鸟粪石沉淀法脱除氨氮对鸡粪厌氧发酵过程的影响
李博文,朱鸿斌,郭建斌,董仁杰
鸟粪石沉淀法脱除氨氮对鸡粪厌氧发酵过程的影响
李博文1,朱鸿斌2,郭建斌1※,董仁杰1
(1. 中国农业大学工学院,北京 100083;2. 中国华电科工集团有限公司,北京 100160)
为缓解鸡粪厌氧发酵过程中产生的氨氮抑制,采用投加镁磷盐的方式,在厌氧发酵过程中原位脱除氨氮,考察鸟粪石沉淀法脱除氨氮对鸡粪厌氧发酵过程的影响及镁磷盐的利用效率。试验向稳定运行的半连续厌氧反应器内投加MgCl2·6H2O和K2HPO4·3H2O,理论脱除速率为3 000 mg/d。第一次加盐脱除氨氮后,试验组反应器内氨氮浓度由2 937 mg/L降低至1 466 mg/L,平均产甲烷量为0.39 L/g,相较对照组的0.33 L/g提高了18%,镁磷盐利用率为91%;第二次加盐脱除氨氮后,试验组氨氮浓度由2 232 mg/L降低至762 mg/L,平均产甲烷量为0.33 L/g,相较对照组的0.30 L/g提高了10%,镁磷盐利用率为90%。研究表明鸟粪石沉淀法能较好的与厌氧发酵过程相耦合,在脱除氨氮缓解抑制的同时,提高系统甲烷产量,并回收部分氮磷资源。
甲烷;发酵;氨氮;鸟粪石
0 引 言
随着中国经济的快速发展,人们对于肉蛋奶等产品的需求也不断提高,畜禽养殖业的快速发展也带来了大量的畜禽粪污[1]。厌氧消化是一种有效的废弃物资源化利用技术,但在厌氧发酵过程中鸡粪及猪粪等畜禽粪污中高含量的尿素、蛋白质等有机质被分解产生氨氮,氨氮浓度通常达到3 000 mg/L以上时,会开始产生氨氮抑制现象[2],降低产气率[3-4]。厌氧反应氨抑制可以通过降低氨氮浓度和提高微生物耐受能力等方法来解除,如稀释、调节进料碳氮比、沉淀、吸附、驯化、添加微量元素等[5-10]。
鸟粪石沉淀法是利用镁磷和氨形成不溶的磷酸铵镁沉淀来除磷脱氮[11],生成的磷酸铵镁含有高含量的P2O5和氮素,是一种优良的缓释肥[12]。Rahman等[13]综述了影响鸟粪石沉淀的因素,其中镁磷摩尔比、pH、投加试剂的种类及其他离子的存在(如Ca2+、Fe2+等)是影响磷酸铵镁反应的主要因素。国内外已有许多研究探索了添加不同镁源或调控反应条件以优化鸟粪石结晶过程来回收氮磷资源,主要集中在污水、厌氧消化液等养分回收领域[14-15]。但是,将鸟粪石沉淀法与厌氧发酵相结合,实现厌氧反应过程实时原位脱氨的研究较少。本研究采用鸟粪石沉淀法在鸡粪中温厌氧发酵过程中脱除氨氮,研究鸟粪石沉淀法脱氨对厌氧消化系统产甲烷的影响。旨在通过鸟粪石沉淀法在解除鸡粪厌氧发酵过程中氨氮抑制的同时,提高厌氧发酵过程产甲烷能力,为鸟粪石沉淀法与厌氧消化过程相结合提供参考依据。
1 材料与方法
1.1 试验材料
试验采用的鸡粪取自中国农业大学蛋鸡养殖基地。为确保进料均匀,将取回的鸡粪加水稀释后过0.850 mm筛,除去羽毛蛋壳及沙子等杂质后置于4 ℃冷库备用。接种污泥取自实验室运行良好的中温鸡粪厌氧反应器,反应器在水力停留时间为15 d,进料TS为5%的条件下稳定运行了150 d,没有发生明显的氨抑制现象。进料鸡粪和污泥的理化指标如表1所示。
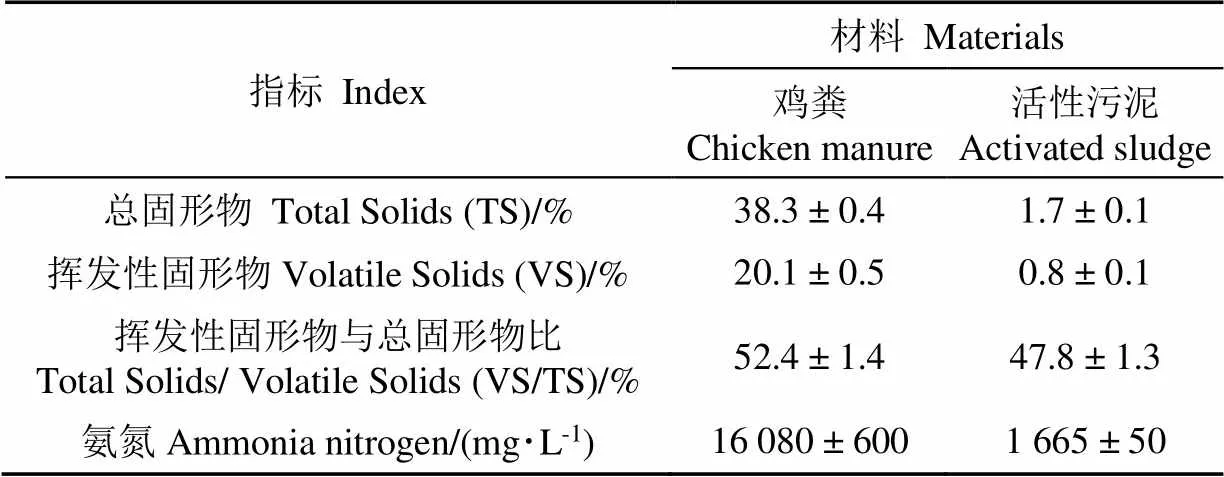
表1 原料及接种污泥的性质
1.2 试验设计
本研究为半连续厌氧发酵试验,采用2个总容积为15 L、有效容积为10 L的CSTR反应器,罐体外层带有水浴夹层,通过水浴锅控制温度,反应器顶部配有搅拌电机及配套的调速控制器。2反应器各接种10 L污泥后启动,搅拌转速120 r/min,在中温条件(35±2)℃下进行。试验的水力停留时间为15 d,初始有机负荷(以体积浓度计)为1.5 g/(L‧d),后续具体运行情况如表2所示。反应器所产沼气通过集气袋收集,每日测定气体产量、甲烷浓度,及发酵液pH值,每3~4 d测定发酵液中的氨氮及有机酸浓度。采用MgCl2·6H2O和K2HPO4·3H2O分别作为镁磷盐添加进行脱氨操作,脱氨期间,每日将43.5 g MgCl2·6H2O及48.8 g K2HPO4·3H2O与进料鸡粪混合后投入试验组反应器内。在此期间,每日对出料中的氨氮及有机酸浓度进行测定。脱氨操作每次仅对单个反应器进行,另一反应器不做处理正常进出料。在第36~41天对反应器A进行脱氨操作,在第56~62 天对反应器B进行脱氨操作,来缓解反应器内氨氮累积产生的抑制现象,第一次加盐时反应器在OLR为2 g/(L‧d)的条件下运行了13 d,此时反应系统氨氮已经达到较高水平,产气量出现下降,第二次加盐时为反应器在OLR为2 g/(L·d)的条件下运行了33 d,此时反应系统已经运行了2个水力停留时间,处于稳定运行状态。
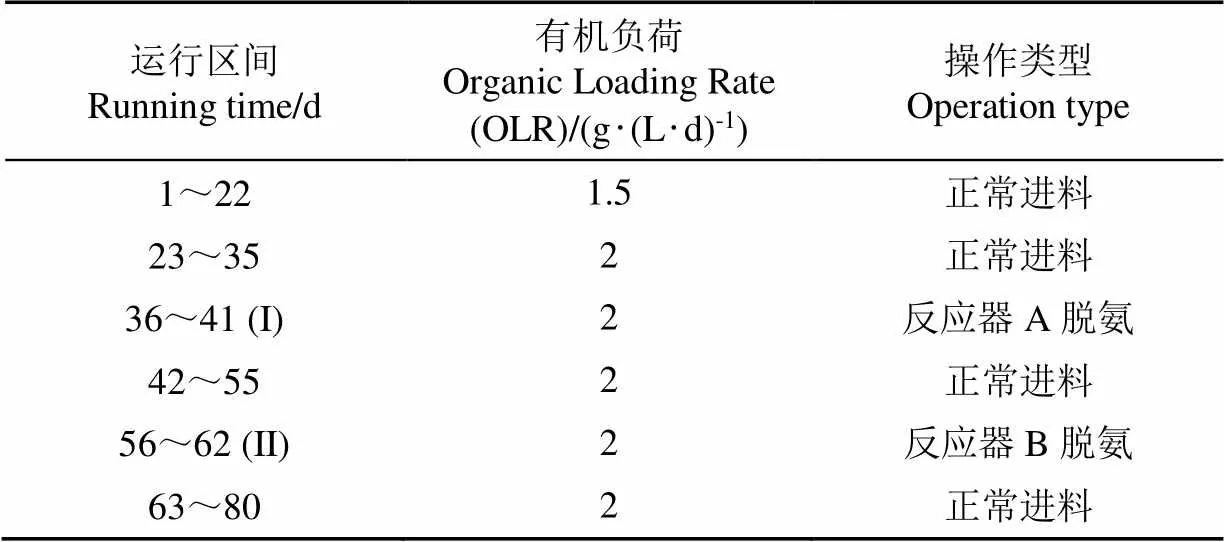
表2 试验运行方案
注:(I)A组脱氨/B组正常进料;(II) B组脱氨/A组正常进料,下同。
Note: (I) Ammonia nitrogen removal for group A/Normal feeding for group B; (II) Ammonia nitrogen removal for group B/Normal feeding for group A, the same below.
1.3 氨氮脱除量计算方法
氨氮脱除量计算方法根据物质守恒定律及经验分析得出。
对反应器进行第次脱除氨氮操作时,反应器内部的氨氮平衡为
根据未处理反应器出料中的氨氮浓度,可推算出每日进料引入的氨氮含量X
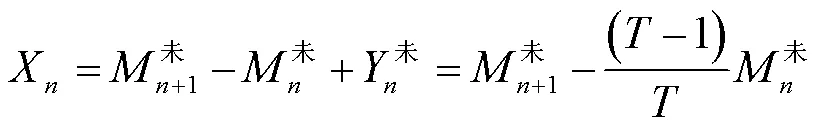
将计算得到的进料氨氮含量带入进行脱除氨氮的反应器的氨氮平衡公式中,可计算得到沉淀脱氨操作脱除的氨氮含量Z
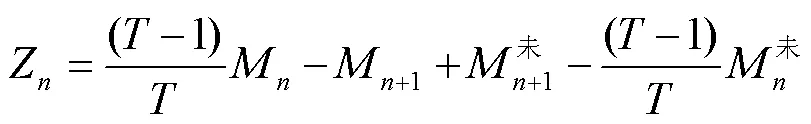
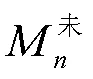
1.4 分析方法
沼气采用湿式气体流量计及沼气分析仪分别测定产气量及甲烷含量。采用玻璃电极法测定发酵液pH值;挥发性脂肪酸(Volatile Fatty Acid /VFA)采用GC测定[16];氨氮采用水杨酸-次氯酸盐光度法测定[17]。
2 结果与讨论
2.1 鸟粪石沉淀法对氨氮浓度的影响及脱氨效率分析
图1反映了两组反应器中氨氮浓度动态变化情况,在反应器初始运行阶段,有机负荷为1.5 g/(L‧d),此时两组反应器内氨氮浓度维持在1 452~1 829 mg/L之间,提升有机负荷至2 g/(L‧d)后,两反应器内的氨氮浓度迅速上升至2 600 mg/L以上,最高时达到3 219 mg/L,对两反应器进行脱除氨氮操作时,氨氮浓度均迅速下降。在试验运行的第36~41天进行第一次加盐,加盐后试验组(反应器A)氨氮浓度从2 937 mg/L降低至1 466 mg/L,随后反应器A中的氨氮浓度在1 122~2 033 mg/L内浮动;在试验运行的第56~62天进行第二次加盐后,试验组(反应器B)氨氮浓度从2 232 mg/L降低至762 mg/L,在停止加盐17 d后与对照组(反应器A)氨氮浓度达到一致。在半连续厌氧消化系统中,含氮有机物分解转化产生氨氮,稳定后反应器中的氨氮浓度处于动态平衡。利用鸟粪石沉淀法脱除氨氮时,氨氮浓度迅速降低,打破了原先的平衡。加盐结束后,系统中的氨氮浓度维持在较低水平,氨氮脱除效果明显。
国内外已有许多利用鸟粪石沉淀法回收废水或沼液中氨氮和磷的相关研究。Li等在pH值为8.5~9的条件下,利用鸟粪石法去除垃圾渗滤液中的氨氮,氨氮的去除率超过96%[18]。Yetilmezsoy等利用鸟粪石法去除鸡粪废水中的氨氮,研究结果表明,在pH 9的条件下的去除效果最好,此时氨氮的去除率为85.4%[19]。郝晓地等对磷酸铵镁沉淀形成的最佳条件进行了研究,试验结果表明,在自来水反应体系中,当pH值在7.0~7.5时,鸟粪石纯度最高,随着pH值升高,沉淀中磷酸铵镁的含量逐渐降低[20]。在本研究中,采用鸟粪石沉淀法原位脱除鸡粪沼液中的氨氮,脱除反应过程中pH值介于6.9~7.8之间,根据氨氮脱除量计算公式推算出第一次沉淀脱除氨氮操作共去除16 462 mg氨氮,而理论氨氮去除量为18 000 mg,盐利用效率为91%。第二次沉淀脱除氨氮操作共去除氨氮18 945 mg,理论氨氮去除量为21 000 mg,盐利用效率为90%,脱除效率与前人的研究结果相似,表明在厌氧消化的过程中利用鸟粪石沉淀法脱除氨氮,可以高效利用试剂以调控消化系统氨氮的含量。同时,在反应器出料的固相中发现了白色颗粒状磷酸铵镁结晶。与前人研究不同的是,本研究在系统pH值为6.9~7.8的条件下也获得了较高的盐利用率,这可能由于系统中的氨氮浓度过高,促使鸟粪石反应发生并消耗掉了大部分的镁磷盐。
2.2 鸟粪石沉淀法对甲烷产量的影响
半连续厌氧发酵试验过程中甲烷产量的变化如图2所示。在1~22 d,两反应器在1.5 g/(L‧d)有机负荷的条件下运行,产气量在0.37~0.55 L/g范围内波动,两组反应器产气量无显著差异,平行性较好,且均未产生抑制现象。在23~35 d,反应器有机负荷提升至2 g/(L‧d),此时氨氮浓度上升到2 600~3 219 mg/L(如图1),产生抑制现象,产气呈现缓慢下降趋势,逐渐由该负荷初期的0.54 L/g下降到第35天的0.43 L/g。在36~41 d时,对反应器A进行连续6 d的脱除氨氮处理,反应器B正常进料,在此期间试验组(反应器A)的产甲烷量由0.44 L/g上升至0.46 L/g,而对照组(反应器B)的产甲烷量由0.43 L/g降低至0.40 L/g。加盐有效缓解了氨氮抑制,加盐期间甲烷产量与未加盐组相比提升了15%。在42~56 d,即停止加盐后的15 d内,试验组(反应器A)的平均产甲烷量为0.39 L/g,而对照组(反应器B)为0.33 L/g,采用配对t检验的方式对15 d内的单位产甲烷量进行统计分析,二者有显著性差异(< 0.05),脱氮后的平均单位产甲烷量相较对照组提升18%。值得注意的是,反应器A在停止加盐后的日产甲烷量显著低于加盐过程中的日产甲烷量,这可能是由于加盐过程中引入了大量的钾离子对厌氧消化系统产生了抑制作用而造成的。Chen等的研究表明,在未驯化的厌氧系统中,钾离子的浓度超过3 g/L就会抑制系统的产甲烷量[21]。在56~62 d,对反应器B进行连续7 d的脱除氨氮处理后,产甲烷量由0.30 L/g下降至0.28 L/g,减少了6.7%,反应器A正常进料,产甲烷量由0.38 L/g下降至0.32 L/g,减少了15.8%。在62~80 d,即停止加盐后的15 d内,试验组(反应器B)的平均单位VS产甲烷量为0.33 L/g,而对照组(反应器A)为0.30 L/g,采用配对t检验的方式对15 d内的单位产甲烷量进行统计分析,二者有显著性差异(< 0.05),进行脱除氨氮后的单位产甲烷量相较对照组提升10%。
两次脱除氨氮后反应器单位产甲烷量提升程度不同可能是由于氨氮浓度不同所致。研究认为,在厌氧发酵过程中,氨氮浓度超过3 000 mg/L时,系统将会受到抑制[22]。完成第一次氨氮脱除后,试验组(反应器A)的氨氮浓度降低至1 500 mg/L左右,而对照组(反应器B)的氨氮浓度在2 800 mg/L左右;而完成第二次氨氮脱除后,试验组(反应器B)的氨氮浓度降低至800 mg/L左右,但此时对照组(反应器A)的氨氮浓度也仅在1 800 mg/L左右。较低的氨氮浓度对厌氧发酵过程的抑制程度也相对较低,从而导致第二次脱除氨氮时的产甲烷量较第一次提升程度低。
两次脱除氨氮的操作均使得甲烷产量有所提升,这与前人的研究结果相似。Romero-Güiza等向稳定运行的中温厌氧反应器中添加5和30 kg/m3由低品位氧化镁配制而成的稳定剂,使甲烷产量增加了25%和40%,与本研究的结果相似[7]。然而,Uludag-Demirer等在研究时发现,在批式厌氧发酵过程中添加镁磷会导致了系统产甲烷量的降低[23],这与本文研究结果相反。这可能是由于试验为批式试验且所用的发酵底物不同导致的。批式发酵过程中无法进行物料交换,容易造成产物抑制,而半连续进料发酵试验中,水解产酸与产甲烷过程同时进行,不断与外界进行物料交换,能有效避免发酵过程中的产物抑制,使发酵过程处于稳定状态[24]。且Uludag-Demirer等的研究中以牛粪为原料,最终发酵液的氨氮浓度也仅在300 mg/L附近,通常不会产生氨氮抑制。因此,添加镁磷试剂,可能会引入较多的阳离子或改变发酵过程的pH从而造成总产气量降低。
2.3 鸟粪石沉淀法对有机酸含量的影响
图3显示了厌氧发酵过程中沼液总有机酸含量变化情况,总有机酸含量是指示厌氧发酵过程稳定及效率的重要指标。试验结果显示,在1~35 d,2反应器正常进料,总有机酸浓度上升至2 500 mg/L左右,出现了一定程度的积累。在36~41 d进行脱除氨氮处理后,试验组(反应器A)中总有机酸含量显著降低,从2 317 mg/L降低至72 mg/L(< 0.05)。后续发酵过程中试验组的总有机酸含量也在很低的水平内波动,未再发生积累。而对照组(反应器B)中总有机酸含量则持续上升至3 730 mg/L(第55天)。在进行第二次氨氮脱除操作后,试验组(反应器B)中的总有机酸含量也发生下降,在第63 天时浓度为2 321 mg/L。在停止加盐后,总有机酸含量继续下降至第79天的25 mg/L,与对照组在相同水平。在厌氧消化系统中,总有机酸含量受到产酸菌和产甲烷菌的双重影响,产酸菌将可溶性有机物先降解成小分子的VFA,再经产甲烷菌将VFA转化为甲烷,在稳定的厌氧消化系统中,各过程处于动态平衡中[22]。两次脱除氨氮处理后,试验组的总有机酸含量均显著降低,推测加入镁磷等试剂脱除氨氮后,系统内产酸菌受到抑制或甲烷菌活性提高或两者同时发生。Romero-Güiza等的研究结果表明,阳离子对于产酸菌可能会产生抑制作用而导致VFA含量的降低[25],在本试验中,停止加盐后,总有机酸的含量保持在较低水平,未发生累积,证明采用鸟粪石沉淀法脱除氨氮时主要是通过恢复产甲烷菌的活性来实现的。
2.4 鸟粪石沉淀法对pH值的影响
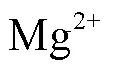
3 结 论
1)研究结果表明,鸟粪石沉淀法与鸡粪厌氧消化过程耦合效果良好。向半连续厌氧消化反应器中投加MgCl2·6H2O和K2HPO4·3H2O,能够有效降低鸡粪中温厌氧消化过程中产生的氨氮,缓解氨抑制,恢复产甲烷菌活性,促进有机酸的转化,进而提高产甲烷量。
2)以3 000 mg/d的去除速率,通过向半连续厌氧消化反应器中投加MgCl2·6H2O和K2HPO4·3H2O的方式来脱除氨氮后,试验组产甲烷量较对照组提高了10%~18%,外源性镁磷盐利用率在90%左右,具有较高的盐利用效率。
3)投加外源性MgCl2·6H2O和K2HPO4·3H2O会释放H+,降低发酵液pH,容易导致厌氧消化系统酸化。前人研究指出鸟粪石回收的最适pH值为8.5~9.0,而本研究中系统pH值在6.9~7.8之间也获得了较高的镁磷盐利用率,这可能由于系统中的氨氮浓度过高,促使鸟粪石反应发生并消耗掉了大部分的镁磷盐。
[1] 罗娟,赵立欣,姚宗路,等. 规模化养殖场畜禽粪污处理综合评价指标体系构建与应用[J]. 农业工程学报,2020,36(17):182-189.
Luo Juan, Zhao Lixin, Yao Zonglu, et al. Construction and application of comprehensive evaluation index system for waste treatment on intensive livestock farms[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2020, 36(17): 182-189. (in Chinese with English abstract)
[2] Morozova I, Nikulina N, Oechsner H, et al. Effects of increasing nitrogen content on process stability and reactor performance in anaerobic digestion[J]. Energies, 2020, 13(5): 1-19.
[3] Molaey R, Bayrakdar A, Sürmeli R Ö, et al. Anaerobic digestion of chicken manure: Mitigating process inhibition at high ammonia concentrations by selenium supplementation[J]. Biomass & Bioenergy, 2018, 108: 439-446.
[4] Calli B, Mertoglu B, Inanc B, et al. Effects of high free ammonia concentrations on the performances of anaerobic bioreactors[J]. Process Biochemistry, 2005, 40(3/4): 1285-1292.
[5] Hejnfelt A, Angelidaki I. Anaerobic digestion of slaughterhouse by-products[J]. Biomass & Bioenergy, 2009, 33(8): 1046-1054.
[6] Vries J W D, Vinken T M W J, Hamelin L, et al. Comparing environmental consequences of anaerobic mono- and co-digestion of pig manure to produce bio-energy-A life cycle perspective[J]. Bioresource Technology, 2012, 125(12): 239-248.
[7] Romero-Güiza M S, Astals S, Chimenos J M, et al. Improving anaerobic digestion of pig manure by adding in the same reactor a stabilizing agent formulated with low-grade magnesium oxide[J]. Biomass & Bioenergy, 2014, 67: 243-251.
[8] Zhang N, Stanislaus M S, Hu X, et al. Strategy of mitigating ammonium-rich waste inhibition on anaerobic digestion by using illuminated bio-zeolite fixed-bed process[J]. Bioresource Technology, 2016, 222: 59-65.
[9] Sung S, Tao L. Ammonia inhibition on thermophilic anaerobic digestion [J]. Chemosphere, 2003, 53(1): 43-52.
[10] Banks C J, Zhang Y, Jiang Y, et al. Trace element requirements for stable food waste digestion at elevated ammonia concentrations[J]. Bioresource Technology, 2012, 104: 127-135.
[11] 李金页,郑平. 鸟粪石沉淀法在废水除磷脱氮中的应用[J]. 中国沼气,2004,22(1):7-10.
Li Jinye, Zheng Ping. Applications of struvite precipitation in removal of phosphorus and nitrogen from wastewater[J]. China Biogas, 2004, 22(1): 7-10. (in Chinese with English abstract)
[12] 陈静霞,李咏梅. 鸟粪石沉淀法预处理高氨氮废水的镁盐研究[J]. 环境工程学报,2011,5(12):2663-2667.
Chen Jingxia, Li Yongmei. Study on magnesium agents for the pretreatment of high-strength ammonia wastewater by struvite precipitation[J]. Chinese Journal of Environmental Engineering, 2011, 5(12): 2663-2667. (in Chinese with English abstract)
[13] Rahman M M, Salleh M, Rashid U, et al. Production of slow release crystal fertilizer from wastewaters through struvite crystallization: A review[J]. Arabian Journal of Chemistry, 2014, 7: 139-155.
[14] Suzuki K, Tanaka Y, Kuroda K, et al. Removal and recovery of phosphorous from swine wastewater by demonstration crystallization reactor and struvite accumulation device[J]. Bioresource Technology, 2007, 98(8): 1573-1578.
[15] Doyle J D, Parsons S A. Struvite formation, control and recovery[J]. Water Research, 2002, 36(16): 3925-3940.
[16] 乔玮,任征然,李晨艳,等. 自搅拌厌氧折流板反应器连续处理猪场废水的效果[J]. 农业工程学报,2018,34(20):210-215.
Qiao Wei, Ren Zhengran, Li Chenyan, et al. Continuous anaerobic treatment of swine wastewater by using self-agitation anaerobic baffled reactor[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2018, 34(20): 210-215. (in Chinese with English abstract)
[17] 国家环境保护总局. 水和废水监测分析方法(第四版)[M]. 北京:中国环境科学出版社,2002
[18] Li X Z, Zhao Q L, Hao X D. Ammonium removal from landfill leachate by chemical precipitation[J]. Waste Management, 1999, 19(6): 409-415.
[19] Yetilmezsoy K, Sapci-Zengin Z. Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer[J]. Journal of Hazardous Materials, 2009, 166(1): 260-269.
[20] 郝晓地,兰荔,王崇臣,等. MAP沉淀法目标产物最优形成条件及分析方法[J]. 环境科学,2009,30(4):1120-1125.
Hao Xiaodi, Lan Li, Wang Chongchen, et al. Optimal formation conditions and analytical methods of the target product by MAP precipitation[J]. Environmental Science, 2009, 30(4): 1120-1125. (in Chinese with English abstract)
[21] Chen Y, Cheng J J. Effect of potassium inhibition on the thermophilic anaerobic digestion of swine waste[J]. Water Environment Research, 2007, 79(6): 667-674.
[22] 野池达野. 甲烷发酵(刘兵,薛咏海)[M]. 北京:化学工业出版社,2014.
[23] Uludag-Demirer S, Demirer G N, Frear C, et al. Anaerobic digestion of dairy manure with enhanced ammonia removal[J]. Journal of Environmental Management, 2008, 86(1): 193-200.
[24] 郭建斌,董仁杰,程辉彩,等. 温度与有机负荷对猪粪厌氧发酵过程的影响[J]. 农业工程学报,2011,27(12):217-222.
Guo Jianbin, Dong Renjie, Cheng Huicai, et al. Effect of temperature and organic loading rates on anaerobic digestion of pig manure[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2011, 27(12): 217-222. (in Chinese with English abstract)
[25] Romero-Güiza M S, Astals S, Mata-Alvarez J, et al. Feasibility of coupling anaerobic digestion and struvite precipitation in the same reactor: Evaluation of different magnesium sources[J]. Chemical Engineering Journal, 2015, 270: 542-548.
[26] Karthikeyan O P, Joseph K. Chemical precipitation of ammonia-N as struvite from landfill leachate effect of molar ratio upon recovery[J]. Journal of Solid Waste Technology & Management, 2008, 34(1): 20-26.
[27] 邓玉营,阮文权,郁莉,等. pH调控对瘤胃液接种稻秸厌氧消化中水解菌及产甲烷菌的影响[J]. 农业环境科学学报,2018,37(4):813-819.
Deng Yuying, Ruan Wenquan, Yu Li, et al. The effect of pH adjustment on hydrolytic bacteria and methanogens during rumen fluid derived anaerobic digestion of rice straw[J]. Journal of Agro-Environment Science, 2018, 37(4): 813-819. (in Chinese with English abstract)
Effect of ammonia nitrogen removal by struvite precipitation method on the anaerobic digestion of chicken manure
Li Bowen1, Zhu Hongbin2, Guo Jianbin1※, Dong Renjie1
(1.,,100083,;2..,.,100160,)
Anaerobic digestion has been widely utilized to dispose of agricultural organic wastes. The renewable energy of methane can be produced during the treatment, together with the digestates rich in the nutrients for the fertilizer. However, the ammonia nitrogen can be tended to accumulate during anaerobic digestion, when using a large proportion of protein-rich substrates, such as chicken manure, pig manure, and kitchen wastes. Once the concentration of ammonia nitrogen reaches over 3 000 mg/L in the anaerobic process, the ammonia inhibition is likely to happen, resulting in the decrease of microorganisms’ activities and methane production during anaerobic digestion. Struvite precipitation can be a useful way to remove the ammonia nitrogen and phosphorus in the digestates and wastewater. Many studies have been reported to optimize the reaction conditions, such as the molar ratio of Mg to P, pH level, and temperature, to recover the struvite. However, there are only a few studies to combine struvite precipitation with anaerobic digestion. This study aims to investigate the effect of in-situ struvite precipitation on the anaerobic digestion of chicken manure. The MgCl2·6H2O and K2HPO4·3H2O were mixed into the feeding substrate in the stable running reactors for 6-7 consecutive days to remove NH4+-N. The theoretical removal rate was at the speed of 3 000 mg/d. Some parameters were detected, including the concentration of ammonia nitrogen, methane yield, total volatile fatty acids (TVFA), and pH during anaerobic digestion. After the first operation of adding MgCl2·6H2O and K2HPO4·3H2O, the concentration of ammonia nitrogen and TVFA were reduced from 2 937 to 1 466 mg/L, and 2 317 to 72 mg/L, respectively, whereas, the methane production was 0.39 L/gVS increased by 18%, compared with the control group (0.33 L/gVS), where the utilization rate of magnesium and phosphate was 91%. After the second operation, the concentration of ammonia nitrogen and TVFA were reduced from 2 232 to 762 mg/L, and 2 321 to 25 mg/L, respectively, whereas, the methane production was 0.33 L/gVS increased by 10% approximately, compared with the control group (0.30 L/gVS), where the utilization rate of magnesium and phosphorus was 90%. The results demonstrated that the addition of exogenous MgCl2·6H2O and K2HPO4·3H2O greatly contributed to mitigating the ammonia inhibition by struvite precipitation during the anaerobic digestion. An optimum pH was 8.5-9 (Li et al, 1990) for the struvite precipitation in the nutrient recovery of wastewater. A high utilization rate of magnesium and phosphorus was also achieved, when the pH of the system was 6.9-7.8, due to the high ammonia nitrogen concentration in the system. As such, it can be widely expected to promote the struvite precipitation to consume most of the magnesium phosphate salts. The exogenous MgCl2·6H2O and K2HPO4·3H2O can release H+ in the system, when the struvite was formed the lower pH to consume the alkalinity in the digester, easily leading to the acidification of anaerobic digestion. Consequently, the amount of exogenous MgCl2·6H2O and K2HPO4·3H2O needs to be controlled within a reasonable range for the stable anaerobic process.
methane; fermentation; ammonia nitrogen; struvite
李博文,朱鸿斌,郭建斌,等. 鸟粪石沉淀法脱除氨氮对鸡粪厌氧发酵过程的影响[J]. 农业工程学报,2021,37(22):220-225.doi:10.11975/j.issn.1002-6819.2021.22.025 http://www.tcsae.org
Li Bowen, Zhu Hongbin, Guo Jianbin, et al. Effect of ammonia nitrogen removal by struvite precipitation method on the anaerobic digestion of chicken manure[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2021, 37(22): 220-225. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2021.22.025 http://www.tcsae.org
2021-08-17
2021-10-29
国家自然科学基金项目(U20A2086);中德国际研究培训项目(粮食-饲料-能源玉米生产系统磷资源高效利,328017493/GRK 2366);中国农业大学2115人才工程资助
李博文,博士生,研究方向为农业废弃物处理与资源化利用技术。Email:bowenlinz@cau.edu.cn
郭建斌,博士,副教授,研究方向为农业废弃物处理与资源化利用技术。Email:jianbinguo@cau.edu.cn
10.11975/j.issn.1002-6819.2021.22.025
S216.4;X705
A
1002-6819(2021)-22-0220-06

