Applications and developments of gene therapy drug delivery systems for genetic diseases
China Pharmaceutical University,Nanjing 211198,China
Keywords:Gene therapy drugs Viral vectors Non-viral vectors Genetic diseases Nano-delivery system
ABSTRACTGenetic diseases seriously threaten human health and have always been one of the refractory conditions facing humanity.Currently,gene therapy drugs such as siRNA,shRNA,antisense oligonucleotide,CRISPR/Cas9 system,plasmid DNA and miRNA have shown great potential in biomedical applications.To avoid the degradation of gene therapy drugs in the body and effectively deliver them to target tissues,cells and organelles,the development of excellent drug delivery vehicles is of utmost importance.Viral vectors are the most widely used delivery vehicles for gene therapy in vivo and in vitro due to their high transfection efficiency and stable transgene expression.With the development of nanotechnology,novel nanocarriers are gradually replacing viral vectors,emerging superior performance.This review mainly illuminates the current widely used gene therapy drugs,summarizes the viral vectors and non-viral vectors that deliver gene therapy drugs,and sums up the application of gene therapy to treat genetic diseases.Additionally,the challenges and opportunities of the field are discussed from the perspective of developing an effective nano-delivery system.
1.Introduction
Typically,the deepened knowledge of genetic materials offers an innovative perspective for understanding disease occurrence and treatment.Genetic diseases are caused by genetic material changes or controlled by disease-causing genes,which are wholly or partly determined by genetic factors.Genetic diseases are classified into monogenic diseases and polygenic diseases.Monogenic diseases are caused by a pair of essential genes in accordance with Mendelian laws of inheritance,such as hemophilia and huntington’s disease [1–4].Polygenic diseases are controlled by two or more pairs of genes affected by the environment,such as diabetes and malignant tumors [5,6].Although drugs can suppress the symptoms of genetic diseases,they cannot achieve the fundamental effect of treatment.On the grounds that the complexity of the pathogenesis and the lack of medicines,genetic diseases have imposed a heavy burden on families and society,so the treatment of genetic diseases hasalways been a concern of biomedical scientists.Researchers have been exploring the solution to genetic diseases by modifying and repairing the disease-causing gene,that is,gene therapy.
Gene therapy is one of the most revolutionary medical technologies developed with DNA recombination technology and gene cloning technology [7] .It is a biomedical treatment method based on changing human genetic material.For genetic diseases,gene therapy can directly repair or even replace the disease-causing genes at the molecular level,restoring defective protein.After decades of development,gene therapy has shown great potential in treating significant diseases caused by genetic defects and genetic abnormalities such as malignant tumors,acquired immunodeficiency syndrome,and cardiovascular diseases.
At present,gene therapy drugs mainly include plasmids DNA,small interfering RNA (siRNA) [8],microRNA (miRNA),short hairpin RNA (shRNA),antisense oligonucleotide (ASO),and CRISPR/Cas9 system.With the development of gene therapy and the improvement of innovative vectors,gene therapy drug products have been resoundingly approved by the Food and Drug Administration (FDA),such as Kynamro,Imlygic,Spinraza,Exondys51,Yescarta,Kymriah,Zolgensma,and so on (Table 1) [9] .Despite the dawn of gene therapy,its delivery systems still face many difficulties,limiting the widespread clinical application.Usually,the target site of activity for gene therapy drugs is a specific organelle or intracellular location.Thus,one of the main challenges of gene therapy is to deliver gene therapy drugs to working sites,but naked gene therapy drugs have the following disadvantages:(1) Easily degraded by RNase in serum;(2)Recognized by serum immunologically active factors and phagocytosed by mononuclear macrophages;(3) Interact with serum proteins;(4) Renal Clearance;(5) Poor tissue targeting and cell uptake capacity;(6) Low endosomal escape and intracellular release efficiency [10] .However,with the development of materials science and nanotechnology,numerous novel viral vectors and non-viral vectors are used for gene therapy drug delivery to solve above obstacles.
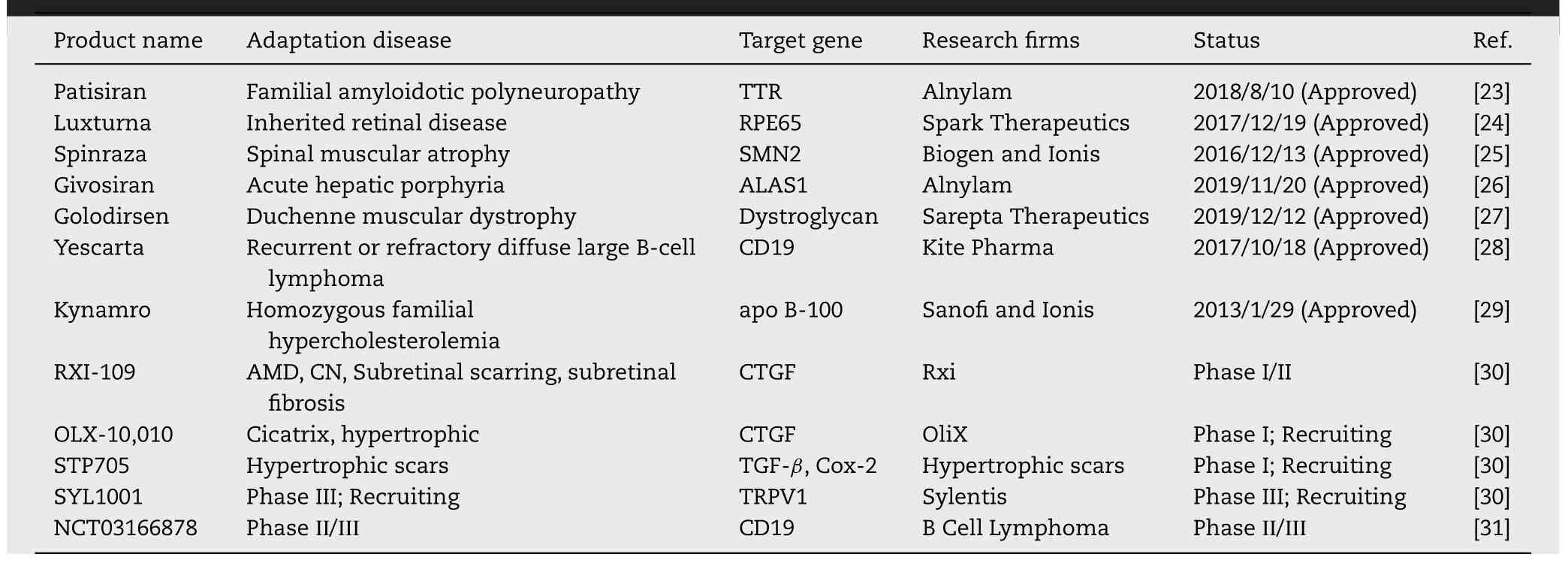
Table 1-Gene therapy drugs approved by the FDA and in clinical trials.
Currently,the main gene delivery systems are viral vectors and non-viral vectors [11–13].Viral vector systems include retroviruses [14],adenoviruses (AdV) [15],adenovirusassociated viruses (AAV) [16,17],lentiviruses (LV) [18],bacteriophages and the like.Most of the gene therapy drugs currently on the market use viruses as vectors.Although viral vectors have the disadvantages of high immunogenicity,safety hazards,and production difficulties,their higher transfection efficiency is the unique advantage for gene delivery.Non-viral vectors are favored by researchers profiting from large gene load,low immunogenicity,and high safety for large-scale production.Commonly used non-viral carriers include cationic liposomes,micelles [19],polymers,and inorganic nanoparticles.With the rise of biomaterials,innovative nanocarriers have been reported,such as DNA nanoclews [20],metal organic frameworks [21],cell membrane,and so on [22] .With the deepening of research,the modified nanocarriers have better performance,which can not only improve the drug loading and gene therapy effect,but also induce higher biological safety and higher targeting characteristics than viral vectors.Therefore,it is necessary to find a suitable carrier loaded with gene therapy drugs to overcome the bottleneck encountered during the delivery process.
In this review,we first introduce the gene therapy drugs and their current application progress.Then we narrate the delivery systems of gene therapy drugs,including virus delivery and non-virus delivery.Next,we systematically summarize the research progress of gene therapy drugs in the field of genetic disease treatment.Finally,the challenges and opportunities associated with gene therapy are discussed from multiple perspectives.
2.Gene therapy drugs
At present,most of the targets of conventional drugs are proteins expressed by disease-causing genes,such as enzyme inhibitors and antibodies.In contrast,gene therapy drugscan correct gene transcription and translation processes at the DNA or mRNA level and occupy a far-reaching clinical treatment position.This section shall discuss several of the most commonly studied gene therapy drugs,including siRNA,miRNA,plasmid DNA (pDNA),ASO,CRISPR/Cas9,and the like.Table 2 illustrates some advances in the use of gene therapy drugs for genetic diseases in recent years.

Table 2-Summary of gene therapy drugs for genetic diseases.
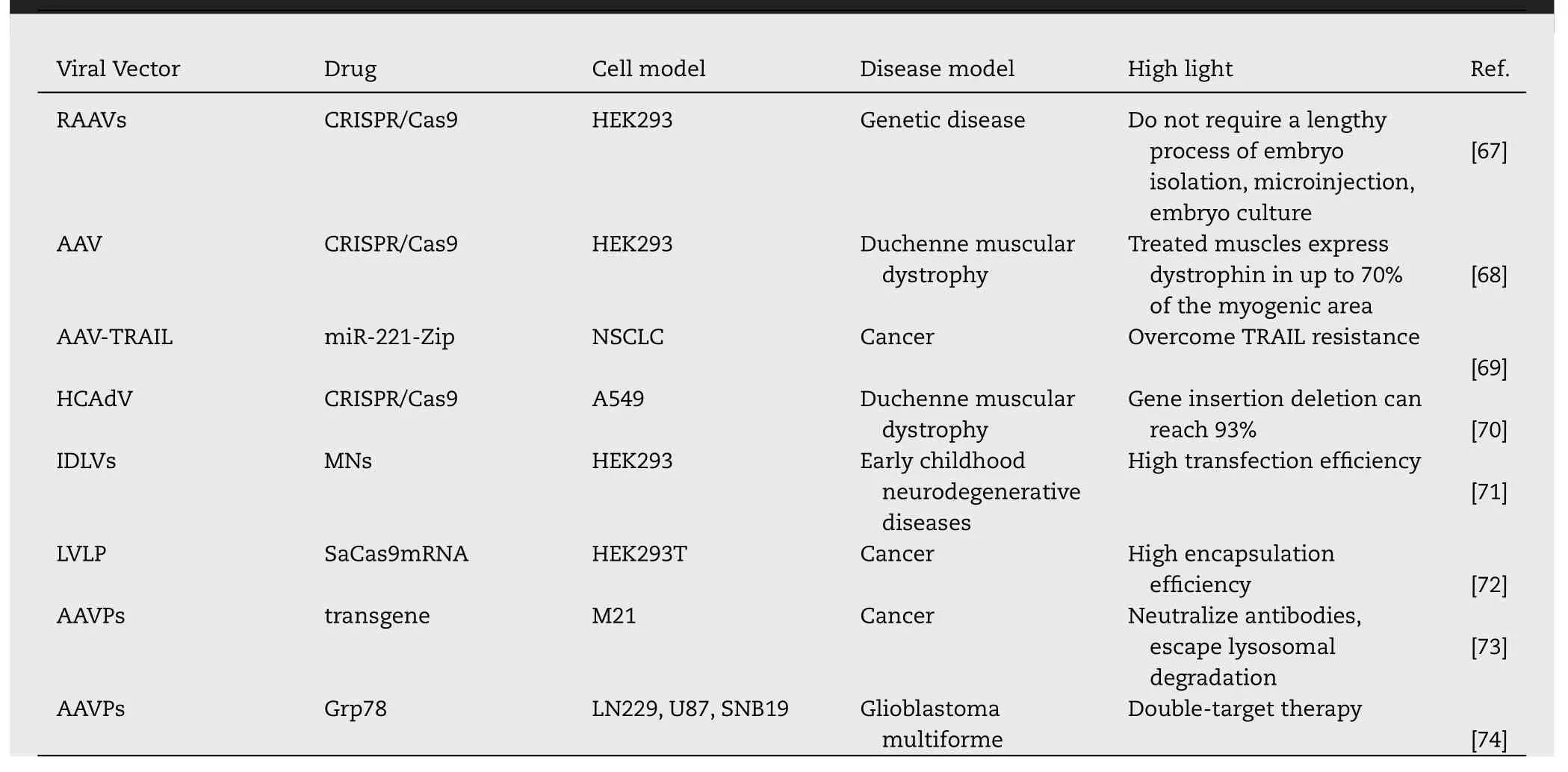
Table 3-Viral delivery strategies for gene therapy drugs.
2.1.Small interfering RNA (siRNA)
RNA interference (RNAi) is a new technology developed in recent years that can block the expression of specific genes and has excellent potential in gene therapy [45] .When the endogenous or exogenous double-stranded RNA enters the cell,it is processed into 21–23 nucleotide siRNA by Dicer enzyme in the cytoplasm.The antisense strand binds to the RNA-induced silencing complex (RISC) containing various nucleases [46] .Next,the siRNA is untwisted and the siRNA targeting sequence on the RISC complex is used as a guide to digest mRNA.The digested fragments are non-specifically degraded by nucleases in the cytoplasm,which silences the gene and prevents specific protein expression.The therapeutic effect of siRNA is through interaction with mRNA rather than protein,which reduces harmful proteins produced before synthesis.Moreover,the regulation of different target genes by changing the sequence of siRNA can be applied to many major human diseases[47] .
SiRNA is easily degraded by enzymes,filtered by the kidneys,and engulfed by host cells.Besides,its immunogenicity and membrane passing capacities are insufficient,limiting its clinical transformation [48] .Nanocarriers have gradually become a potential tool for siRNA system gene therapy to deliver siRNA to specific cells efficiently.Non-viral carriers of siRNA include cationic lipid polymers,polypeptides,polyethyleneimine (PEI) and nucleic acid self-assembly carriers [49] .The nucleic acid selfassembly carrier can overcome the rigid structure of siRNA,increase the negative charge of siRNA to increase the loading of siRNA,and significantly improve gene silencing efficiency.Bujold et al.assembled a prismatic DNA nanocage and wrapped siRNA in a responsive gated DNA strand structure to avoid nuclease degradation.The anti-apoptotic factors Bcl-2 and Bcl-xl antisense nucleotide chains were used to control the release of siRNA.The FRET method at the molecular level proved that the assembled DNA nanocage could effectively release therapeutic genes such as siRNA (Fig.1) [50] .

Fig.1-Schematic illustrations of the siRNA-encapsulating prism and release mechanism.Reprinted with permission from [50] .Copyright 2016 American Chemical Society.
2.2.MicroRNA (miRNA)
MiRNA is a non-coding small RNA of about 19–22 nt encoded by the cell genome of eukaryotic organisms.It regulates gene expression by promoting the degradation of target mRNA or inhibiting mRNA translation,ultimately affecting the development,proliferation,differentiation,and apoptosis of cells [51] .The mechanism of miRNA is to combine with miRNA-induced silencing complex(miRISC) on the target mRNA to form an asymmetric RISC.Studies have shown that miRNA recognizes the 3 ′ untranslated region (3UTRs)of target mRNA,and binds to miRISC under the catalysis of RISC endonuclease,thereby inhibiting the translation of target mRNA [52] .
MiRNA plays a vital role in the treatment of genetic diseases,especially tumors.Zhao et al.sequenced the miRNAs involved in the pathogenesis of hepatoma carcinoma cells and found that miR-451a expression was significantly down-regulated in hepatoma carcinoma cells.As a tumor suppressor,miR-451a acts on the downstream essential target lipoprotein 1 (lpin1) and promotes the apoptosis of hepatoma carcinoma cells and endothelial cells [53] .Li et al.found that the overexpression of miRNA-218 reduced the kidney damage and the levels of IL-6,TNF-α
,IL-1β
,and MCP-1 in the mouse podocyte model of diabetic nephropathy [44] .The dual-luciferase reporter gene assay confirmed that miR-218 targeted the mRNA encoding IKK-β
and regulated NFκ
B-mediated inflammation.Furthermore,miRNA can treat cardiovascular diseases by regulating heart development,remodeling and regeneration,endothelial function,and angiogenesis [54] .Samanta et al.reported that miR-124 and miR-135a are involved in regulating blood pressure by weakening the signal transduction of the renin-angiotensintestosterone system [55] .Many miRNAs,including miR-17-3p,miR-31,and miR-126,regulate vascular inflammation by regulating E-selectin expression,intercellular cell adhesion factor 1,and vascular cell adhesion factor 1 [56] .Moreover,Dwivedi reported that specific miRNAs (miR-941,miR-589) in the blood could be used as clinical biomarkers to diagnose major depression [57] .2.3.Antisense oligonucleotide (ASO)
ASO is a molecular drug that inhibits gene expression by specifically binding to the target gene DNA or mRNA,and regulates it at the gene level.The mechanism of ASO is through complementary binding to the target mRNA nucleotide sequence,and part of the DNA sequence from synthetic antisense oligonucleotides binds to the target mRNA to form a DNA-RNA hybrid.Finally,it attracts endogenous RNaseH
to this site,shears and degrades the target mRNA,thereby reducing the expression of the target gene.Up to now,there are four antisense oligonucleotide drugs approved by the FDA for marketing:fomivirsen sodium,mipomersen sodium,eteplirsen and nusinersen sodium.Eteplirsen became the first phosphoramidite morpholino oligonucleotide approved for the treatment of duchenne muscular dystrophy (DMD).Subsequently,nusinersen sodium is approved by the FDA for the treatment of spinal muscular atrophy (SMA).ASO lays the foundation for the rapid development of the nucleic acid drug market,and promotes nucleic acid to become the third promising drug after small molecule drugs and protein drugs.ASO drugs have a place in clinical applications and studies are continuing and making progress.Yang et al.studied biodegradable lipid nanoparticles to deliver ASO to cultured human cells and animal models.The proprotein convertase subtilisin/kexin type 9 (PCSK9) was selected as the therapeutic target,and the results showed that the delivery system had a high gene knockout efficiency.This bioreducible lipid is a valuable candidate for ASO delivery and provides new ideas for the development of ASO-based therapies [43] .Leitner et al.prepared a positively charged ethyl cellulose nanoemulsion and successfully complexed with ASO.In vitro
transfection tests showed that transfection efficiency of 40% could be obtained when the N/P ratio in serum-free medium was 28.The complexes are expected to take effectin vitro
diagnostic tests andin vivo
gene therapy [44] .2.4.CRISPR/Cas9
CRISPR/Cas9 system is mainly composed of Cas9 protein and single-stranded guide RNA (sgRNA).In the presence of protospacer adjacent motif (PAM),Cas9 protein can reach different target sites through base complementary pairing under the guidance of sgRNA,and cleave the target gene to achieve DNA double strand break (DSB) [58] .At present,the delivery of CRISPR/Cas9 system can be divided into three levels based on plasmid,RNA and protein.Delivery of plasmids encoding Cas9 protein and sgRNA does not require multiple transfections,which is convenient and stable;delivery of mRNA encoding Cas9 protein has lower off-target rate than delivery plasmid;direct delivery of Cas9 protein has low immunogenicity.The pDNA-based CRISPR/Cas9 system has become an extremely attractive tool due to its simple operation and low cost.However,the size of the encoded pDNA and the transcription process will significantly hinder the delivery of the system and reduce the efficiency of gene editing.For the CRISPR/Cas9 system,the most efficient way is to skip pDNA or mRNA expression on Cas9 protein and sgRNA and directly deliver the Cas9/sgRNA ribonucleoprotein (RNP) to minimize off-target effects.Viral vectors are most commonly used to deliver the CRISPR/Cas9 system,followed by physical methods such as electroporation,microinjection,and tail vein high pressure injection.Ghassemi et al.constructed plasmids encoding Cas9 protein and double sgRNA,aiming to target the exon 2 and 3 sites of the brokenHbb-bs
gene.The recombinant plasmid was delivered to the fertilized eggs by microinjection,and then the fertilized eggs were implanted into pregnant mice for progeny screening and genotyping.Finally,aβ
-thalassemia mouse model withHbb-bs
gene knockout was established(Fig.2) [59] .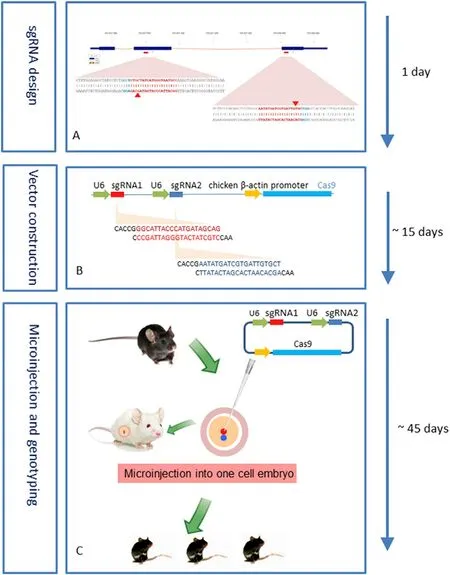
Fig.2-General overview and timing.(A) sgRNA design.(B)Vector construction.(C) Microinjection and genotyping.Reprinted with permission from [59] .Copyright 2019 Elsevier.
With the development of nanotechnology in recent years,non-viral vectors of the CRISPR/Cas9 system have effectively delivered to cells and tissuesin vivo
andin vitro
.Mout et al.used arginine gold nanoparticles (ArgNPs) to achieve the co-delivery of Cas9 protein and sgRNA,effectively delivering RNP to the cytoplasm.Subsequently,it was transported to the nucleus,which achieved a delivery efficiency of up to 90% and a gene editing efficiency of 30% [60] .Among organic materials,cationic liposomes are widely used due to their high gene load and transfection ability.Cho et al.designed the sgRNA targeting dipeptidyl peptidase-4 gene (DPP-4) and encapsulated with lecithin cationic liposomes.Intravenous injection into type 2 diabetic mice could significantly destroy the DPP-4 gene,accompanied by normalization of blood glucose levels and reduction of liver and kidney damage[61] .In order to better achieve the delivery of CRISPR/Cas9 system,the modification of Cas9 protein and sgRNA has also been widely studied.The modified Cas9 protein and sgRNA can reduce the off-target rate and increase stability.Ferdosi et al.combined the hydrophobicity ofT
cell receptor contact residues with HLA,and identified two immunodominant SpCas9T
cell epitopes of HLA-A * 02:01 through an enhanced prediction algorithm.The study found that SpCas9 protein modification can eliminate immunity dominant epitope while retaining its function and specificity [62] .In addition to modifying Cas9 protein,sgRNA also can be shortened from 20 nt to 17 nt to reduce the off-target rate.After synthesizing 100 nt full-length sgRNA,Hendel et al.performed three different chemical modifications of 2 ′-O-methyl (M),2 ′-Omethyl 3phosphorothioate (MS),or 2-O-methyl 3thioPACE(MSP) at both termini.The results showed that chemical modification of sgRNA could enhance genome editing efficiency of human primaryT
cells,CD34hematopoietic stem cells,and progenitors cells [63] .2.5.Other gene therapy drugs
ShRNA consists of two short inverted repeats and a stemloop sequence in the middle to form a hairpin structure.With relatively high viral infection efficiency,shRNA remains stable through virus-mediated transduction.After transduction,the Dicer enzyme in the cell is used to trigger gene silencing in the nucleus.Compared with siRNA,although it has a higher curative effect and a longer lasting effect,the transfection efficiency is low and preparation is time-consuming.Zhang et al.used cationic liposomes to deliver TLR4 shRNA (VA-lipshRNA-TLR4),induced mitochondrial apoptosis of pancreatic stellate cells,and achieved the purpose of treating pancreatic fibrosis [64] .
Inserting a therapeutic gene into a plasmid and introducing it into a target cell is considered a safer and more convenient treatment method.The gene plasmid containing the foreign antigen is directly introduced into the animal body so that it can express the antigen protein in the host cell and induce the human body to produce an immune response.The pCMFO/DMT complex prepared by Tian et al.resulted in a longer duration of DNA release in the DNA-DMT complex.The results showed that mice vaccinated with pCMFO/DMT caused a biased response to Th1 and a significant increase in CFMO-specific IL2Tcell response in the spleen.pCMFO/DMT is a very promising tuberculosis vaccine and requires further preclinical and clinical trials [65] .
According to the physical properties and mechanism of action,gene therapy drugs vary in drug delivery and gene knockout efficiency in diverse cell lines targeting various genes [66] .Therefore,it is necessary to select suitable gene therapy drugs for specific cell lines and target genes.From the perspective of drug knockout efficacy,the CRISPR/Cas9 system can remove the DNA molecules of the target gene to achieve gene knockout without any mRNA background,while the other gene drugs can only target mRNA molecules interfere at the RNA level with uncertain effects.From the perspective of drug delivery,most gene drugs are negativelycharged to hinder drug delivery efficiency,and particle size is an nonnegligible factor.Among gene drugs,nucleic acids of small molecules such as siRNA,miRNA,shRNA,ASO are convenient in terms of drug delivery efficiency thanks to their small size,but the sizeable molecular weight of CRISPR/Cas9 system or pDNA is an urgent problem to be solved in the delivery process.In addition to CRISPR/Cas9 with nuclear localization signals (NLS) to assist the system in entering the nucleus,other free gene drugs based on RNA background cannot achieve target delivery.In short,the current various delivery tools adopt the strategy of maximizing strengths and avoiding weaknesses for each gene therapy drug to accelerate the pace of gene therapy.
3.Viral delivery strategies for gene therapy drugs
In recent years,viral vectors of gene therapy drugs have been widely usedin vivo
andin vitro
due to their high transfection efficiency and stable transgene expression.Commonly used viral vectors include AAVs,AdVs,LVs and bacteriophages.The latest research progress of non-viral vectors is summarized in Table 3 .3.1.Adeno-associated virus (AAV)
AAV is a non-enveloped double-stranded DNA virus with good biological characteristics,genetic stability,high gene transduction efficiency and large-scale ease of use.However,the packaging limit of AAV is only 4.5 kb,the production process is complicated,and the cost is high,so the application of AAV vectors has certain restrictions.In the research of AAV for gene therapy,it is necessary to improve the efficiency of gene therapy and reduce cytotoxicity as much as possible to explore the optimal targeting strategy of AAV vector-mediated gene therapy.There are currently three strategies for optimizing the targeting of AAV infection:genetic modification;targeted regulation at the transcription level;covalent coupling modification on capsid protein.
Genetic modification is the most commonly used method to confer the targeting ability of AAV vectors.Yoon et al.transduced the CRISPR/Cas9 system into mouse embryos by recombinant adeno-associated viruses (rAAVs) to targetTyr
locus,resulting in genetically modified mice.rAAVs do not require a lengthy process of embryo isolation,microinjection,embryo culture and transfer to pseudo-pregnant females,thereby facilitating gene editing in mice and other mammals more conveniently [67] .Gene therapy using AAV vectors has broad prospects for treating DMD.Bengtsson et al.used AAV to construct a Cas9 and sgRNA system that can deliver muscletargeting genes,edit mutations in dystrophic mdx4cv mice,and complete HDR-mediated Duchenne muscle nutritionin vivo
.The results indicate that AAV-mediated musclespecific gene editing has excellent potential for treating neuromuscular diseases (Fig.3) [68] .Ma et al.studied AAVmediated TNF-related apoptosis-inducing ligand (TRAIL) and miR-221-Zip co-expressed gene therapy to overcome the drug resistance of TRAIL-induced apoptosis of liver cancer cells.AAV-TRAIL combined with miR-221-Zip combination therapy,has become a new method of therapeutic intervention for liver cancer [69] .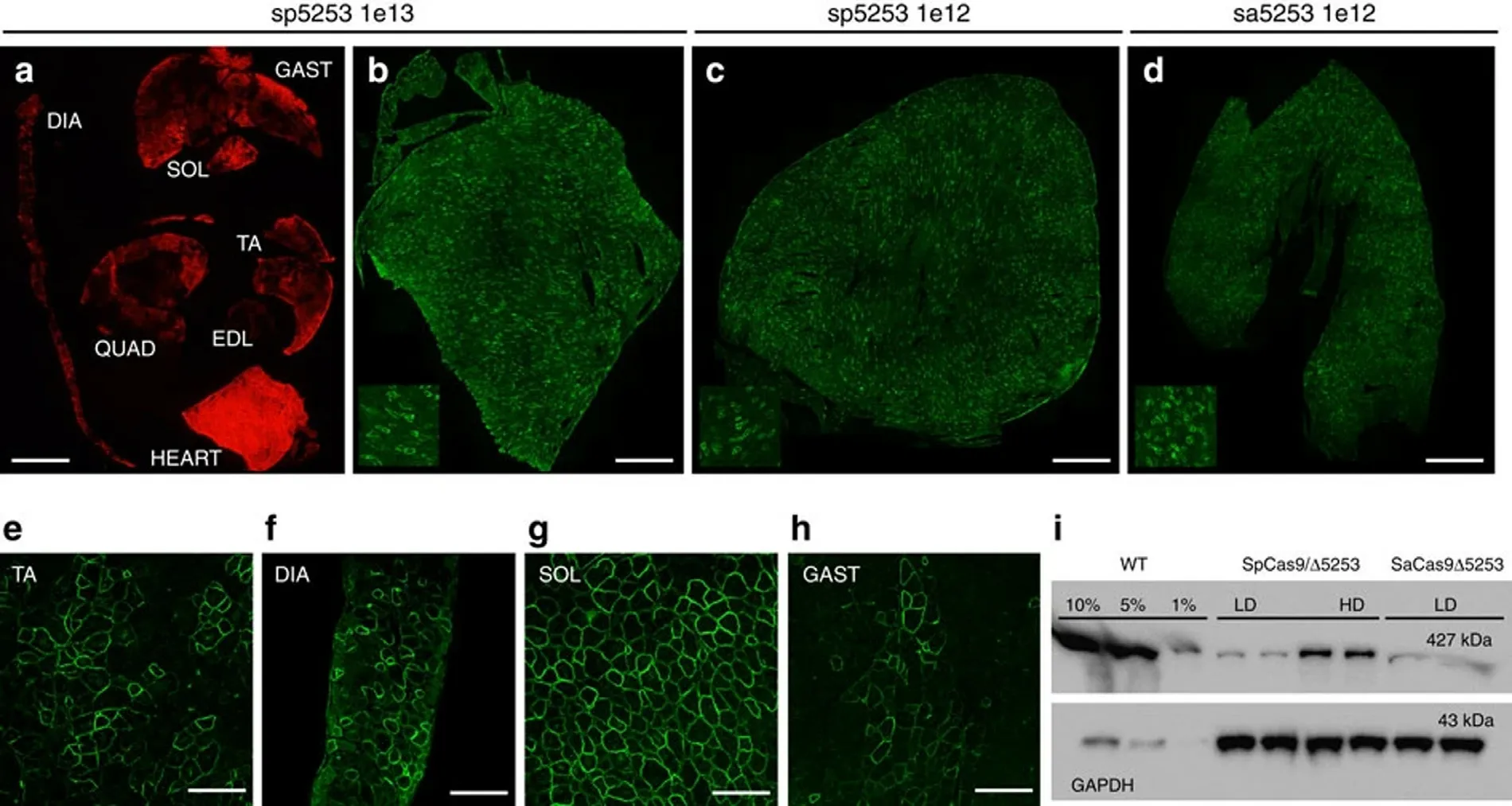
Fig.3-Systemic gene editing results in widespread dystrophin expression.Reprinted with permission from [68] .Copyright 2017 Springer Nature.
3.2.Adenovirus (AdV)
AdV is an unenveloped double-stranded DNA virus with an icosahedral nucleocapsid structure.Due to the wide range of AdV hosts,easy purification,genetic stability,and large foreign gene loading capacity,about 25% of the viral vectors used in clinical gene therapy are AdV vectors.Ehrke-Schulz et al.used high-capacity adenoviral vectors (HCAdV) as vectors,which did not express any viral genes that reduced immunogenicity.This study constructed human papilloma virus (HPV) oncogeneE6
,DMD dystrophinrelated gene,HIV co-receptor CC chemokine receptor type 5 (CCR5) gene-targeted CRISPR/Cas9 delivery system.The highest gene insertion-deletion can reach 93%,but the HCAdV delivery CRISPR/Cas9 system has not yet been appliedin vivo
[70] .Tan et al.immunized transgenic mice by constructing a recombinant AdV vector carrying the hepatitis B core antigen (HBcAg) and TPPII genes (Adv-HBcAg-TPPII) to evaluate the specific immune response induced by them and the antiviral ability of the transgenic mice.The results show that Adv-HBcAg-TPPII can break the immune tolerance,stimulate the activity of HBV-specific cytotoxicT
lymphocytes,and has a better therapeutic effect in transgenic mice [75] .3.3.Lentivirus (LV)
LV is a spherical structure composed of single-stranded RNA with an encapsulation volume of about 8 kb and has been widely used to deliver gene therapy drug systems.LVs are the same as AdVs,which delete all genes so as not to activate the immune system.As a delivery vehicle for genetic drugs,LV integration of the host genome may lead to unnecessary non-target insertion mutations,which poses a safety risk.At present,most laboratories cannot prepare non-genomic integrated LV vectors,so LV is used less frequently than AAV and AdV,and it is most commonly used to establish disease models.
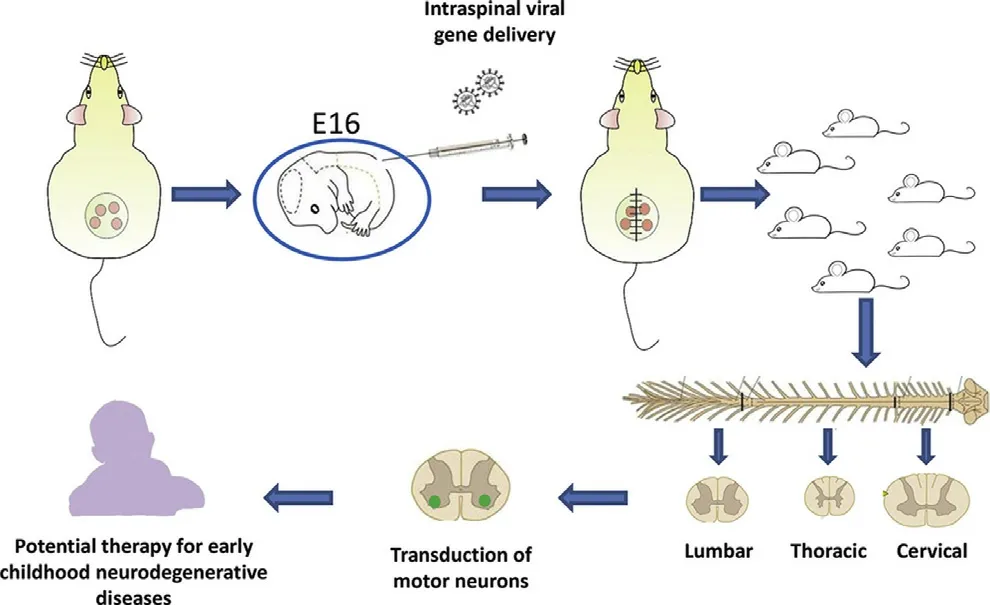
Fig.4-Schematic diagram of gene editing of integrated lentiviral vector.Reprinted with permission from [71] .Copyright 2018 Elsevier.
Integration-deficient lentiviral vectors (IDLV) retains the high transduction efficiency of LVs and reduces insertion mutations.Ahmed et al.confirmed that IDLVs could efficiently transduce spinal motor neurons,which may provide a new strategy for treating inherited early childhood neurodegenerative diseases (Fig.4) [71] .Zhao et al.studied the expression of silenced CLDN1 in two breast cancer cells(MDA-MB-231 and MCF7) by LV-mediated RNA interference.The results indicate that CLDN1 RNA interference mediated by lentiviral vectors can control tumor cell growth by inhibiting epithelial-mesenchymal transition (EMT),demonstrating great potential in breast cancer gene therapy [76] .Lu et al.developed a lentivirus-like bionanoparticle (LVLP) vector that can encapsulate up to 100 mRNAs expressing SaCas9 protein.Compared with cells transduced with adeno-associated virus or LV expressing SaCas9,cells that were transduced with LVLP containing SaCas9 mRNA had a lower off-target rate[72] .
3.4.Bacteriophages
Phage vectors can achieve targeted gene delivery,and can be used for gene therapy target discovery and multidrug resistance research.However,due to its narrow host selectivity and lower gene delivery efficiency than animal viruses,research applications are relatively few,and most are used in combination with other viral vectors.Suwan et al.modified the viral capsid structure of phage vectors and synthesized a multifunctional mixed adeno-associated virus/phage (AAVP) vector.This directed evolutionary genetic strategy can give the next generation of AAVP particles the ability to neutralize antibodies,escape lysosomal degradation and avoid the pre-and post-internalization barriers of transduction,improving delivery efficiency [73] .Przystal et al.inserted the human AAV single-stranded genome into the M13 phage single-stranded genome to form a bacteriophage capsid (AAVP) containing the AAV genome.This phage vector can double-target therapeutic gene glioma,which provides new ideas for the treatment of glioblastoma multiforme (GBM)[74].
4.Non-viral delivery strategies for gene therapy drugs
Although viral vectors efficiently deliver gene therapy drugs through cell transduction,their safety issues such as high immunogenicity and high mutation risk also limit their clinical transformation.Generally,to enhance transfection efficiency,the ingenious design of non-viral vectors makes up for the defects of gene therapy drugs.Besides,the ideal gene therapy drug carrier should have the ability to protect the nucleic acid drugs,extend halflife,improve targeting,enhance endosomal escape,and intracellular release.Compared with viral vectors,non-viral vectors are more adaptable,can overcome the problems mentioned above simultaneously and have a broader range of applications.Herein,the delivery strategy is outlined and divided into synthetic nanocarriers and endogenous nanocarriers according to the source of carrier materials(Table 4).
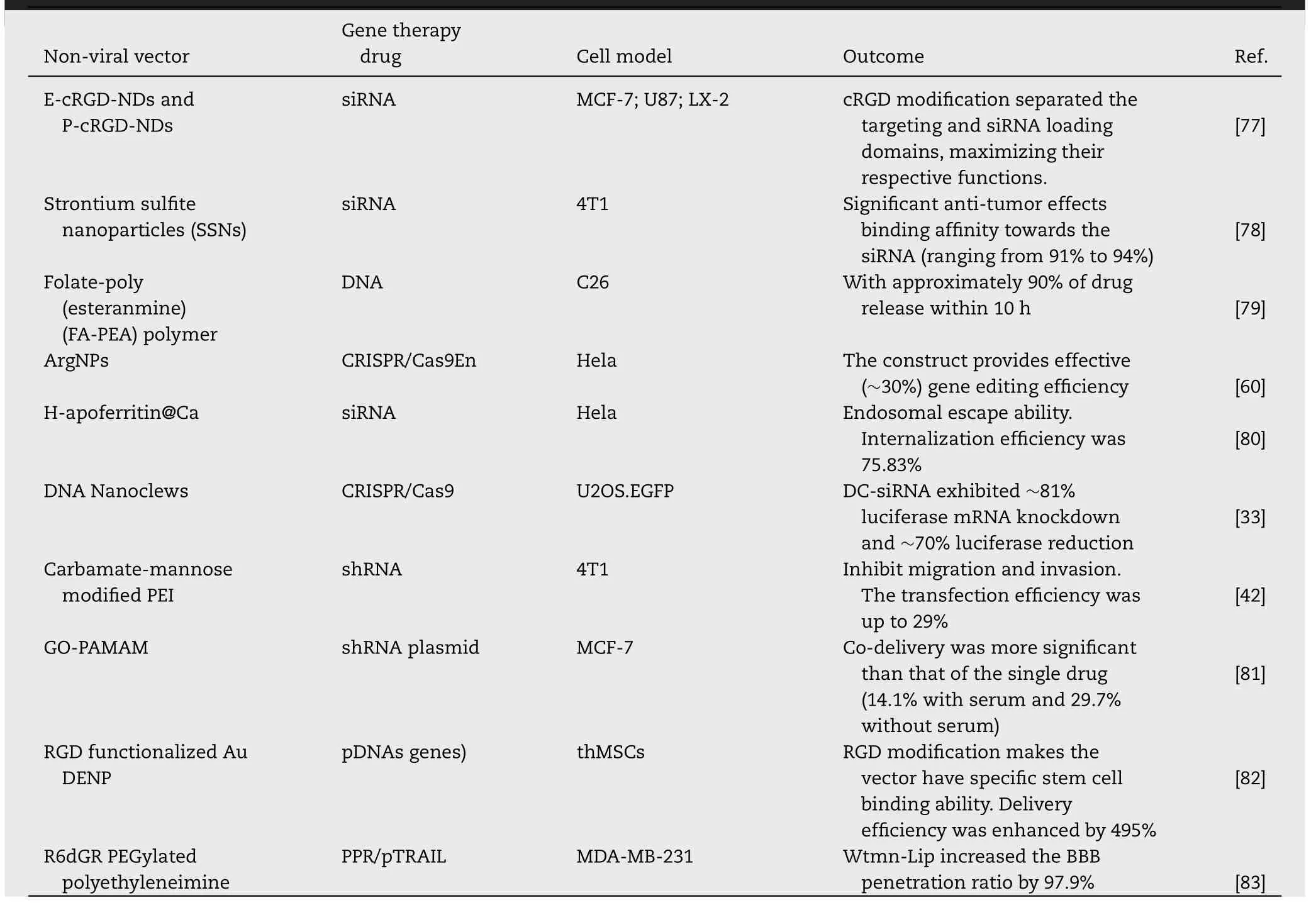
Table 4-Non-viral vector delivery strategies for gene therapy drugs.
4.1.Synthetic nanocarrier
Synthetic nanocarriers are the most widely used nonviral nano-delivery carriers.Compared to endogenous nanocarriers,raw materials are easy to obtain and can be produced in large quantities at low cost.At present,synthetic nanocarrier technology is relatively mature,and corresponding gene therapy drugs have been launched,such as Patisiran [84] .
4.1.1.Liposome
Liposomes are formed by the orientation of phospholipid biomolecules that can encapsulate fat-soluble and watersoluble drugs,which deliver genetic drugs to the body through fusion with cell membranes.Liposome nanoparticles(LNPs) have the advantages of drug targeting and good biocompatibility,reducing drug toxicity,overcoming drug resistance,and facilitating endosomal escape [85] .Therefore,LNPs have been widely used in the delivery of gene therapy drugs,of which Lipofectamine,TurboFectand Stemfecthave been commercialized.Cationic liposomes formed by a positively charged amphoteric compound and a neutral lipid are the main force to deliver gene therapy drugs.The positive charge carried by the cationic liposome can interact with the negative charge on the target gene to form a stable complex,increasing the circulation time and enhancing the transfection efficiency.To enhance the delivery efficiency of gene therapy drugs,a large number of ingenious designs of liposomal non-viral vectors have begun to emerge,including exosome-liposome hybrid nanoparticles[86],DOTAP liposome [87],hyaluronic acid-modified cationic noisome [88] .Especially,Li et al.developed efficacious LNPs with>
25% GFP knockout efficiencies that can load up to 90% of Cas9/sgRNA RNPs,confirming that nanoparticles loaded with the CRISPR/Cas9 system have higher targeted gene knockout capabilities and lower toxicity compared with free CRISPR/Cas9.The cationic liposome delivery system provides a new way to deliver gene editing tools for disease modeling and treatment (Fig.5) [89] .4.1.2.Cationic polymer
The cationic polymer is formed by polymerizing short-chain repeating units with a highly controllable structure and can be precisely designed according to the requirements of the responsiveness of the contained drugs.With its advantages of good biocompatibility,biodegradability and structural controllability,polymers are widely used for gene therapy drug delivery.However,the cytotoxicity of cationic polymers is positively correlated with molecular weight and charge.Common cationic polymer gene carriers include PEI,polyaminoamine,polyaminoesters and natural biocompatible polymer materials.The principle of action is that the positively charged polymer and the negatively charged nucleic acid are combined by electrostatic action to deliver the gene therapy drugs to the target cell to achieve a therapeutic effect.Wang et al.delivered a therapeutic gene encoding human tumor necrosis factor-related apoptosisinducing ligand (PPR/pTRAIL) with R6dGR peptide-modified PEGylated PEI for breast cancer metastasis treatment,which showed that PEI-PEG-R6dGR/pTRAIL exhibited multiple anti-tumor functions [83] .In addition to PEGylation to enhance transfection efficiency,the functional design of cationic polymer carriers has received attention.Duan et al.reported a highly branched poly(β
-amino ester)s (BPAENB) with the multivalent arrangement of cationic groups that provides better nucleic acid binding capacity.BPAENB exhibited remarkably higher DNA/siRNA transfection,notably outperforming commercial reagents PEI 25k and Lipofectamine 2000 [90] .Polyamidoamine (PAMAM) is a kind of cationic synthetic dendrimer with high branching degrees,low dispersion,and high functionality.The surface of the dendrimer has a modified amino group that is protonated under physiological conditions to be positively charged.It can be combined with negatively charged DNA,RNA,and mucopolysaccharides onthe cell surface through electrostatic interaction.Gu et al.designed a graphene oxide functionalized PAMAM dendrimer(GO-PAMAM) to achieve co-delivery of doxorubicin (DOX)and MMP-9 shRNA plasmids to treat breast cancer.The delivery system has high drug loading,high gene delivery efficiency (14.1% with serum and 29.7% without serum),good biocompatibility,and significantly inhibits the expression of MMP-9 protein in MCF-7 cells (Fig.6) [81] .

Fig.5-(A) Synthetic route employed for lipidoids synthesis.(B) Intracellular delivery of Cas9:sgRNA RNP complex loaded LNPs for gene editing.(C) Chemical structures of amine head (R) groups.Reprinted with permission from [89] .Copyright 2018 Royal Society of Chemistry.
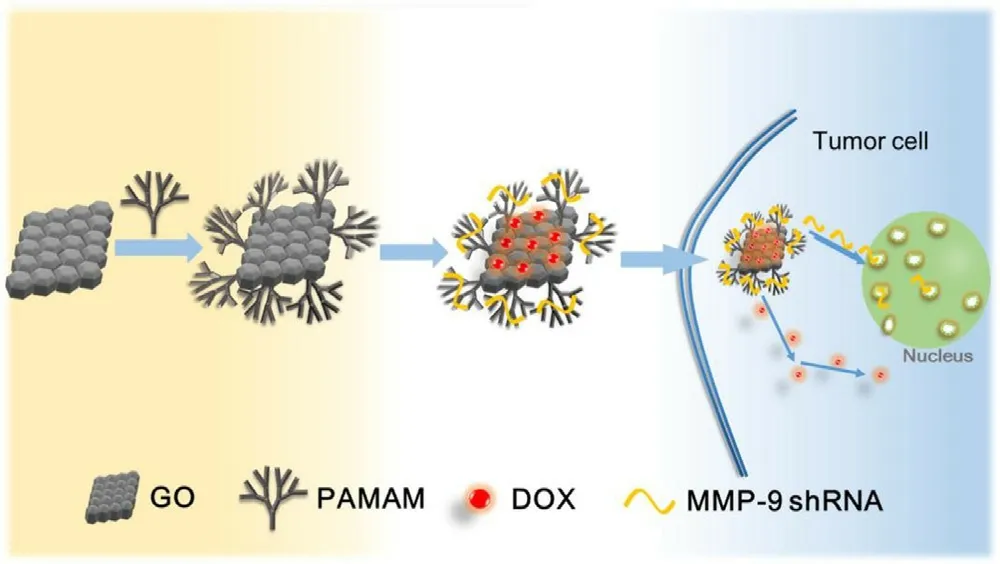
Fig.6-Schematic diagram of gene editing of MMP-9shRNA.Reprinted with permission from [81] .Copyright 2017 Elsevier.
4.1.3.Inorganic nanocarrier
Inorganic nanocarriers have broad application prospects,such as uncomplicated preparation,convenient storage,large specific surface area,good stability,and easy-tomodify features.However,its biosafety is still a controversial topic,which requires long-term and in-depth research.Surface modification can improve the transfection efficiency and reduce the toxicity of nanocarriers.Currently,gold nanoparticles (AuNPs),carbon nanotubes (CNTs) [91],graphene [92],quantum dots (QDs) [93],up-conversion nanoparticles (UCNs) [94],and silica nanoparticles (MSN) are widely used in gene therapy drugs delivery [95] .
AuNPs are classical inorganic nanomaterials,which have the advantages of low toxicity,high drug loading,customizable size and shape,good chemical stability and biocompatibility,becoming a good platform for gene therapy drug delivery through chemical modification [96] .Kong et al.constructed arginine-glycine-aspartate (Arg-Gly-Asp,RGD)peptide modified dendrimer-trapped gold nanoparticles(Au DENPs),which can efficiently transfect plasmid DNA carrying both the enhanced green fluorescent protein and the luciferase (pEGFPLuc) reporter genes into human mesenchymal stem cells (hMSC).The gene delivery efficiency using K1 (mPEG),K2 (mPEG+AuNPs),K3 (mPEG+RGD),and K4 (mPEG+AuNPs+RGD) was enhanced by 104%,264%,180%,and 495%,respectively,indicating that the coexistence of RGD and AuNPs allows the designed carrier to bind stem cells specifically [82] .
With the development of nanomaterials,new types of nanomaterials emerge endlessly and play essential roles in gene delivery.Karim et al.constructed strontium sulfate nanoparticles as new pH-responsive inorganic nanocarriers to effectively transport siRNA into breast cancer cells.The study demonstrated significant binding affinity (ranging from 91% to 94%) towards the siRNA,suggesting that the potential ability of the nanoparticles in carrying siRNAs [78] .Alsaiari et al.used structural carriers (zeolitic imidazole frameworks,ZIFs)prepared with imidazole groups and zinc ions to encapsulate large-diameter Cas9 protein and sgRNA effectively into CC-ZIF nanoparticles.The protonation of the imidazolyl group at pH promoted the escape of CC-ZIF endosomes and transport to the nucleus and finally reduced EGFP protein expression by 37% [97] .
4.2.Endogenous nanocarriers
The endogenous carriers have a perfect natural structure for assembling and isolating the contents,and their inherent biological functions make them have a specific targeting nature delivery.More and more evidence showed that endogenous vectors in drug delivery strategies will become a breakthrough progress,and even have the potential for personalized gene therapy.Compared with synthetic nanocarriers,endogenous nanocarriers have the advantages of low immunogenicity,high stability in blood,and high efficiency of drug delivery.
4.2.1.Exosome
Exosomes are a kind of vesicles secreted by cells at the nanometer scale (40–100 nm),which play an important role in transporting materials and signal communication between cells.Exosomes have the advantages of being similar to the cell membrane,small in size,negatively charged,avoiding phagocytosis,producing immune escape,prolonging circulation time,and penetrating deep tissues.Since the potential biological functions of exosomes from different cell sources are quite different,the source of exosomes is controversial,and there is a risk of promoting tumor growth and immunosuppression.Gyorgy et al.constructed an exosome AAV vector by coupling exosomes with adenoassociated viruses.Strikingly,exo-AAV9 transduced almost 95% of inner hair cells (IHCs) and outer hair cells (OHCs).The conventional AAV1-GFP vector transduced approximately 20% of IHCs and OHCs,whereas exo-AAV1-GFP transduced up to 65% of IHCs and 50% of OHCs,indicating that exo-AAV1-GFP is more effective than conventional AAV1-GFP and it could be a significant transgenic vector for deafness [98] .Exosomes serve as membranes to encapsulate gene drugs allowing high encapsulation efficiency.Zhao et al.constructed cationic bovine serum albumin (CBSA) conjugated siS100A4 and exosome membrane coated nanoparticles with high encapsulation efficiency (EE) of 86.70 ±1.22% [99] .Even for the CRISPR/Cas9 system with large molecular weight,exosomes also show a better loading advantage.Ye et al.engineered a modified exosome by fusing the exosomal membrane protein CD63 with GFP,which verified that Cas9 proteins were captured and efficiently loaded into exosomes rather than a random package to increase the efficiency of loading [100] .
4.2.2.Ferritin
Ferritin is a protein widely found in various animals and plants,mainly composed of a mineral core and subunit shells.The ferritin that removes the mineral core is called apoferritin (AFn).It is a hollow globular protein shell with an inner diameter of 8 nm and an outer diameter of 12 nm.Its cage-like structure and unique pH-dependent depolymerization/recombination self-assembly properties make it more convenient for drug loading.Furthermore,ferritin can achieve active targeting by specifically recognizing highly expressed transferrin receptors (TfR1) on the surface of various tumor cells.With the above advantages,ferritin has become a recognized and ideal multifunctional nanodrug carrier,which has been used in the delivery of gene therapy drugs in recent years.Our group recently calcified the inner surface of heavy chain ferritin (HFn) for lysosomal escape,carrying siRNA to avoid lysosomal degradation,and finally used for gene silencing.The results showed that the mineralized HFn could be used as a lysosomal escape vector,and the protein expression of HFn@Ca/siEGFR was approximately decreased to 84%,indicating the HFn@Ca can be a potential gene drugs vector [80] .Nevertheless,thein vivo
fate of ferritin and its derivatives,the kinetic characteristics of drug release from the nanocage,and the modification of ferritin nanocage to increase its immunogenicity have seriously hindered the development of ferritin nanocage drugs.4.2.3.Red blood cells membrane
Red blood cells (RBC) are the most abundant blood cells in the blood,and the red blood cell membrane (RBCM)extracted from them can be used as a carrier for gene therapy drug delivery.Hao et al.coated the nano-scale RBCM on the surface of the gene complex through electrostatic interaction to prepare a bionic gene delivery system,thereby prolonging its circulation time in the body and improving its biocompatibility.The phagocytic rate of these biomimetic gene delivery systems reduced by 52% compared with the control group,which will open up a new avenue for gene delivery systems by RBC membrane camouflage (Fig.7) [101] .Wang et al.reported a nanoworm involving RBCM cloaking of charge-reversible polyplexes of siRNA and polycation.RBCM cloaking charge-reversible polyplexes protected siRNA from RNaseA
degradation and prolonged blood circulation.The characteristics of charge reversal endow siRNA with the characteristics of loading and releasing,which solves the main problems of delivery process [102] .Due to the outstanding biocompatibility,biodegradability,and half-life extension of RBCM,various studies have achieved good results.However,in some cases,the critical host defense function of phagocytes is hindered,so whether this platform can be used in clinical practice remains considered.4.3.Innovative delivery strategy
At this stage,the development of new nanocarriers provides new possibilities for the delivery of gene tharapy drugs.Sun et al.prepared DNA nanoclews (DNA NC) by rolling circle replication.The purified Cas9 protein and sgRNA are coincubatedin vitro
to form RNP,and the sgRNA in RNP is combined with DNA nanoclews according to the principle of base pairing.After successful loading,the positively charged PEI is coated outside the DNA nanoclews to promote the escape of lysosomes [20] .Recent studies have shown that DNA nanoclews are also used for the successful delivery of siRNA.In vitro
experiments have shown that such spherical DNA nanostructures show effective gene knockdown in miRNA and protein levels [33] .Such innovative carriers also include arrestin domain containing protein 1 [ARRDC1]-mediated microvesicles (ARMMs) [103],multifunctional peptides [104],cell penetrating peptide modified carriers [105] .From the perspective of enhancing drug transfection efficiency by peptide coupling,Mulholland et al.prepared nanoparticles via condensation of siFKBPL with RALA;a novel,cationic 30 mer amphipathic peptide [106] .Coincidentally,Li et al.conjugated different amounts of REDV-G-TAT-G-NLS-Cys (TPG) peptide onto PLGA-g-PEI amphiphilic copolymers in which the TP-G peptide and PEI could cooperatively and strongly condense pZNF580.The results showed significantly high internalization efficiency,endosomal/lysosomal escape and nucleus location of pZNF580 through TP-G peptide conjugated gene delivery system [107] .5.Diseases treated with gene therapy drugs
5.1.Cancer therapy
Cancer is one of the major diseases that endanger human health.Current cancer treatment methods,such as chemotherapy,radiotherapy,and surgical treatment,may reduce body immunity but not satisfactorily achieve restricting tumor growth and spread.With the development of DNA recombination technology,gene therapy has become a new method of treating tumors.RNA interference technology has an apparent silencing effect on endogenous genes and exogenous genes.The treatment of tumors caused by the interaction of stages of disease provides the possibility.Wu et al.used RNAi technology to silence the proto-oncogenePIK3CA
andPIK3CB
genes to suppress human colon and rectal cancer (CRC).The results show that PIK3CA and PIK3CB can be used as potential therapeutic drug targets for colorectal cancer,alleviating the resistance of cancer cells during chemotherapy,which can provide personalized treatment options for CRC patients[108] .Gene editing technology can regulate the expression of genes in prostate cancer cells and provides great help for exploring the functions of related genes in prostate cancer.The regulation of the signaling pathway is of great significance to the occurrence and development of cancer.Plant homeodomain finger protein 8 (PHF8)is a histone demethylase that can promote tumor cell mesenchymal transformation and cell cycle progression and inhibit cell apoptosis.Maina et al.found that PHF8 played an important regulatory role in the hypoxia signaling pathway of prostate cancer.They used RNA interference to knock down the expression of PHF8 in LNCaP
and knocked out the expression of PHF8 in 293T cells using the CRISPR/Cas9 system.Finally,they found that the hypoxia signaling pathway was significantly suppressed.This work revealed the epigenetic mechanism of the hypoxic signal and laid the foundation for further evaluation of the role of PHF8 inhibitors in the treatment of prostate cancer[109] .
Fig.7-Preparation process of NP/pZNF580/RBCs and their gene delivery by crossing extracellular and intracellular barriers.Reprinted with permission from ref [101] .Copyright 2018Royal Society of Chemistry.
5.2.Duchenne muscular dystrophy
CRISPR/Cas9 system-mediated gene editing can restore the coding frame of the DMD gene by introducing small insertions or deletions and induce exon skipping and correct nonsense mutations through gene recombination.With the continuous development and improvement of the CRISPR/Cas9 gene editing system,the application of this technology for the repair of DMD mutations has rapidly developed.Some researchers have edited and repaired different DMD mutations in pluripotent stem cells (IPSCs) derived from DMD patients.Among them,Young et al.established the CRISPR/Cas9 system to repair the 725 kb mutant fragment between exons 45–55 through non-homologous end joining(NHEJ),which is used to correct 60% of the serious reads in the code mutations of DMD patients.This method is the current CRISPR/Cas9-mediated DMD deletion based on the largest fragment repaired by NHEJ.In vivo
studies in mice have shown that cardiomyocytes and skeletal muscles differentiated from IPSC cell clones by this gene editing method have the expression and function of dystrophin,which improves the stability of the membrane and the complexity of glycoproteins(Fig.8) [110] .5.3.Parkinson’s disease
The advantage of gene therapy is that it can deliver gene therapy drugs to specific brain regions to change functions and treat Parkinson’s disease (PD) while avoiding off-target effects.Currently,there are three main gene therapy strategies for the treatment of PD:(1) restoring the synthesis of striatum dopamine;(2) providing neurotrophic support to promote the survival of neurons in neurodegenerative sites;(3) regulating the subthalamic nucleus excitatory neuron activity [111] .In order to study the mechanism of PARK17in vivo
,Ishizu et al.constructed Vps35 D620N knock-in mice to express homologous mutant proteins with endogenous expression.CRISPR-Cas9 technology knocks out Vps35 in exon 15 of the mousePARK17
gene,which can significantly reduce the release of midbrain substantia nigra dopamine,and has a specific effect on alleviating PD[112] .
Fig.8-Schematic diagram of CRISPR/Cas9-mediated DMD deletions.Reprinted with permission from ref [110] .Copyright 2016Elsevier.
5.4.Other genetic diseases
HemophiliaA
(HA) is anX
-linked recessive genetic disease.The leading cause is the lack of coagulation factor (F
) Ⅷin the body,which leads to coagulation dysfunction.Park et al.used CRISPR/Cas9 technology to genetically edit theF
Ⅷ gene of the induced IPSCs to restore the inverted chromosome fragments to the wild state,differentiate the IPSCs into endothelial cells,and transplant the endothelial cells into mice with hemophilia.The mice began to produceF
Ⅷ,and bleeding symptoms were also controlled[113] .Huntington’s disease (HD) is an autosomal dominant genetic disease,and the most fundamental treatment is gene therapy.HD is an autosomal dominant genetic disease,and the most fundamental treatment is gene therapy.It currently includes gene editing that directly corrects huntington’s gene mutations at the DNA level and inhibits the production of mutant huntinatin (mHTT) proteins at the mRNA level.Merienne et al.introduced a new CRISPR/Cas9 gene editing system containing gRNA targeting theHTT
gene (sgHTT)and gRNA targeting theCas9
gene (sgCas9),making the system excise the Cas9 gene through sgCas9 while excising theHTT
gene.This technology can significantly reduce the off-target effect while suppressing the expression of mHTT[114] .In addition to the above genetic diseases,gene therapy clinical experimental studies including diabetic retinopathy(DR),glaucoma,β
-mediterranean anemia,age-related macular degeneration (AMD),and cardiovascular disease(CVD) have made some progress that are not described in detail here.6.Conclusions and future perspective
In this review,we discussed the current widely used gene therapy drugs and research progress.The delivery methods of gene therapy drugs for viral vectors and non-viral vectors are summarized,which provides a new direction for the delivery of gene therapy drugs.In addition,we also summarized the diseases of gene therapy,providing a reference for the direction of gene therapy.The critical point of gene therapy is to select specific genes that have therapeutic effects on the disease based on understanding the molecular mechanism of disease occurrence.The choice of gene carrier is crucial in the process of gene therapy.Only when gene therapy drugs are used to complement their weaknesses can the progress of gene therapy research go further.The primary problem to be solved for gene therapy drugs is delivery,which is the bottleneck of gene therapy today.Our review summarizes the latest delivery strategies and is committed to the development of effective gene editing strategies.
Gene therapy has become more mature and improved with the emergence of multiple biological technologies,such as second-generation sequencing and gene editing.The genetic diversity of genetic diseases,the complexity of pathogenesis and the innate differences of individuals make it difficult to apply gene therapy in clinical trials and actual treatment.Exploring and studying various targets of genetic diseases has also been the direction of the medical field and scientific researchers.In terms of safety,the use of viral vectors in gene therapy is currently controversial.Although viral vectors are highly efficient and easy to integrate into host cells,the infectivity and parasitism of the virus also need to be focused on,and the problem of off-target has always been gene therapy difficulties.On the other hand,due to the relatively high cost of gene therapy research and development and drug delivery methods,its treatment is expensive.The reform and improvement of gene therapy pricing and payment system are a big challenge,and it is also the only way for gene therapy to enter the market in an all-round way.Taking into account the complexity of human genetic information,gene therapy drugs may also bring unpredictable consequences.Currently,there is no guarantee that such changes in gene structure will never lead to the loss of unknown human functions.Therefore,gene therapy is not only a medical method but also involves ethical issues.Moreover,gene therapy drugs still have obstacles such as lack of efficient delivery systems,lack of sustained and stable expression,and host immune response.In the future,gene therapy research will develop in two directions:one is to deepen basic research,seek more effective drug treatment targets,and solve the delivery problem of gene therapy drugs;the other is to optimize the implementation plan in the clinical trial process,formulate objective evaluation standards and make treatment effects more precise.In short,with the development of molecular biology,molecular genetics,clinical medicine,and emerging nanomaterials,gene therapy will continue to mature and be widely used in clinical practice.
In conclusion,gene therapy drugs have broad application prospects in the treatment of genetic diseases.We believe that with the progress of research and the exploration of new vectors,gene therapy may substantially impact the future treatment of genetic diseases.
Declaration of Competing Interest
The authors declare no competing financial interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.51472115) and Double Firstclass Innovation Team of China Pharmaceutical University(CPU2018GY40).
 Asian Journal of Pharmacentical Sciences2021年6期
Asian Journal of Pharmacentical Sciences2021年6期
- Asian Journal of Pharmacentical Sciences的其它文章
- Advances in photosensitizer-related design for photodynamic therapy
- Aspects of high-performance and bio-acceptable magnetic nanoparticles for biomedical application
- Nanomedicine potentiates mild photothermal therapy for tumor ablation
- Carrier-free prodrug nanoparticles based on dasatinib and cisplatin for efficient antitumor in vivo
- Liposomal remdesivir inhalation solution for targeted lung delivery as a novel therapeutic approach for COVID-19
- Iron-doxorubicin prodrug loaded liposomenanogenerator programs multimodal ferroptosis for efficient cancer therapy
