narrow snout (vs.robust and wide),larger and laterally compressed eyes (ED 15.2% HL,VED 17.5% HL vs.not laterally compressed,<12%),more cross-bands on the body and tail (66 on body,26 on tail vs.L.fasciatus19–37 on body,7–21 on tail;L.gongshan32–40 on body,13–15 on tail;L.liuchengchaoi40–45 on body,10–15 on tail;L.ruhstrati33–46 on body,14–28 on tail;L.chapaensiscomb.nov.23–37 on body,11–16 on tail;L.synaptor30 or 31 on body,9 on tail),different-shape and width of the bands (narrow (mostly single-scale broad) and strongly serrated vs.broader (mostly two-to three-scale broad and less serrated) and less serrated),and a distinct body coloration (Tawny Olive (Color 17) with Jet Black (Color 300)bands vs.Jet Black (Color 300) with white or yellowish bands).Additionally,L.serratussp.nov.differs fromL.fasciatus,L.gongshan,andL.ruhstratiby having divided cloacal plate (vs.entire),presence of neck collar-band in adult (vs.absence),and a distinct ventral ornamentation pattern (randomly speckled vs.regular transverse bands (L.fasciatus,L.gongshan) or mostly uniform white (L.ruhstratiandL.chapaensiscomb.nov.)); from all butL.fasciatusandL.chapaensiscomb.nov.by having more IL-aCS (5 vs.4); from all butL.chapaensiscomb.nov.by having non-overlapping SC (84 vs.L.gongshan92–96;L.liuchengchaoi68–77;L.ruhstrati97–114;L.synaptor68 or 69); fromL.liuchengchaoiby having fewer VEN (198 vs.202–206); fromL.chapaensiscomb.nov.andL.synaptorby having loreal entering orbit(vs.separated) and a divided cloacal plate (vs.entire).For the junior synonym ofL.fasciatusthat is currently available,L.serratusdiffers fromDinodon yunnanensisWerner,1922 by more DSRH (19 vs.17),smooth or feebly keeled dorsal body scale rows (strongly keeled),a divided cloacal plate (vs.entire),and more cross-bands on the dorsum (66 vs.23).
For remaining three species that have genetic data and are in the same clade (i.e.,L.butleri,L.pictus,andL.cavernicolus),L.serratussp.nov.differs from all by having a distinct body coloration (Tawny Olive (Color 17) with Jet Black(Color 300) bands vs.Jet Black (Color 300) with white or yellowish bands).Additionally,the new species differs fromL.pictusby more DSRH (19 vs.17),a divided cloacal plate (vs.entire),more cross-bands on the body (66 vs.28 or 29) and tail (26 vs.13),much narrower cross-bands (mostly single scale-broad,rarely two vs.2–4 scale broad),and by the presence of collar band in adult (vs.absence); fromL.cavernicolusby having smooth dorsal scales (vs.keeled),fewer SL (8 vs.9 or 10),and more NDB (66 vs.36–45); and fromL.butleriby having smooth dorsal scales (vs.keeled)and a divided cloacal plate (vs.entire).
For all the remaining 55 species of the genus,L.serratussp.nov.differs from all by having a distinct dorsal coloration(Tawny Olive (Color 17) with Jet Black (Color 300),strongly serrated bands vs.black or brownish with white,yellow,or red cross-bands that are less serrated or smooth,or with no complete cross-bands but reticulated ornamentations).Additionally,L.serratussp.nov.differs from all except 18 species (i.e.,L.cardamomensis,L.carinatus,L.flavozonatus,L.futsingensis,L.hypsirhinoides,L.jara,L.laoensis,L.mackinnoni,L.meridionalis,L.nympha,L.orientalis,L.sealei,L.septentrionalis,L.sidiki,L.striatus,L.tessellatus,L.tiwarii,andL.zawi) by having a divided cloacal plate (vs.entire).For the excluded 17 species,the new species differs fromL.cardamomensis,L.carinatus,L.flavozonatus,L.meridionalis,L.nympha,L.sealei,andL.sidikiby having smooth dorsal scales (vs.keeled); fromL.futsingensis,L.hypsirhinoides,L.jara,L.laoensis,L.mackinnoni,L.striatus,L.tessellatus,andL.zawiby having more DSRH (19 vs.17); fromL.orientalisby the presence of preocular scale (vs.absence); and fromL.tiwariiby having fewer ventral scales (198 vs.218–237).
Description of holotype:KIZ 038335,Adult male,medium sizedLycodon,SVL 480mm,TaL 148mm.Body slender; tail long,TaL 23.6% of ToL; head elongated,flat,snout narrow,HW 9.7mm,HL 12.2mm,distinct from neck; eye large,slightly compressed laterally,ED 2.4mm,VED 2.8mm,ED 19.9% HL,VED 23.0% HL; pupil vertically oriented.Rostral pentagonal,broader than height,visible from above; nasal laterally elongated, divided, anterior one bordering rostral, first supralabial,and internasal,posterior one bordering first and second supralabials, loreal, internasal, and prefrontal;internasal pentagonal; prefrontal paired,hexagonal,larger than internasal; loreal elongated tear shape,entering orbit,bordering posterior nasal, prefrontal, second and third supralabials,and preocular; preocular single; supralabials 8/9,third to fifth entering orbit on left,third to sixth entering orbit on right; postocular 2; temporal 2+2 (including paraparietal),inferior one of first pair much larger; frontal pentagonal,spearlike tip pointing posteriorly; supraocular elongated; parietal paired in V-shape,inlaying spear-tip of frontal anteriorly,bordering supraocular and superior postocular anteriorly,paraparietal,and 7 nuchal scales posteriorly; paraparietal single, enlarged. Infralabials 10/10, anterior most pair enclosing mental and meeting medialposteriorly; anterior 5 infralabials bordering anterior chin shield on both sides,fifth and sixth bordering posterior chin shield on both sides; 2 pairs of chin shield,anterior pair much elongated,meeting medially,forming V-shape and inlaying meeting tip of first pair of infralabials anteriorly; posterior chin shield much narrower and shorter,separated from each other by rather wide section of mental groove.Dorsal body scales smooth,19 rows onehead-length behind neck,17 rows midbody,15 rows onehead-length before vent.Single preventral; ventral 198,angular; cloacal plate divided; subcaudal paired,84 excluding tail tip.DHB 10.4mm,2.1% SVL; PBB at 5th ventral scale.
Maxillary teeth 12 (fifth lost),forming 4 distinct groups separated by three gaps on both sides.Six teeth (first to sixth)in first group:first four gradually increase in size,followed by much enlarged sixth; single (seventh) tooth in second group,also much enlarged,same size as sixth; single (eighth) tooth in third group,same size as fourth; last four (ninth to twelfth) in last group,ninth and tenth same size as fourth,eleventh and twelfth same as second.Three gaps present between sixth and seventh teeth (about 1.8 times regular width),seventh and eighth (twice regular width),eighth and ninth (twice regular width).
The hemipenis only partially everted,single; very proximal end free of spines; remaining part filled with small to medium sized spines.
Coloration:In life,the background coloration of the dorsal and lateral surfaces of the head and body is Tawny Olive(Color 17).The dorsal surface of the head is speckled with Dark Neutral Gray (Color 299),particularly on the frontal and parietal scales.A single Jet Black (Color 300) collar-band on neck,66 cross-bands of the same color are present on the dorsum,and another 26 cross-bands are present on the tail.Cross-bands on the anterior 1/7 of SVL are broader,expending across two dorsal scales in width,and the remaining bands are rather narrow,expanding only a single dorsal scale in width.All bands are strongly serrated.The immediate bordering margins of each black band are Pale Buff(Color 1).Ventral surface of the head is white.The immediate bordering regions between infralabials and between infralabials and chin shields are speckled with Dark Neutral Gray (Color 299).The ventral surface of the body and tail is white:the anterior 1/7 of the SVL is uniform white with no patterns,and the remaining section of the ventral body and the tail is speckled with Dark Neutral Gray (Color 300),with the tail more heavily speckled.Coloration and ornamentation patterns remain mostly the same after short-term preservation(one month).
Distribution,natural history and conservation:BesidesL.multizonatus,L.serratussp.nov.is the only known species of the genus that inhabits hot-dry valley habitats at high elevation in the northern HMR.CurrentlyL.serratussp.nov.is known from the type locality in Yunnan Province only,however,an individual of the same species was photographed but not captured in Derong County of Sichuan Province,which is about 21 km linearly southeast from the type locality (personal communication with Mr.Di-Hao Wu).The habitats consist of open rock outcrops and low bushes,and the annual precipitation is very low (Figure 6A).While the distribution range of the species remains unknown,habitat destructions from road constructions were observed at and near the type locality of the new species (Wang et al.,2021).We recommend Data Deficient (DD) for the IUCN status of the new species,and we call for population studies to assess its conservation status in the near future.
The new species is sympatric withDiplodermasp.,Gekko scabridus,andScincella monticola(Wang et al.,2021; Yang &Rao,2008),and the holotype ofL.serratussp.nov.was found at night searching for food on a bush,where several individuals ofDiplodermasp.were sleeping on.As the genusLycodonis known to feed predominantly on lizards (Zhao et al.,1998),it is likely that these sympatric lizard species constitute main preys of the new species.Other herpetofauna that are sympatric with the new species includeElaphe carinata,E. taeniura,Protobothrops xiangchengensis,Amolops jingshaensis,Bufo gargarzans,andScutigersp..
Lycodon obvelatussp.nov.(Figures 4B,5B; Table 4)
Zoobank LSID:D15F4F07-FADF-43D5-94B8-EA746655727B
Chresonyms:Lycodon fasciatus:Deng et al.,1991; Wu et al.,1997; Zhao et al.,1998; Zhao,2002,2004,2006
Proposed Chinese common name:隐士白环蛇 (Pinyin:Yin Shi Bai Huan She)
Proposed English common name:Recluse Wolf Snake
Holotype:KIZ 040146,adult male,collected by Kai Wang and Ben-Fu Miao from Panzhihua City Park,Panzhihua,Sichuan,China (N26.5751º,E101.7174º,WGS84,elevation 1 243 m a.s.l.) on 19 April,2018.
Etymology:The Latin species name,obvelatus,means“hidden” or “concealed”,which not only describes the taxonomic confusions of the cryptic new species overL.fasciatus,but it also highlights the fact that new species can be hidden even in major urban areas.
Diagnosis:Lycodon obvelatussp.nov.can be diagnosed from congeners by a combination of the following characters:(1) body size small,ToL 551 mm; (2) tail moderate,TaL 18.9% ToL; (3) dorsal scale rows 17-17-15,all smooth; (4)VEN 199; (6) SC 76; (7) cloacal plate entire; (8) loreal long and narrow,entering orbit; (9) SL 8,2-3-3; (10) IL 8(4 or 5);(11) preocular single, in contact with supraocular and prefrontal; (12) postocular 2; (13) temporal 2+2 or 2+3; (14)paraparietal enlarged,single; (15) frontal bordering 4 nuchal scales; (16) maxillary teeth 11 in four groups (7-1-1-2),seventh largest,first gap widest,four times wide as distance between first two teeth; (17) hemipenis single clavate,nip at distal end,spinose except very proximal end; spines larger toward proximal end; (18) distinct collar band present on occipital head,Salmon Color (Color 251); (18) dorsal Jet Black(Color 300) in life,with 31 Salmon Color (Color 251) crossbands on dorsum,13 on tail; (19) cross-bands with serrated edges,2-or 3-scale broad medially,widen slightly toward ventrolateral side; (20) first dorsal cross-band at 4th ventral scale,DHB 14.1 mm; and (21) ventral pale Salmon Color(Color 251),with more or less regular black transverse bands and some irregular speckles.
Comparisons:The new species is morphologically most similar and was confused asL.fasciatus,but it can be differentiated from the latter by having smooth dorsal scales(vs.keeled),fewer infralabials (8 vs.9 in most individuals),and the presence of distinct collar band on head in adults (vs.absence) (Figure 5).Lycodon obvelatussp.nov.further differs fromDinodon yunnanensis,which is still considered the junior synonym ofL.fasciatusbut believed to be valid,by having smooth dorsal scales (vs.keeled),more ventral scale(199 vs.193),more dorsal cross-band on body (32 vs.23),and fewer supralabials (8 vs.9).
For species that are also similar toL.fasciatus,L.obvelatussp.nov.differs fromL.gongshanby having smooth dorsal scales (vs.keeled),fewer subcaudals (76 vs.92–96),and a smaller body size (ToL 551mm vs.maximum 963mm); fromL.liuchengchaoiby having smooth dorsal scales (vs.keeled),an entire cloacal plate (vs.divided),and fewer dorsal cross-bands(31 vs.≥40); fromL.pictusby having fewer ventrals (199 vs.212–218),presence of collar-band in adults (vs.absence),and a distinct coloration (dorsal Jet Black (Color 300),with Salmon Color (Color 251) cross-bands vs.dorsal Brick Red(Color 36) to Warm Sepia (Color 40),with dirty white cross bands); and fromL.synaptorby having loreal entering orbit(vs.separated from orbit by preocular),smooth dorsal scales(vs.keeled),and wider dorsal cross-band (2-or 3-scale broad dorsally vs.single scale broad).
Lycodon obvelatussp.nov.differs fromL.serratussp.nov.by having fewer infralabials (8 vs.10),fewer ASR (17 vs.19),far fewer dorsal cross-bands (31 on dorsum,13 on tail vs.66 on dorsum,26 on tail),a distinct coloration (dorsal Jet Black (Color 300) with Salmon Color (Color 251) cross-bands vs.dorsal dirty Tawny Olive (Color 17) with Jet Black (Color 300) cross-bands),and wider cross-bands (expending 2-or 3-scale wide dorsally vs.mostly single-scale broad) (Figure 3).
For remaining species,L.obvelatussp.nov.differs from all members of theL.ruhstratispecies group (L.cathaya,L.chapaensiscomb.nov.,L.futsingensis,L.multifasciatus,L.ophiophagus,L. paucifasciatus,L. ruhstrati, andL.septentrionalis) andL.alcalai,L.banksi,L.bibonius,L.cardamomensis,L.carinatus,L.chrysoprateros,L.davidi,L.ferroni,L.flavozonatus,L.gammiei,L.kundui,L.muelleri,L.rufozonatus,L.rosozonatus,L.solivagus,L.stormi,L.travancoricus,andL.zoosvictoriaeby having loreal entering orbit (vs.separated); fromL.effraenisby the presence of loreal scale (vs.absence); fromL.subannulatusby having more DSRH (17 vs.15) and DSRM (17 vs.15); fromL.albofuscus,L. aulicus,L. capucinus,L. flavicollis,L.flavomaculatus,L.hypsirhinoides,L.jara,L.laoensis,L.mackinnoni,L.meridionalis,L.multizonatus,L.nympha,L.orientalis,L.sealei,L.sidiki,L.striatus,L.subcinctus,L.tessellatus,L.tiwarii,andL.zawiby having an entire cloacal plate (vs.divided); fromL.anamallensisby fewer temporals(2+2 or 2+3 vs.3+4); and fromL.philippinusby more MT (11 vs.8) and fewer ventral scales (199 vs.216–225).
Description of holotype:KIZ 040146,adult male,medium sizedLycodon,ToL 551 mm,SVL 447 mm.Body slender,tail moderate,TaL 18.9% ToL; head moderate,flat,snout narrow,HL 11.3 mm,HW 9.4 mm,distinct from neck; eye large,not laterally compressed,ED 2.1 mm,18.6% HL; pupil vertically oriented.Rostral pentagonal,broader than height,slightly visible from above; nasal divided,anterior half rectangular,small,bordering rostral,first supralabial,and internasal,posterior half hexagonal,much larger,bordering first and second supralabials, loreal, internasal, and prefrontal;prefrontal paired,hexagonal,much larger than internasal,separated from orbit by preocular; loreal much elongated,entering orbit,bordering posterior nasal,prefrontal,second and third supralabials, and preocular; preocular single;supralabials 8/8,third to fifth entering orbit; postocular 2;temporals 2+3/2+2,inferior one of first pair much larger;frontal pentagonal, spear-like tip pointing posteriorly;supraocular elongated; parietal paired in V-shape,relatively wide, inlaying spear-tip of frontal anteriorly, bordering supraocular and superior postocular anteriorly,paraparietal,and four smaller nuchal scales posteriorly; paraparietal single,enlarged.Infralabials 8/8,anterior most pair enclosing mental and meeting medioposteriorly; anterior 5 infralabials bordering anterior chin shield on left,4 on right; fifth and sixth bordering posterior chin shields on left,fourth and fifth on right; two pairs of chin shield,anterior pair wider,meeting medially,forming Vshape and inlaying meeting tip of first pair of infralabials anteriorly; posterior chin shields much narrower and elongated,separated from each other by rather wide section of mental groove.Dorsal body scales smooth,17 rows onehead-length behind neck,17 rows midbody,15 rows onehead-length before vent.Single preventral; ventral 199,angular; cloacal plate entire; subcaudal paired,76 excluding tail tip.DHB 14.1 mm,3.1% SVL,PBB at 4th ventral scale.
Maxillary teeth 11,forming 4 distinct groups separated by three gaps.Seven teeth in first group:first five gradually increase in size,followed by much enlarged sixth and seventh;smaller eighth tooth in second group,same size as third; ninth tooth in third group,same size as eighth; last two (tenth and eleventh) in last group,same size as fifth.Three gaps present,namely between seventh and eighth teeth (largest,about 4 times regular teeth width),eighth and ninth (1.5 times regular width),ninth and tenth (twice regular width).
Hemipenis only partially everted,single clavate,nip at distal end,spinose except very proximal end; spines enlarged toward proximal end; very proximal end free of spines.
Coloration:In life,the dorsal and lateral surfaces of the head are Jet Black (Color 300),except the anterior portion of the head:the internasal,prefrontal and anterior frontal are speckled with Pale Neutral Gray (Color 296); and the posterior half of nasal,loreal,and first four supralabials are nearly uniform Pale Neutral Gray (Color 296).A distinct collar band on occipital region of the head,dirty Salmon Color (Color 251).Dorsal surface of the body is Jet Black (Color 300).Salmon Color (Color 251) cross-bands are present on the dorsal and lateral surfaces of body and tail.Cross-bands have jagged edges,and they are two-to three-scale broad dorsally and are further widen ventrolaterlly.A total of 31 cross-bands are present on the body,and 13 are on the tail.Starting at the fifth cross-bands from the head,most Salmon Colored (Color 251)cross-bands of the body has a transverse row of black speckles running through the middle,some of which even forms a narrow and almost complete black transverse streak(i.e.,in number 20 and 21 cross-bands from the head).The ventral surfaces of the head,body,and tail are pale Light Flesh Color (Color 250) to white.Anterior five infralabials,mental,and anterior portion of the anterior chin shields are Medium Neutral Gray (Color 298).Dark Neutral Gray (Color 299) to Jet Black (Color 300) cross-bands,transverse groups of patches,or irregular speckles are present on the ventral body,with the anterior nine cross-bands clearly defined.A total of twelve Jet Black (Color 300) cross-bands are present on ventral tail.
The ornamentations remain the same after two-year of preservation,but coloration fades away.Specifically,the Salmon Color (Color 251) of dorsal cross-bands becomes pale Light Flesh Color (Color 250),and the ventral color becomes almost Light Buff (Color 2).
Distribution,natural history,and conservation:AlthoughL.obvelatussp.nov.is currently only known from the Panzhihua City Park,it is possible that the new species is also found in the nearby regions in Panzhihua and in the adjacent northcentral Yunnan Province (i.e.,in Yongren County).The habitat consists of both natural and horticultural plants of both deciduous and evergreen species,and roads and other tourist infrastructures fragmented the habitats (Figure 6B).The holotype was found actively hunting for geckos on a stone parapet at night.Other reptiles that are sympatric in the city park includeNaja kaouthia,Ptyas nigromarginata,Elaphe taeniura,Achalinussp.,Pareassp.,Indotyphlops braminus,Diploderma dymondi,Gekkosp.,Hemidactylus bowringii,andSphenomorphus indicus; and amphibian includesKaloula verrucosa,Polypedatessp.,Odorrana grahami, andDuttaphrynus melanostictus.
Although the type locality is at the center of a major city(about 10.8 million people),the oasis in the city park provides habitats for a surprisingly diverse group of reptiles and amphibians.The natural habitats around the Panzhihua City have been deforested in the mid 1900s,and the selfrecovering process of the fragile valley ecosystem is particularly slow.The City Park of Panzhihua preserved few of the remaining natural montane evergreen forests in the area,which provide important habitats for local wildlife.The discovery of the new species highlights the conservation importance of the remaining habitats in the city park.Unfortunately,the current maintenance practice of the park is not ecofriendly, with rapid developments for tourist infrastructure,replacements of native plants with exotic horticultural plants,and the wide usage of pesticides.We recommend the park modify its current practices and conserve the remaining natural habitats for the native wildlife.
DISCUSSION
Additional cryptic diversity in the northern HMR
The discovery of our two new species supports the notion that the reptilian diversity in the northern HMR is still underestimated.As the suitable habitats of reptiles (i.e.,hotdry valleys) in the HMR are isolated and fragmented by continuous mountain ranges over 4 000 m of elevation,populations in different river valleys are allopatric to each other,despite the short linear distance among them (Figure 1).Therefore,it is likely that nearby valleys along the upper Mekong,Salween,and Yalong Rivers also harbor additional undiscovered diversity of the genusLycodon.Further surveys are needed to better inventory of the reptilian diversity and assess their conservation statuses in the northern HMR.
Problematic records of Lycodon species in China and SE Asia
Lycodon fasciatus and L. liuchengchaoi:For the recognized species of the genusLycodonin China,great confusions exist in published literature regarding the taxonomic identification and the resulting distribution range,particularly forL.fasciatus(Vogel & David,2010; Vogel & Luo,2011).Much similar to other groups of reptiles from the HMR that represent species complexes (i.e.,Gloydius strauchi,Diploderma flaviceps; Shi et al.,2018; Wang et al.,2019a,2021),L.fasciatuswas and still is considered as a widespread taxon,despite increasing evidence suggesting the existence of cryptic diversity (Vogel & David,2010; Vogel & Luo,2011;Zhang et al.,2011b).As the results of taxonomic confusions,misidentifications and erroneous records of species are prevalent in literature.
Kang et al.(2009) reportedL.fasciatusas a new record of snake in Hunan Province based on specimens from Hupingshan Nature Reserve.Later Bai et al.(2018) reportedL.liuchengchaoifrom the very same nature reserve.Closer examination of the corresponding descriptions reveals that the referred specimens by Kang et al.(2009) and Bai et al.(2018)both possess a divided cloacal plate,which matches the diagnosis ofL.liuchengchaoibut notL.fasciatus(Zhang et al.,2011b).In addition to the presence of a yellow collar-band on the neck in figures of both Kang et al.(2009) and Bai et al.(2018),which again contradict to the diagnosis ofL.fasciatusbut align withL.liuchengchaoi,it is clear that the previous record ofL.fasciatusfrom Hunan Province by Kang et al.(2009) represent a misidentification ofL.liuchengchaoi.

Figure 6 The habitats at the type locality of Lycodon serratus sp.nov.near Geyading Village,Deqin County,Yunnan Province,China (A) and L.obvelatus sp.nov.in Panzhihua City Park,Panzhihua,Sichuan,China (B) (Photos by Zhong-Bin Yu and Ben-Fu Miao)
Li et al.(2012) first recordedL.fasciatusfrom Guangdong Province,and the authors stated that the tail length of Guangdong specimens is 24.8%–25.8% of the total length in sub-adults,which are much longer than the trueL.fasciatus(≤22.5%; Vogel & Luo,2011; Table 4).Later Guo et al.(2013)provided the genetic data ofL.fasciatusfrom Guangdong,but at the time there is no genetic data from topotypicL.fasciatusto compare against.Recently,Peng et al.(2018) reportedL.liuchengchaoifrom Guangdong based on morphological and molecular evidence of cytbgene,and the authors stated that the cytbdata of their specimens ofL.liuchengchaoifrom Guangdong is nearly identical to the published sequence ofL.liuchengchaoion GenBank and share the same haplotype with previously published data ofL.fasciatusfrom Guo et al.(2013).As results,Peng et al.(2018) confirms the previous record ofL. fasciatusin Guangdong represents misidentification ofL.liuchengchaoi.However,Peng et al.(2018) did not submit their new data to GenBank,nor did they conduct phylogenetic analyses of the mentioned samples.
Our phylogenetic study of available sequences supports the conclusion by Peng et al.(2018),where the Guangdong samples of “L.fasciatus” from Guo et al.(2013) are nested within available data ofL.liuchengchaoi; and with the newly available topotypic samples ofL.fasciatus,the Guangdong samples are confirmed to be paraphyletic with respect to the trueL.fasciatusfrom Yunnan (Figure 2).In addition to our revision of “L.fasciatus” in Panzhihua,it is clear that the current records of “L.fasciatus” outside of Yunnan Province in China (i.e.,in Anhui,Gansu,Guizhou,Hubei,Shaanxi,and Zhejiang; Zhao et al.,1998) are distant from the range of the trueL.fasciatus,and these questionable records likely represent either misidentifications of recognized congeners,or additional cryptic diversity that warrant further investigations.Future studies should focus on confirming the taxonomic statuses of the questionable records of “L.fasciatus” outside of Yunnan Province in China.Currently,L.fasciatussensu stricto has been confirmed in Yunnan Province of China and Myanmar (Vogel & Luo,2011; present study).
RegardingL.liuchengchaoi,Li et al.(2020) reported a sample of “L.liuchengchaoi” from “Sanjiazhai” in Yunnan Province,which would expand the distribution range of the species further southwestward and represents a new record of herpetofauna of Yunnan Province.However,our phylogeny shows that the referred sample of “L.liuchengchaoi” by Li et al. (2020) is phylogeneticlly distinct from the trueL.liuchengchaoi,and it actually represents a misidentification ofL.multizonatusinstead (Figure 2).With this correction of taxonomy,this record in Yunnan still represents a range extension of theL.multizonatusand a new record of Yunnan Province.However,Li et al.(2020) did not provide complete information regarding the county or prefecture of the locality name “Sanjiazhai”.As multiple localities in Yunnan Province are under this very same name,the distribution ofL.multizonatusin Yunnan remains unknown.Future studies should verify the record.
For the remaining confirmed record ofL.liuchengchaoi,it is important to note that there are considerable discrepancies of key morphological characters between the type series of the species and the later reported records in China,particularly regarding the number of dorsal cross-band and the state of cloacal plate (Peng et al.,2018; Zhang et al.,2011b).Future population-level studies are needed to better understand the morphological variation and diagnosis ofL.liuchengchaoi.
Lycodon ruhstrati in Yunnan Province:Guo et al.(2007)first reportedL.ruhstratias the new record of reptilian fauna of Yunnan Province from the Gaoligong Mountains in far western Yunnan.However,examination of the description and photos by Guo et al.(2007) reveals that all three referred specimens by Guo et al.(2007) do not agree with the diagnosis ofL.ruhstrati:the first specimen (HNU 200 505 001) has a much shorter tail (TaL/ToL 18.7%),fewer ventral scales (VEN 203),fewer subcaudal scales (SC 68),and distinctively banded ventral surface of the body throughout (vs.in trueL.ruhstrati,TaL/ToL 20.8%–24.8%,VEN 214–233,SC 90–116,and ventral body either uniformly colored or speckled without distinct cross-bands; Vogel et al.,2009); and the remaining two specimens (HNU 200 505 002 and 200 609 001) both have loreals entering orbits (vs.in trueL.ruhstratinot entering orbit;Vogel et al.,2009; Zhao et al.,1998).Furthermore,the later two specimens have much longer tails (TaL/ToL 21.5–25.5%)and more subcaudals (92–94) than the first specimen.Therefore,even based on the reported morphological data by Guo et al.(2007) alone,it is clear that the three referred specimens are neither trueL.ruhstrati,nor do they even represent the same taxa:HNU 200 505 001 is similar toL.chapaensis,while HNU 200 505 001 and 200 609 001 matches diagnosis ofL.gongshan.
A year after Guo et al.(2007),Yang & Rao (2008) also recordedL.ruhstratifrom Yunnan.This time the record is based on a different vouchered specimen,which has no detailed locality information (KIZ 8 300 012,“from Yunnan”;Yang & Rao,2008).Unfortunately,we could not locate the referred specimen at KIZ (possibly lost),but upon review of the description by Yang & Rao (2008),we found that the specimen does not agree with the diagnosis of the trueL.ruhstrati,including having fewer SC (81 vs.90–116),different dorsal scale texture (feebly keeled vs.distinctively keeled),and by the presence of white collar-band on neck (vs.absence in adults).Therefore,based on the current published data,all reported voucher specimens of “L.ruhstrati” from Yunnan do not agree with the diagnosis of trueL.ruhstrati,and there is no evidence confirming the presence ofL.ruhstratiin Yunnan Province as of to date.
Lycodon gongshan in Yunnan and Sichuan:Lycodon gongshanwas described based on morphological characters only,and the type series was collected from far western Yunnan Province in the Dulongjiang Valley and adjacent Nujiang valley (Vogel & Luo,2011).Later Guo et al.(2015)recorded the species from Lincang,Southwestern Yunnan Province and provided genetic data of the species.Our newly collected topotypic materials from Dulongjiang confirm the taxonomic identification by Guo et al.(2015) (Figure 2;Table 2).Furthermore,our phylogenetic analyses confirm that our non-vouchered genetic sample from Yunlong Nature Reserve in Yunlong County,Dali is alsoL.gongshan,which expand its range eastwards (Figure 1).
Although our results expand the range ofL.gongshanfurther eastwards,the species is still endemic to Yunnan only,and the existing records of the species in Sichuan Province require further confirmation.Chen et al.(2018a) recordedL.gongshanbased on two specimens from Hongbao Village and Daheishan National Forest in Panzhihua,Sichuan.However,the images that Chen et al.(2018a) provided show obvious difference from the type series ofL.gongshanin terms of ornamentation pattern,and the recorded numbers of dorsal cross-bands do not match with the bands of the actual specimen in the photographs.Based on morphological data alone,we cannot assign these two specimens to our new speciesL.obvelatusfrom Panzhihua City (i.e.,Hongbao individuals have keeled dorsal scales,where dorsal scales ofL.obvelatusis smooth).It is likely that there are two species ofLycodonin Panzhihua,similar to the genusDiploderma(i.e.,D.dymondiis found in Panzhihua City,whereD.swildis found in Hongbao Village; Wang et al.,2019b),but whether the Hongbao population represents morphological variation ofL.gongshanor a distinct new species would require future confirmation.
Remaining records of “L. septentrionalis” in SE Asia and validity of L.ophiophagus:Lycodon septentrionalishas been recognized to have a wide distribution range,from the Himalaya (i.e.,India (Boulenger,1893; Smith,1943) and Bhutan (Tshewang & Letro,2018)) across Myanmar (Dowling& Jenner,1988) and Yunnan of China (Zhao et al.,1998;Zhao,2006) to Southeast Asia (including Vietnam (Smith,1943; Van Sang et al.,2009),Laos (Deuve et al.,1961),and Thailand (David et al.,2004)).Similar to the above-discussed congeners that also have wide distribution ranges,the current records ofL.septentrionalislikely contain misidentifications of different lineages,particularly in Southeast Asia.With our resurrection ofL.chapaensis,it leaves the remaining records ofL. septentrionalisin Laos, Vietnam, and Thailand questionable.The taxonomic position of the Southeast Asian populations of “L.septentrionalis” should be reconsidered in future studies.
Additionally, our morphological comparison shows overwhelmingly similar morphology betweenL.chapaensisandL.ophiophagus.The only differences are the relative tail length (17.1%–20.5% inL.chapaensisvs.20.1%–22.8% inL.ophiophagus) and number of subcaudal scales (74–85 vs.87–90).However,given the small sample size (n=2) and the lack of molecular data ofL.ophiophagus,we cannot conclude on its taxonomic validity.Future integrative taxonomic studies are needed to confirm the validity ofL.ophiophaguswith respect toL.chapaensis.
Records of L.aulicus and L.capucinus in China:Owning the nearly indistinguishable morphology and the lack of genetic materials from topotypic individuals,taxonomists have not reached agreements regarding the validity ofL.capucinus:whether it is junior synonym ofL.aulicus,valid but only as a subspecies,or valid as a full species (O’Shea et al.,2018;Ota,2000; Siler et al.,2013; Wostl et al.,2017).Although the overall distributions ofL.capucinusandL.aulicushave been relatively consistent in literature (L.aulicusis from South Asia,whereL.capucinusis from Southeast Asia,and both species are hypothesized to be sympatric in Myanmar; David & Vogel,1996; Lanza,1999; Smith,1943),the distribution of both species near the hypothesized contacting region remain unclear,particularly in China (O’Shea et al.,2018).
While consideringL.capucinusas a subspecies ofL.aulicus,bothL.a.capucinusand the nominate subspeciesL.a.aulicushave been recorded from Hong Kong (Pope,1935;Romer,1979).Most of the later authors did not consider the subspecies or species status ofL.capucinus,and onlyL.aulicushave been recorded from China,with its distribution ranging from southwestern Yunnan,Fujian,to Guangdong Provinces (Wang et al.,2020b; Zhao & Adler,1993; Zhao et al.,1998; Zhao,2006).In contrary,Zhang et al.(2011b) only recordL.capucinusfrom China,without discussing the past record ofL.a.aulicusfrom Hong Kong (Pope,1935) or the possible distribution ofL.aulicusfrom the Myanmar border regions in Southwest Yunnan.
Images of live individuals of theL.aulicus-capucinuscomplex from China-Myanmar border in Yunnan and from Hong Kong show nearly identical ornamentation patterns(Figure 7),which matches the current diagnosis ofL.capucinus(O’Shea et al.,2018).Unfortunately,vouchered genetic materials of theL.aulicus-capucinuscomplex from China and from the type localities of the two corresponding names are still unavailable to date.Given theL.aulicuscapucinuscomplex is known by its profound variability in ornamentation patterns (O’Shea et al.,2018),we cannot determine the taxonomic identity of the Chinese populations with confidence.Based on the current diagnosis of both species,we here consider the Chinese populations asL.capucinus,and we propose to maintain its Chinese common name as 白环蛇.Later taxonomic studies are needed to further verify the validity ofL.capucinusand confirm the identity of the related Chinese populations.
Updated key and distribution of the genus Lycodon in China:To facilitate future taxonomic studies of the genusLycodonin China,we provide an updated dichotomous key and the distributions of the 20 recognized species ofLycodonspecies in China.The distribution data are based of Zhao et al.(1998) and are further modified with new findings in this present study and additional literatures published after 1998(Appendix III).“?” indicates possible but not yet confirmed records based on photographic evidence or published sequences with vague locality and no morphological data; and“!” indicate possible erroneous records that warrant future confirmations.“Dorsal background coloration” is defined as the same coloration of the dorsal surface of the head.
Key to the species of Lycodon in China:
1a) Dorsal background coloration yellowish brown,dark brown,or reddish brown; dorsal cross-bands Jet Black(Color 300),relatively narrow and serrated,not widen towards ventrolateral sides; cloacal plate divided; loreal entering orbit....................................................................2
1b) Dorsal background coloration blackish,with white,gray,yellowish,pinkish,or reddish dorsal cross-bands,usually widen toward ventrolateral sides; or dorsal brownish with no cross-bands but reticulated patterns; cloacal plate divided or entire; loreal entering orbit or not......................3
2a) Head distinct from neck; eyes laterally compressed; dorsal scale rows 19 at one-head length behind neck; first five infralabials in contact with anterior chin shield; black crossbands on the anterior dorsum strongly serrated,mostly single scale broad,rarely two..........................L.serratus(Yunnan; Sichuan?)
2b) Head indistinct from neck; eyes not laterally compressed;dorsal scale rows 17 at one-head length behind neck; first four infralabials in contact with anterior chin shield; black cross-bands on the anterior dorsum less serrated,mostly 2-or 3-scale broad....................................L.multizonatus(Gansu,Sichuan,Yunnan?)
3a) Dorsal scale rows 19 at one-head length behind neck..............................................................................4
3b) Dorsal scale rows 17 at one-head length behind neck.................................................................................5
4a) Dorsal scale rows 19 at mid-body; dorsal cross-bands wide,28–35 on dorsum,8–13 on tail....................................................................................................L.rosozonatus(Hainan)
4b) Dorsal scale rows mostly 17 at mid-body,rarely 19; dorsal cross-bands narrow,51–87 on dorsum,12–30 on tail......................................................................L.rufozonatus(Anhui,Beijing,Chongqing,Fujian,Gansu,Guangdong,

Figure 7 Photos of live Lycodon aulicus-capucinus complex from China
Guangxi,Guizhou,Henan,Hebei,Hubei,Hunan,Heilongjiang,Jilin,Jiangsu,Jiangxi,Liaoning,Sichuan,Shandong,Shanghai,Shanxi,Shaanxi,Taiwan,Tianjin,Yunnan,Zhejiang)
5a) Cloacal plate entire.............................................................6
5b) Cloacal plate divided.....................................................16
6a) Dorsal body scale smooth................................................7
6b) Dorsal body scale feebly keeled or strongly keeled.....................................................................................10
7a) Ventrals 212–218; subcaudals 90 or 91; infralabials 9 or 10; loreal entering orbit; maxillary teeth 13 or 14......................................................................................L.pictus(Guangxi)
7b) Ventrals≤210; subcaudals<90; infralabials ≥8; loreal entering orbit or not....................................................8
8a) Infralabials 8; loreal always entering orbit; maxillary teeth 11; dorsal cross-bands Salmon color (Color 251) in life.....................................................................L.obvelatus(Sichuan; Yunnan?)
8b) Infralabials 9 or more; loreal mostly not entering orbit;dorsal cross-bands Pale Rose Pink (Color 243),with dense but faint gray speckles........................................9
9a) Dorsal cross-bands connecting with each other laterally,separating ground black coloration into ellipse patches;maxillary teeth 10.............................................L.cathaya(Guangxi)
9b) Dorsal cross-bands separated from each other; maxillary teeth 12–15..................................................L.futsingensis(Fujian,Guangdong,Guangxi,Jiangxi,Zhejiang,Hong Kong)
10a) Loreal entering orbit; dorsal cross-bands wide (usually 3-scale wide) with strongly jagged edges.....................................................................................................................11
10b) Loreal not entering orbit; dorsal cross-bands narrow(usually 1-to 2-scale wide) with smother edges (exceptL.ruhstrati)..................................................................12
11a) Tail long,TaL 23.1%–23.2% ToL in males,21.5% in female...............................................................L.gongshan(Sichuan?,Yunnan)
11b) Tail short,TaL 19.8%–22.5% in males,19.0%–21.9% in females...............................................................L.fasciatus(Yunnan,Anhui!,Gansu!,Guizhou!,Hubei!,Shaanxi!,Zhejiang!)
12a) Dorsal cross-bands bright Sulphur Yellow (Color 80) in life,50–96 on dorsum.................................L.flavozonatus(Anhui,Chongqing,Fujian,Guangdong,Guangxi!,Guizhou,Hainan,Hunan,Jiangxi,Sichuan,Zhejiang)
12b) Dorsal cross-bands white or gray,<50 on dorsum...............................................................................13
13a) Dorsal cross-bands dirty white (speckled with Drak Neutral Gray (Color 300)) or Cinnamon Drab (Color 259),increasingly more dirty posteriorly,19–46 on dorsum,3–4 dorsal-scale wide for most parts; intercepted black segments rather short.........................................L.ruhstrati(Anhui,Beijing,Chongqing,Fujian,Gansu,Guangdong,Guangxi,Guizhou,Hainan,Henan,Hong Kong,Hubei,Hunan,Jiangsu,Jiangxi,Sichuan,Shaanxi,Taiwan,Tianjin,Zhejiang)
13b) Dorsal cross-bands clear white,25–31 on dorsum,1 or 2 scale wide on most parts; intercepting black segments long.........................................................................14
14a) Body size small,ToL 463–487mm; subcaudal 68 or 69;maxillary teeth 10,forming three groups,group one and three each with two significantly enlarged teeth.........................................................................................L.synaptor(Yunnan)
14b) Body size large,ToL >1 000 mm; subcaudal 74–85........................................................................................15
15a) Medial 5–7 rows of dorsal scale keeled; maxillary teeth 8................................................................L.septentrionalis(Tibet)
15b) Dorsal body scale completely smooth or only very posterior portion of vertebral row feebly keeled; maxillary teeth 11 or 12...............................................L.chapaensis(Yunnan)
16a) Dorsal body scales smooth and glassy................................................................................................................17
16b) Medial rows of dorsal body scales strongly or feebly keeled......................................................................18
17a) Frontal in contact with preocular; reticulated patterns absent on body...................................................L.laoensis(Yunnan)
17b) Frontal not in contact with preocular; light reticulated patterns present on lateral and sometimes dorsal body.................................................L.aulicus-capucinuscomplex(Fujian,Guangdong,Hong Kong,Yunnan)
18a) Loreal not entering orbit; dorsal cross-bands Sulphur Yellow (Color 80) to Olive Sulphur Yellow (Color 90);distinct speckles and reticulated patterns present on dorsal and lateral head; ..........................................L.meridionalis(Guangxi,Yunnan)
18b) Loreal entering orbit; speckles and reticulated patterns absent on dorsal and lateral head....................................19
19a) Preocular absent; prefrontal entering orbit; dorsal crossbands white....................................................L.subcinctus(Fujian,Guangdong,Guangxi,Hainan)
19b) Preocular present; prefrontal not entering orbit; dorsal cross-band creamy Dark Spectrum Yellow (Color 78) to creamy Light Chrome Orange (Color 76)............................................................................................L.liuchengchaoi(Beijing?,Guangdong,Henan,Hunan,Sichuan,Shanxi,Shaanxi,Zhejiang)
NONMENCLATURAL ACTS REGISTRATION
The electronic version of this article in portable document format represents a published work according to the International Commission on Zoological Nomenclature (ICZN),and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone (see Articles 8.5–8.6 of the Code).This published work and the nomenclatural acts it contains have been registered in ZooBank,the online registration system for the ICZN.The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefixhttp://zoobank.org/.
Publication LSID:urn:lsid:zoobank.org:pub:0273816E-2B90-4683-B0C8-0208BCB05ED5
Species LSID:see Taxonomic accounts
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Collections of all animals used for this present study obey the Wildlife Protection Act of China.Collection permits were issued by Kunming Institute of Zoology,Chinese Academy of Sciences (BBCJ-2014-001).
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
K.W.and J.C.conceived the study.K.W.and Z.B.Y.conducted the field surveys.Z.B.Y.and V.G.collected morphological data.Z.B.Y.collected genetic data.K.W.analyzed the data and prepared the manuscript,with other authors’ inputs.All authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENT
We thank Mr.Di-Hao Wu for providing locality information of the new species; Mr.Ben-Fu Miao,Mr.Hui-Ming Xu (Yunlong National Nature Reserve),and Mr.Wen-Jie Dong (KIZ) for their assistances in the field; and Mr.Chao Wu,Mr.Wei-Liang Xie,Mr.Shao-Bing Hou (KIZ),Mr.Jin-Long Ren (CIB) and Mr.Franco Leung Ka Wah (CIB) for providing photos ofLycodonspecies.
Appendix I
Detailed locality information in Figure 1.
Lycodon chapaensiscomb.nov.(triangle):1.Laocai,Tonkin,Vietnam; 2.Daweishan,Honghe Prefecture,Yunnan,China; 3.Xichou County,Wenshan Prefecture,Yunnan,China; 4.Mengzi,Yunnan,China; 5.Jingdong County,Puer,Yunnan,China; 6.Tengchong County,Baoshan,Yunnan,China; 7.Yunlong County,Dali,Yunnan,China; 8.Lushui,Nujiang Prefecture,Yunnan,China; 9.Fugong,Nujiang Prefecture,Yunnan,China; 10.Dulongjiang,Nujiang Prefecture,Yunnan,China.
L.fasciatus(circle):1.Mogok,Mandalay,Myanmar; 2.Mindat,Chin State,Myanmar; 3.Longchuan,Dehong Prefecture,Yunnan,China; 4.Menglian County,Puer,Yunnan,China; 5.Tengchong,Baoshan,Yunnan,China; 6.Jingdong County,Puer,Yunnan,China; 7.Mengla,Xishuangbanna,Yunnan,China; 8.Kunming,Yunnan,China (type locality of junior synonym,Dinodon yunnanensis)
L.gongshan(square):1.Dulongjiang,Gongshan County,Yunnan,China; 2.Xiaoheishan,Longling County,Yunnan,China; 3.Yunlong Nature Reserve,Dali,Yunnan,China.
Lycodon multizonatus(trapezoid):1.Pengba,Luding County,Sichuan,China.
Lycodon septentrionalis(pentagon):1.Khasi Hills,Meghalaya State,India; 2.Medog,Nyinchi Prefecture,Tibet,China.
Lycodon synaptor(hexagon):1.Dongchuan,Yunnan,China.
Appendix II
Examined specimens of recognized species.Museum abbreviations see method.
Lycodon chapaensiscomb.nov.(n=8):MNHN-RA-1 933.001 1 (holotype),from 20 km SW of Lao-Kay (=Lao Cai),Tonkin,Vietnam; KIZ 027593,Tengchong,Yunnan,China; KIZ 035594,Yongping,Dali,Yunnan,China; KIZ 006753,Mengzi,Yunnan,China; KIZ 035045,Lushui,Gongshan,Yunnan,China; KIZ 038282,Fugong,Nujiang,Yunnan,China; KIZ 035113,Dulongjiang,Gongshan,Yunnan,China; KIZ 034331,Xichou,Wenshan,Yunnan,China.
L.fasciatus(n=4):KIZ 74II0262,74II0263,Tengchong County,Yunnan,China; 74I0145,Husa,Longchaun County,Yunnan,China; 75I473,Menglian County,Yunnan,China.
L.gongshan(n=4):KIZ 730 034 (holotype),730 008 (paratype),35 112 (topotype),35 114 (topotype),Dulongjiang,Gongshan County,Yunnan,China.
L.liuchengchaoi(n=1):HENU 001,Nanyang,Neixiang County,Henan,China.
L.multizonatus(n=4):CIB 9 964 (holotype),CIB 9 965 (paratype),Pengba,Luding County,Sichuan,China; KIZ 01623,Luding,Sichuan,China; KIZ 0911051,Shimian,Sichuan,China.
L.ruhstrati(n=8):NMW 22 794:1,22 994:3,22 794:4,22 794:10,22 794:15,22 794:18,FMNH 140 167,140 168,CAS 18 874,all from Taiwan.
L.septentrionalis(n=2):CIB 117 521,CIB M20150607,Medog,Tibet,China.
L.synaptor(n=1):BMNH 1 905.1.30.63 (holotype),Dongchuan,Yunnan,China.
Appendix III
Literature used for the updated distribution ofLycodonspecies in China.For full citation refer to the literature cited section.
L.aulicus-capucinuscomplex:Ades & Kendrik,2004
L.cathaya:Wang et al.,2020a
L.fasciatus:Yan et al.,2004; Kang et al.,2009; Li et al.,2012
L.flavozonatus:Orlov& Ryabov,2004; Luo et al.,2010
L.futsingensis:Vogel et al.,2009; Zhang et al.,2011a; Peng et al.,2015
L.gongshan:Vogel & Luo,2011; Guo et al.,2015; Chen et al.,2018a
L.liuchengchaoi:Zhang et al.,2011b,2015; Peng et al.,2014,2017,2018; Bai et al.,2018; Zhao et al.,2018; Zhang,2019
L.multizonatus:Lei et al.,2014; Yao&Gong,2012; Li et al.,2020
L.meridionalis:Orlov& Ryabov,2004
L.pictus:Jessen et al.,2020
L.ruhstrati:Yuan& Wei,2000; Wang et al.,2003; Ades& Kendrik,2004; Wang& Zheng,2005; Chen et al.,2006; Guo et al.,2007; Yang& Rao,2008; Zhang et al.,2017
L.rufozonatus:Zhao,2006
L.septentrionalis:Chen et al.,2018b
L.synaptor:Vogel& David,2010
- Zoological Research的其它文章
- The forty-year journey of Zoological Research:advancing with the times
- Chromosomal assembly of the Antarctic toothfish(Dissostichus mawsoni) genome using third-generation DNA sequencing and Hi-C technology
- Geographical range evolution of the genus Polypedates (Anura:Rhacophoridae) from the Oligocene to present
- Molecular and morphological evidence for a new species of the genus Typhlomys (Rodentia:Platacanthomyidae)
- Pitfalls of barcodes in the study of worldwide SARSCoV-2 variation and phylodynamics
- Dynamic evolution of transposable elements,demographic history,and gene content of paleognathous birds

