金属相1T´MoS2增强类石墨相C3N4的可见光催化性能
贾并泉,叶 斌,赵 伟,许钫钫,黄富强,2,3
(1.中国科学院上海硅酸盐研究所,高性能陶瓷和超微结构国家重点实验室,上海200050;2.中国科学院大学,材料科学与光电子工程中心,北京100049;3.北京大学化学与分子工程学院,稀土材料化学与应用国家重点实验室和北京分子国家重点实验室,北京100871)
1 Introduction
Group-Ⅵtransition metal dichalcogenides(TMDs)such as MoS2are promising for applications in diverse fields like secondary battery[1,2],electrocatalysis[3,4],photocatalysis[5,6]and solar cell[7].It exists several poly⁃morphs including 2H,1T phases and so on,depending on the coordination modes between the transition metal and chalcogen atoms[8—10].These modes are thought to highly correlate with their performance in applications.Among them,the octahedral coordinated one(1T phase)is metallic compound,showing superior performance for electrocatalysis[11,12]and energy storage[13,14]than trigonal coordinated 2H phase,because the charge trans⁃fer resistance is dramatically reduced in the metallic phase.However,the 1T phase of MoS2is metastable and suffers from easily converting to the stable 2H phase[15],which restricts its further development.
1T′MoS2with disordered octahedral coordination has relatively high stability and good conductivity compared with 1T MoS2[9,16].As is reported[15],1T′MoS2is highly efficient for the hydrogen evolution reaction with a small overpotential,which not only serves in electrocatalysis but also makes sense in photocatalysis.Among traditional strategies to improve photocatalytic activity,noble metal loading including Pt,Pd,RuO2and IrO2plays a important role in the efficient carriers′separation and reaction overpotential reduction[17—20].However,it still remains a challenge to take non-noble-metal materials as cocatalysts for photocatalytic activity enhancement.Hence,1T′MoS2may be an ideal choice to replace noble-metal compounds to obtain good photocatalysts.
Herein,we provide a facile route to prepare graphitic carbon nitride(g-C3N4)/1T′MoS22D nanocomposites.g-C3N4as a traditionally well studied photocatalyst has response to visible light and should be a good model to hybridize with 1T′MoS2to promote photocatalytic reactions.The activity of the hybrids was evaluated by organic contaminant decomposition and hydrogen evolution under visible light.The as-prepared 2D nanocomposites exhibit enhanced photocataytic activity,owing to effective carriers’separation between g-C3N4and 1T′MoS2.The stability,kinetics model and possible photocatalytic mechanism were also investi⁃gated for the photocatalysts to illustrate their prospect of applications.
2 Experimental
2.1 Reagents and Instruments
Urea(CH4N2O,98%),molybdenum powder(Mo,200 mesh,99.99%),sulfur(S,99%),and lithium sulfide(Li2S,99%)were purchased from Aladdin.Hydrochloric acid(HCl,36%)were purchased from Sinopharm Chemical Reagent Company.
The phase and crystallinity analysis of CNH/MoS2nanocomposites were performed by X-ray diffraction(XRD)on a Bruker D8 Advanced diffractometer(Bruker,Germany)with CuKαirradiation(λ=0.15406 nm),and the scanning angle ranged from 10° to 80° of 2θ.Sample morphology was examined using a JEM2100F transmission electron microscope(TEM,JEM,Japan)under an acceleration voltage of 200 kV.The height of nanosheets was examined using a Horiba SmartSPM-1000 atomic force microscope(AFM,Horiba,France).The chemical composition of the samples was analyzed by X-ray photo-electron spectroscopy(XPS,Thermofisher Scientific,America)on a RBD upgraded PHI-5000CESCA system(Perkin Elmer)with MgKradiation(hν=1253.6 eV).UV-Vis diffuse reflectance spectra were measured at room temperature on a Hitachi U3010 spectrophotometer in the 300—800 nm range.Raman spectra were recorded in a Thermal Dis⁃persive Spectrometer(Horiba,France)using a 7 mW laser with an excitation wavelength of 633 nm.The pho⁃toluminescence(PL)spectra were measured with a JobinYvon Fluoromax-4 spectrofluorometer(Horiba,France).
2.2 Synthesis of 1T′MoS2 Nanosheets
First,the LiMoS2crystals were obtained by solid state reaction from a mixture of Mo,Li2S and S with a molar ratio of 1∶1∶1.05 at 850℃for 16 h.Then,5 mg LiMoS2was placed into 200 mL of H2O with 100µL of 1 mol/L HCl.Finally,the solution was shaked under ultrasonic for 30 min to get 1T′MoS2nanosheets.The cleaning procedure was applied by centrifugment at 15000 r/min for 5 min and ultrasonic dispersion for 3 times.After washing,the 1T′MoS2nanosheets were redispersed in 10 mL of H2O for further use.
2.3 Synthesis of g-C3N4 Nanosheets
The g-C3N4nanosheets was fabricated by thermal condensation of urea.18 g of urea was placed in a 60 mL crucible with a cover in a muffle furnace at 550℃for 4 h.After cooling down to room temperature,the obtained yellow solid was grounded into powder in a mortar and calcinated at 550℃for another 4 h to get g-C3N4nanosheets.
2.4 Synthesis of g-C3N4/1T′MoS2 2D Nanocomposites
200 mg of g-C3N4nanosheets were added into 30 mL of 1 mol/L HCl for ultrasonic to get a dispersed solu⁃tion.After washing with H2O until the pH of solution reaching 7,the protonated g-C3N4was freeze-dried and denoted as CNH.100 mg of CNH were redispersed in 200 mL of H2O with stirring to get a stable sol,followed by dropping of the solution containing 0.5 mg 1T′MoS2nanosheets.After stirring for another 30 min,the products were collected by centrifugment and freeze-dried,which were denoted as CNH/MoS2-0.5%.And the other composites:CNH/MoS2-0.1%,CNH/MoS2-0.2%,CNH/MoS2-1% and CNH/MoS2-2% were also pre⁃pared by adding different mass ratios of 0.1%,0.2%,1%and 2%of MoS2,respectively.
2.5 Photocatalysis Experiments
Methyl orange(MO)were chosen to evaluate the photocatalytic activity of the CNH/MoS2nanocomposites under visible light irradiation(λ>400 nm).Irradiation was carried out using a 300 W Xe lamp with a UV cutoあfilter to completely remove any radiation below 400 nm.A typical experiment was conducted as follows:50 mg of catalyst was dispersed in 50 mL of MO(10 mg/L)aqueous solution.Visible-light irradiation was conducted after the suspension was magnetically stirred for 30 min in the dark to get equilibrium of adsorptiondesorption between dye and catalyst.During irradiation,4 mL aliquots was taken from the mixture at an aptotic interval and centrifuged.The temperature of the reaction solution was maintained at room temperature by a flow of water.The remnant liquid was spectrophotometrically monitored for MO concentration analysis by a UV-Vis spectrometer.
H2production experiment was performed using a top-irradiation Pyrex reaction cell.Photocatalyst powder(100 mg)was dispersed by ultrasonication for 2 min into an aqueous solution(200 mL)that contained trietha⁃nolamine(20 mL)as the sacrificial reagent.Then the suspension was degassed thoroughly with pure N2.The solar light irradiation was from a 300 W Xe lamp using a light reflector to remove UV light.The temperature of the reaction solution was maintained at room temperature by a flow of water.The amount of H2evolved was de⁃termined by an Agilent 7820A GC equipped with a TDX-01 column connected to a TCD.The photocatalytic activities were compared on the basis of the average H2evolution rate in the first 5 h.
3 Results and Discussion
The synthetic procedure for CNH/MoS22D nanocompsites is shown in Scheme 1.The lone-pair electrons of the nitrogen in g-C3N4complex with H+in the solution and lead to the further exfoliation of oxygen etched g-C3N4.After adding 1T′MoS2,the electrostatic interaction between CNH and MoS2help to obtain CNH/MoS22D nanocomposites.The thickness of 1T′MoS2detected by AFM(Fig.S1,see the Supporting Information of this paper)is about 3.3 nm,showing the ultrathin morphology and large lateral area to reunite with g-C3N4.The interaction between MoS2and g-C3N4is shown in Scheme 1,H+cations localized on the sites of N after etching by HCl promote to get positive charged g-C3N4,which lead to electrostatic interaction with negative MoS2sheets.

Scheme 1 Fabrication process of CNH/MoS2 2D nanocomposites
The XRD patterns of the nanocomposites are presented in Fig.1(A).Two remarkable diffraction peaks located at about 13.5° and 27.5° can be assigned to(100)and(002)planes of g-C3N4(JCPDS No.87-1526)[21].The diffraction peaks of 1T′MoS2are not obvious because of little ratio and good dispersity in the composites.However,the Raman results of 1T′MoS2are shown Fig.S2(see the Supporting Information of this paper).The distinctJ1,J2,J3andA1gpeaks appeared while the symbolic peaks for 1T-MoS2and 2H-MoS2are nearly absent,illustrating a relatively pure phase of 1T′MoS2inthe 2D nanocomposites[16].
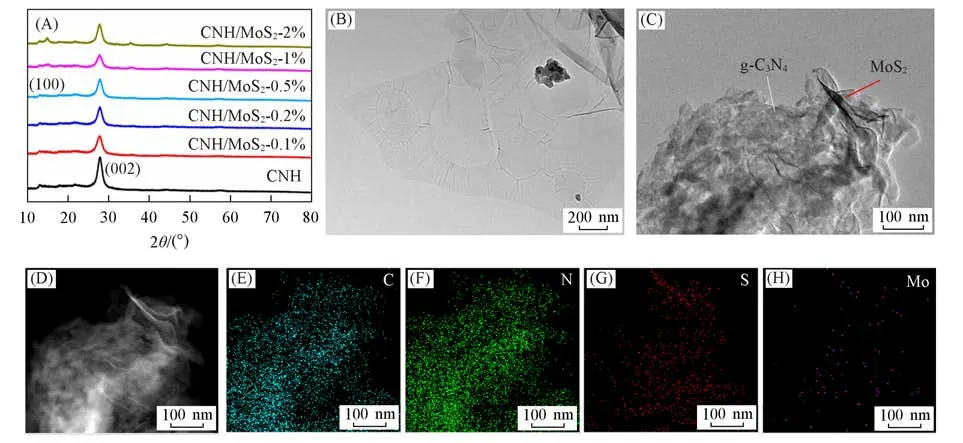
Fig.1 XRD patterns of CNH and CNH/MoS2 2D nanocomposites(A),TEM images of 1T´MoS2(B)and CNH/MoS2⁃0.5%(C),HADDF image(D)and EDS mapping of CNH/MoS2⁃0.5%(E—H)
The morphology of CNH,1T′MoS2and CNH/MoS2are shown in Figs.S3(see the Supporting Information of this paper),1(B)and 1(C).The CNH shows the morphology of nanosheets at about 100 nm at lateral.1T′MoS2exhibits a morphology of ultrathin nanosheets larger than that of CNH while the composites shows the stacked nanosheets.The EDS mapping illustrates that the CNH and MoS2are uniformly distributed in the composites.The detailed content of MoS2in different catalysts are shown in Table S2(see the Supporting Information of this paper).These results not only prove the success of the fabrication for CNH/MoS2,but also become the foundation for the fast electron transportation.
Further structure charactrizations are performed by XPS and shown in Fig.2.The C1sspectra[Fig.2(B)]shows two peaks at 288.1 and 284.6 eV.The former is identified as sp2-bonded carbon(N—C=N),while the other is corresponding to graphitic carbon[22].High-resolution spectrum of N1s[Fig.2(C)]is fitted into four peaks:the main peak at 398.6 eV can be considered as hybridized aromatic N bonded to carbon atoms(C=N—C),which is attributed to 3-s-triazine rings of CNH.Two peaks at 399.6 eV and 400.9 eV are corresponding to the N in the form of N—(C)3and H—N—C,respectively.The final weak peak at 404.5 eV may originate from the—NH2or=NH groups[21,23].Apart from these,three peaks located at 230.7,227.5 and 224.6 eV are ascribed to Mo3d3/2,Mo3d5/2in 1T′MoS2and S2s[Fig.2(D)],respectively[16].The other two peaks with higher binding energy are corresponding to 2H MoS2,whose ratio is lower than 20% in the 1T′MoS2.However,a bit red shift happens for the peaks of Mo3din 1T′MoS2[16],which comes from electron shar⁃ing between MoS2planes and CNH because of abundant electrons in N.Hence,a conclusion can be drawn that electron transportation exists between these two kinds of nanosheets.

Fig.2 XPS survey spectrum(A)and high resolution XPS spectra of C1s(B),N1s(C)and Mo3d(D)of CNH/MoS2⁃0.5%
According to the above characterization results,CNH/MoS2composites are successfully prepared.Then,the UV-Vis DRS spectra of the composites are shown in Fig.3(A).After hybridization,the catalysts exhibit the improved sunlight harvest compared with that for CNH,owing to heavy absorption of visible light for MoS2[9].The PL emission spectra of the CNH and its composites in Fig.3(B)claim that the intensity of the PL peak for CNH is decreased after hybrid with 1T′MoS2,which illustrates the effective photogenerated carriers’separation.These results claim that the composites take use of sunlight more efficiency than the CNH.Thus,the photocataytic activities of these composites are evaluated by the degradation of MO and H2evolution under visible light.As shown in Fig.4(A)and 4(B),the CNH/MoS22D nanocomposites show better photocatalytic activity in H2evolution under visible light than that of CNH.In addition,CNH/MoS2-0.5% exhibits the best activity with 6.24µmol∙g—1∙h—1while 4.64µmol∙g—1∙h—1for CNH-Pt-0.5%.It claims that 1T′MoS2can work as the candidate replacing Pt to boost photocatalytic activity.

Fig.3 UV⁃Vis DRS(A)and PL emmision(B)spectra of CNH and CNH/MoS2 catalysts

Fig.4 H2 evolution with different catalysts under visible light(A)and Bar plot showing the H2 evolution rates of different catalysts(B)
Apart from the H2evolution reactions,degradation reactions were also studied.As shown in Fig.5(A)and 5(B),the photocatalytic activity of the CNH/MoS2composites are higher than that of the CNH alone in MO degradation.Among the catalysts,CNH/MoS2-0.5%exhibits the best photocatalytic activity,which reached a degradation level of 90.7% in 12 min under visible light.Fig.5(B)shows the reaction process of MO under visible light over CNH/MoS2nanocomposites fitted with the Pseudo-first-order kinetics model.The rate con⁃stants of all these catalysts for the degradation of MO are exhibited on Table S1(see the Supporting Information of this paper).From the table,the photocatalyst CNH/MoS2-0.5% shows more superior activity than others.Its rate constants is 0.19 min—1for MO degradation,which is nearly four times of that for CNH.A remarkable improvement is achieved after hybridization.Afterwards,it is high electron conductivity of 1T′MoS2that help to hinder recombination of carriers in CNH planes.The improved visible light absorption and carriers’separa⁃tion efficiency boost photocatalytic activity of the catalysts.However,the excessive 1T′MoS2in the compos⁃ites may lead to carriers’recombination in MoS2planes,bringing decrease in activity for CNH/MoS2-2%.Hence,CNH/MoS2-0.5% exhibits the best photocatalytic activity among the composites under visible light.Then,the stability of the catalysts is also another important factor to evaluate photocatalytic performance.In Fig.5(C),CNH/MoS2-0.5% is applied for 5-time MO degradation reactions and few decays can be seen after photocatalytic cycling.Raman spectra are also used to detect the phase of MoS2.As shown in Fig.S3(see the Supporting Information of this paper),the MoS2in the CNH/MoS2-0.5%nanocomposites still keep 1T′phase without changing into 2H phase,which illustrates the stability of 1T′MoS2in the degradation reactions.

Fig.5 Photocatalytic degradation rates of MO with different catalysts under visible light(A),plots of ln(c/c0)against reaction time(B),bar plot showing the photodegradation rate of MO for 5 cycles using the CNH/MoS2⁃0.5% catalyst under visible light(C)and the MO degradation curves of the hybrid catalyst(D)
It is known to all that the active species,such as˙OH,O2˙-and the photogenerated holes(h+),play an important role in the degradation reactions.Nevertheless,not all of them take part or dominate in the photocat⁃alytic reactions.In order to figure out the mechanism of the photocatalytic degradation reactions,contrast tests were performed to study the active species for CNH/MoS2nanocomposites.Isopropanol(IPA,5 mmol/L),ben⁃zoquinone(BQ,1 mmol/L)and disodium ethyl-enediamine tetraacetate(EDTA-2Na,1 mmol/L)were used as scavengers for˙OH,O2˙-and h+,respectively[24].From Fig.5(D),after adding BQ,the degradation rate of MO decreased from 90.7%in 12 min to 45%under visible light.Besides,11.5%and 10%occurred in the re⁃action with EDTA-2Na and IPA,respectively.Therefore,it is demonstrated that O2˙-radicals dominate in the process of photocatalytic MO degradation as oxidants while˙OH and h+count a little in the reactions.
Hence,the degradation mechanism can be described in Fig.6.According to the scheme,the predomi⁃nant reaction occurred as the following equations:

According to the band edge position of ultrathin g-C3N4reported before,the VB position is around 1.6 eV with its CB position atca.−1.3 eV(vs.NHE)[21,25,26],exhibiting stability[27]under sunlight and appropriate conduction/valance band potentials for hydrogen/oxygen evolution[21,28].The Femi level of MoS2is located at about—1 eV[29],which leads to the injecting of photogenerated electrons from CNH into the MoS2.Thus,it is able to realize O2evolution or water decomposition without sacrificial agent.However,the low crystallinity of intraplate g-C3N4suffers from low carriers’mobility and always leads to low activity in H2/O2evolution.After⁃wards,it still remains a challenge to obtain ultrathin nanosheets of g-C3N4with good crystallinity.

Fig.6 Scheme illustration and energy band of the charge transfer behaviors of CNH/MoS2 2D nanocomposites under visible light
3 Conclusions
In summary,CNH/MoS2nanocomposites were prepared through the facile electrostatic self-assemble strategy,which exhibited a remarkable improvement in visible-light-driven photocatalysis compared with pure g-C3N4.The synergistic effects between ultrathin 1T′MoS2and g-C3N4nanosheets realize fast electrons transportation,which includes better light absorption and superior charge separation,resulting in improved photocatalytic performance.During the photocatalytic degradation reactions,1T′MoS2in thenanocomposites exhibits good stability andthe degradation curve of the CNH/MoS2catalysts fit with the pseudo-first-order kinetics model.The mechanism study indicates that the O2˙-radicals play the key role in photocatalysis.The H2evolution experiment illustrates that 1T′MoS2has the potential to replace noble metal in the photocataly⁃sis.Although the photocatalytic activity of H2evolution still needs to be improved,the achievements in this work may shed light on the application of 1T′MoS2for solar energy conversion.

This work is supported by the National Natural Science Foundation of China(Nos.21871008,21801247,21905292),the Science and Technology Commission of Shanghai,China(No.18YF1427200),the Key Research Program of Chinese Academy of Sciences(No.QYZDJ-SSW-JSC013)and the Shanhai Science and Technology Innovation Action Plan,China(No.20DZ1204400).
——李振声

