Preparation and evaluation of mucoadhesive bioflexy films from Cocos nucifera biopolymer using Tiagabine moiety
Sugandha Varshney,and N.V.Satheesh Madhav
Abstract—This research work deals with formulation and evaluation of nanosized Tiagabine loaded bio-flexy films consisting of Cocos nucifera biopolymer (isolated from coconut kernels).Prepared formulations were administered through soft palatal route for brain targeting for epilepsy treatment.Soft palate,part of oral mucosa serves as novel drug delivery platform and mucoadhesive site for systemic drug delivery.It provides sustained and controlled drug delivery system,does not interfere with patient’s regular activities like talking,eating,drinking,etc.It bypasses firstpass metabolism in the liver,reduces dose frequency and minimizes drug’s side effects.Tiagabine,anticonvulsant drug possesses t1/2:7-9 h (low); Protein binding:96%; Water solubility:22 mg/L,acts as selective gamma amino butyric acid (GABA) reuptake inhibitor.Cocos nucifera biopolymer used as bio-excipient to prepare bio-flexy films due to its biodegradability,biocompatibility,non-toxicity,nonirritantancy on soft palatal surface along with inbuilt filmability,mucoadhesive properties.Nanosized drug loaded bio-flexy films were formulated using standard solvent casting method.Bio-flexy films were prepared using varying ratios of nanosized Tiagabine:isolated Cocos nucifera biopolymer (FCT1-FCT6).These prepared formulations were compared with same ratios of nanosized Tiagabine:sodium carboxyl methyl cellulose standard polymer flexy films (FET1-FET6).
Key words—Nanosized Tiagabine,Soft palatal delivery,Mucoadhesive,Cocos nucifera,Biopolymer,Bio-flexy films
INTRODUCTION
Soft palate is a flexible,muscular flap which is a part of oral mucosa.It extends postero-interiorly from posterior edge of hard palate into pharyngeal cavity.It can serve as Novel Drug Delivery Platform and mucoadhesive site for systemic drug delivery [1].Soft palate drug delivery may serve as a unique smart route for brain targeting of drugs via intra and inter-neural pathway,crossing blood brain barrier (BBB),decreases drug’s side effects and increases its therapeutic effect.It has non-keratinized histology,sustained and controlled drug delivery system.Tongue activity,salivary secretion does not affect drug performance via soft palatal mucosa.It does not interfere with patient’s regular activities like talking,eating,drinking,etc.It has enriched blood and neural supply,drug directly reaches systemic circulation,non-invasive,no bone,non-mobile with high mucoretentivity,bioavailability.It delivers drugs both systemically and locally.Therapeutic potential of many drugs can be improved by soft palatal route.It is innervated by lesser palatine nerve; Greater palatine nerve; Mandibular branch of trigeminal nerve; Nasopalatine nerve; Glossopharyngeal nerve; Motor nerves as well as middle meningeal artery,Ascending palatine branch of facial artery,accessory meningeal artery,ascending pharyngeal artery,greater palatine branch of maxillary artery [2].It has thickness:158-224 μm,pH:7.34 ± 0.38,blood flow:0.89 mL/min/cm,Surface area:200 cm2.Soft palatal mucosa is richly vascularized.Permeability is 4-4000 times more than that by skin.It closes off the nasal passages during swallowing.No mechanical irritation due to its smooth surface,good flexibility,devoid of bones.Drug delivery occurs in controlled,sustained,retentive,systemic manner.It offers optimal site-specific delivery as pH value nearer to blood.low enzyme activity avoids the acid hydrolysis of drug.Enhances the drug bioavailability and provides rapid drug transportation to systemic route.It provides steady infusion of drugs for sustained time period.Pre-systemic elimination is bypassed.Orally inefficient drugs like potent peptide,protein drug molecules can be feasibly delivered by this route.Better patience compliance occurs by this route due to ease of application and removal of dosage form from administration site.It has high patient compliance.Adverse effects and therapeutic failures frequently associated with intermittent dosing are avoided,drug toxicity is reduced.Drugs having erratic absorption from the stomach or intestine can be administered by this route.It overcomes the inconvenience caused by pain,tissue damage that occurs in drug administration via parenteral route.The soft palatal region being flexible and mobile tissue can be easily accessed for placing the dosage form.Nanosized drug can directly reach to site of action i.e.,brain via interneural and intra neutral pathway [3].
Epilepsy is a chronic neurological disorder affecting 65 million people globally as stated by World Health Organization.Epilepsy can be idiopathic i.e.,with no identifiable cause or symptomatic or secondary epilepsy i.e.,due to known cause such as brain damage,genetic abnormality,head injury,stroke,meningitis,encephalitis,brain tumor etc.There is no cure for epilepsy,but the disorder can be managed with medications and other strategies.It is characterized by recurrent seizures in which involuntary movements involving partial or complete brain occur that might lead to unconsciousness.It occurs due to excessive electrical discharges in brain cells.Two or more unprovoked seizures leads to epilepsy.There are two types of neurotransmitters in brain.Excitatory or glutamatergic neurotransmitter and inhibitory or gamma amino butyric acid (GABA) minergic neurotransmitters.Excess of excitatory neurotransmitter discharges lead to epilepsy [4].
Antiepileptic Tiagabine enhances activity of GABA,acts as selective GABA reuptake inhibitor.It is available in tablets and capsules dosage forms.Side effects include sudden unexpected death in epilepsy.Thus,if its dose and frequency of drug administration are reduced,its side effects can also be minimized [5].
Synthetic polymers used in manufacture of tablets.,capsules of Tiagabine contain harmful chemicals,thus if they are replaced by biopolymers,it will be safer and economical.
In this research work biopolymer obtained from Cocos nucifera kernels was found to be cost effective,inert as well as biodegradable.Cocos nucifera belongs to family arecaceae and contains vitamins like vitamin B1,vitamin B2,vitamin B3,vitamin B5,vitamin B6,vitamin B9,vitamin C,minerals like sodium,potassium,magnesium,calcium,iron,carbohydrates like sucrose,fructose,glucose,myoinosital,galactose-62.4 mg/100 g,raffinose-0.003-0.03 mg/100 g,proteins-3.6-4.5%,amino acids as primary metabolites,crude fiber-34.7%,saturated fatty acids-90%,unsaturated fatty acids-10%,phenolic compounds,peroxidase,polyphenol oxidase [6].Biopolymer serves as a suitable bio-excipient for the formulation of bio-flexy films.
Formulations were prepared by economical solvent casting technique.Tiagabine is anticonvulsant drug,Cocos nucifera biopolymer act as mucoadhesive,film forming cum retarding agent.Pectin is used as film initiator.Dextrose and fructose are used as flexicizer.Glycerin is used as plasticizer,and distilled water is used as solvent.
These formulations were screened for mucoadhesivity,mucoretentivity,permeability,in-vitro performance.The optimized formulations showing t1/2of more than 90 h.were selected as best formulations.Best selected formulations will be further used for in-vivo studies.
Existing epilepsy treatment requires prolonged oral therapy of anticonvulsants active pharmaceutical ingredient(API) molecules with increased dose frequency mode.This causes unexpected adverse reactions,side effects and withdrawal symptoms in patients due to cumulative dose dumping.Soft palatal route therapy avoids these drawbacks of oral therapy.This study screens the mucoadhesivity,feasibility of isolated biopolymer and also enlightens the potentiality of oro-soft palatal mucosa as a novelistic drug delivery platform for direct brain targeting of drugs in order to elicit anticonvulsant properly.
MATERIALS AND METHODS
Drug:Tiagabine (procured from Sun Pharmaceuticals Industries Ltd.,Gujarat)
Polymers:Coconut kernels bought from local market
Sodium Carboxyl Methyl Cellulose (Central drug House(P) Ltd.New Delhi
The experimental work was carried out using Double distilled water.
Isolation of biomaterial from Cocos nucifera [6]
Procured coconuts from local market.Removed outer skin of Cocos nucifera kernels,weighed 500 g of tender pulp.Prepared slurry using 300 mL of distilled water,filtered milky white slurry using muslin cloth.Added 1000 mL of propan-2-one to the filtrate,in proportion of 1:2.Refrigeration at 2-8°C for 24 h.Centrifuged mixture at 3500 rpm for 15 min.Separated supernatant,collected the residue.Naturally dried isolated biomaterial,powdered,passed through sieve NO.120.Optimized 6 times,calculated % yield.Stored in well closed container for further use.
Physicochemical characterization of isolated biomaterial [7]
The physiochemical characterization of isolated biomaterial was performed like color,odor,solubility,melting point and various chemical tests were performed.
(a) Texture,(b) Color,(c) Odor were physically monitored.
(d) Color changing point and melting point:determined by capillary method by using melting point apparatus.The bio-polymer was filled inside a capillary tube and inserted in melting point apparatus and the temperature was determined by means of thermometer.
(e) Solubility:determined in different solvents(chloroform,methanol,distilled water,acetone,carbon tetrachloride).
(f) Test for carbohydrates:Molisch reagent test:2 mL of biopolymer solution (0.1 g dissolved in 2 mL of distilled water) was taken in a test tube.2 drops of Molisch reagent(Solution of α-naphthol in 95% Ethanol) was added.Solution was then poured slowly into a test tube containing 2 mL of concentrated sulphuric acid.Two layers were formed.Observed color change.
(g) Test for proteins:Biuret test:This test determines the presence of peptide bonds in protein content in isolated biomaterial.2 mL of biomaterial solution (0.1 g dissolved in 2 mL of distilled water) was taken in a test tube.1 mL of sodium hydroxide solution (1%) followed by 1% copper (II)sulphate solution was added drop wise and shook the test tube vigorously.Allowed the mixture to stand for 5 min and observed the color change.In this test,Cu (II) ions in alkaline conditions form a violet chelate complex with peptide bonds.The chelate complex absorbs light at 540 nm so it appeared violet.Monitored the color change.
(h) Test for starch:2 mL of biomaterial solution (0.1 g dissolved in distilled water (2 mL)) was taken in a test tube.1-2 drops of iodine solution were added.Then observed the color change.
(i) Test for reducing sugar:2 mL of biopolymer solution(0.1 g dissolved in 2 mL of distilled water) was taken in a test tube.Added 1 mL each of Fehling’s A (7 g CuSO4·5H2O dissolved in distilled water containing 2 drops of dilute sulfuric acid) and Fehling’s B (35 g potassium tartrate,12 g of sodium hydroxide in 100 mL of distilled water).The test tube was placed in a water-bath at 60 °C.Observed color change.

Fig.1 Shear stress apparatus for determination of mucoadhesivity of isolated biopolymer
Spectral studies of isolated biopolymer [7,8]
Infra-Red (IR) spectroscopyThe IR spectroscopy of isolated biopolymer in solid form was performed by employing potassium bromide (KBr) disc method.1 mg of sample:100 mg of KBr were finely admixed in mortar.10 tons pressure was applied by help of hydraulic pump to form 1-2 mm pellet.Recorded IR spectrum of the pellet at 4000-200 cm-1.Similarly recorded IR spectra of pure drug.
Differential scanning calorimetry (DSC)Difference in heat from sample and reference was recorded against temperature for determining glass transition temperature(GTT or Tg).Specifications:Model-JADE DSC,Heat flow:50-250 °C,rate of 10 °C/min,Nitrogen flow rate:20 mL/min.Similarly,DSC of pure drug was also recorded.
Nuclear magnetic resonance (NMR) spectral analysisIt depends on the phenomenon of nuclear magnetic Resonance and provides detailed information about chemical environment of molecule.Dimethyl sulfoxide(DMSO) was solvent used.The spectrometer was connected to 5 mm flow cell.Applied high flow rates to the sample,activated a valve switch so as to stop the flow for fast measurement.As the valve switches back,again rinsed the flow cell with the reaction mixture.Spectrum was recorded using automation computer.
Scanning electron microscope (SEM) AnalysisSEM was used to perform morphological examination of isolated biomaterial.Small quantity of biomaterial was kept on aluminum studs coated with gold employing a sputter coater under vacuum (pressure:1 mm Hg).Recorded the image.
In-vitro mucoadhesivity of isolated biopolymer using modified shear stress apparatusVarying concentrations biopolymer solutions ranging from 1%,2%,4%,6%,8% to 10% were prepared and placed drop by measuring weight required for breaking adhesive bonds between the biomaterial and the glass plate after speci fi ed contact time between 0 to 30 min.Reported the results and plotted the graphs (Figure 1).
Standard graph of drug [9]
Preparation of standard curve of Tiagabine in distilled waterDissolved 10 mg of Tiagabine in 30 mL of distilled water taken in a 100 mL volumetric flask.Diluted up to the mark with distilled water to prepare 100 μg/mL stock solution.Prepared dilutions of concentrations (0.5,1,2,3,4 and 5 μg/mL) in 10 mL volumetric flasks.Made up the volume up to 10 mL with distilled water (λmax= 257 nm).Measured absorbance against solvent blank.
Preparation of standard curve of Tiagabine in phosphate buffer pH 7.4Dissolved 10 mg of Tiagabine in 30 mL of phosphate buffer (pH 7.4) taken in a 100 mL volumetric flask.Diluted up to the mark with distilled water to prepare 100 μg/mL stock solution.Prepared dilutions of concentrations (0.5,1,2,3,4 and 5 μg/mL) in 10 mL volumetric flasks.Made up the volume up to 10 mL with distilled water (λmax= 257 nm).Measured absorbance against solvent blank.
Drug excipient interaction studies [10]
In this study 3 different ratios of Drug:isolated biopolymer i.e.,1:1,1:3 and 3:1 were taken.Absorbance was measured and compared with that of pure Tiagabine.
(a) Dry method:In three petridishes Tiagabine:Cocos nucifera biopolymer (1:1,1:3 and 3:1) were taken in dry form,at room temperature for 2 h.Diluted the mixtures by 2 mL of methanol.Absorbance was measured to observed shift in λmaxwith that of pure drug and reported.
(b) Wet method:In three petridishes Tiagabine:Cocos nucifera biopolymer (1:1,1:3 and 3:1) were taken,moistened with 1 mL distilled water and dried for 30 min in at 50 °C in oven.Diluted the mixtures by 2 mL of methanol.Absorbance was measured to observed shift in λmaxwith that of pure drug and reported.
(c) Colorimetry method:On glass plate Tiagabine:Cocos nucifera 1:1 were mixed with potassium permanganate.Scrapped and color change was observed.Diluted with distilled water,analyzed by U.V.Similarly repeated with Tiagabine:distilled water and Tiagabine:potassium permanganate.
Significance of determination of drug-biopolymer interaction by dry and wet methodsThere was no drugisolated biopolymer interaction in both dry and wet methods.Being of natural origin,it was assured that isolated biopolymer was inert in both conditions depicting storage and oral delivery respectively.Thus,to confirm inertness and non-reactive properties of biopolymer with drug,these two methods were performed.The drug was intact,safe and did not got affected with biopolymer.
Preparation of Tiagabine from Tiagabine hydrochloride by precipitation method

Table 1 Formulation of nanosized Tiagabine loaded bio-flexy films using Cocos nucifera biopolymer
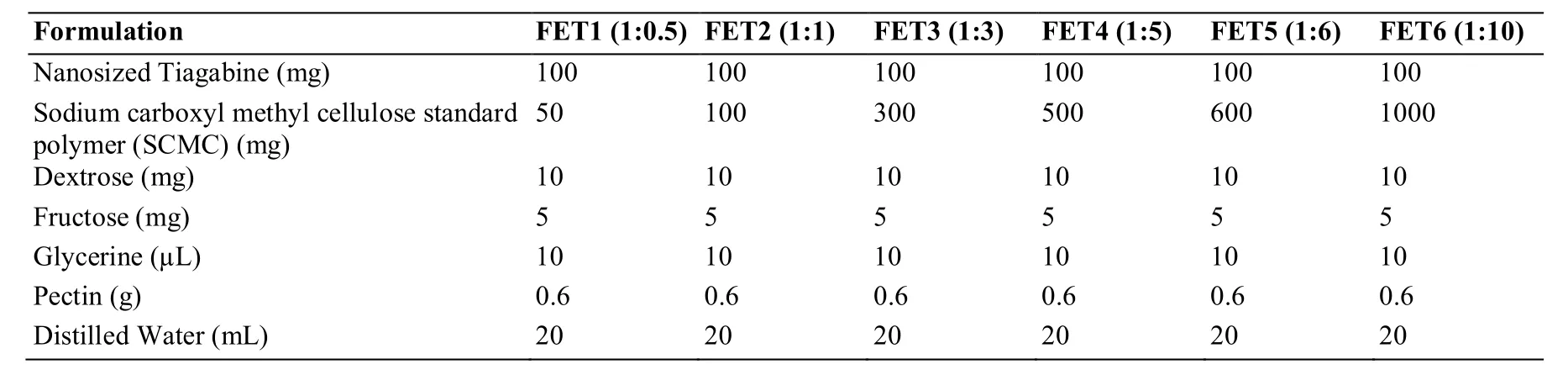
Table 2 Formulation of nanosized Tiagabine loaded flexy films using sodium carboxyl methyl cellulose standard polymer
Tiagabine exist as salt form i.e.,in Tiagabine hydrochloride form.Thus,to increase the absorption,bioavailability of drug and to avoid probable side effects it is converted to its pure form by using precipitation method.Took 20 mL of distilled water in a test tube,added 100 mg of Tiagabine hydrochloride and shaken vigorously.Sonicated for 1 cycle (3 min) using ultrasonic bath sonicator.Added dropwise 10 mL of sodium hydroxide solution (1N) to drug solution.Precipitate was obtained.Centrifuged mixture at 3,500 rpm for 15 min.Filtered,washed using 10 mL distilled water and air dried.Analyzed isolated Tiagabine so obtained using ultraviolet visible spectroscopy (U.V.) spectrophotometer.
Nanosizing of Drug
Solvent evaporation methodAdmixed 100 mg Tiagabine with 10 mg of dextrose,5 mg of fructose and 10 mL of methanol in mortar pestle.Sonicated the mixture for 5 cycles (180 sec/cycle).Diluted the mixture with 50 mL of distilled water and sonicated up to 15 cycles.% transmittance,absorbance,% blockage (100 - % transmittance) was measured after every 5 cycles.Dried the residue obtained and stored for further use [10].
Sonication methodAdmixed 100 mg Tiagabine with 10 mg of dextrose,5 mg of fructose and 10 mL of distilled water in mortar pestle.Sonicated the mixture for 5 cycles(180 sec/cycle).Diluted the mixture with 50 mL of distilled water and sonicated up to 15 cycles.% transmittance,absorbance,% blockage (100 - % transmittance) was measured after every 5 cycles.Dried the residue obtained and stored for further use [10].
The main reason of nanosizing Tiagabine by two different methods was comparing novel sonication method with published standard solvent evaporation method.
Nano size range determination by preliminary U.V.spectroscopic methodThe concept of Tyndall effect dominates transmittance.On passing of light of specified wavelength through the media containing particles less than or greater than the specified particle range,the % Transmittance denotes particles lies above the size range at particular range while the % blockage represents particles beyond the size range [10].
Permeation study of Tiagabine using Madhav Satheesh apparatus
Permeation study of nanosized Tiagabine was performed by using Madhav Satheesh (M.S.,Patent of Prof.(Dr.) N.V.Satheesh Madhav) apparatus.Nanosized Tiagabine (10 mg)was added in donor compartment while phosphate buffer(pH 7.4) was filled in receiver compartment.Tied egg shell membrane over donor compartment.Study was conducted for up to 48 h.Nanosized drug would permeate through egg membrane into phosphate buffer.Samples of 5 mL each were withdrawn at specific time intervals from 10 min up to 48 h and immediately restored with the same volume of fresh phosphate buffer each time.The amount of drug permeated was assessed by measuring the absorbance at 396 nm using U.V.spectrophotometer and compared with that of control i.e.,without nanosized drug and reported the same.
Formulation of bio-flexy films (solvent casting method) [11]
Triturated 100 mg of nanosized Tiagabine(anticonvulsant) with 50 mg of biopolymer (retarding agent,film forming and mucoadhesive agent) (in ratio of 1:0.5)for 2 min in pestle mortar.10 mL of distilled water (solvent)was added.To this dispersion,with continuous stirring incorporated 5 mg of fructose (flexicizer),10 mg of dextrose (flexicizer) and 10 μL of glycerine (1% solution v/v) (plasticizer).Added 0.6 g of pectin (film initiator).Further triturated the mixture for 5 min.Made up the volume up to 20 mL using distilled water.Subjected the mixture to magnetic stirring for 15 min,sonicated for 5 cycles (each cycle 3 min).Clear dispersion was obtained.Dried at room temperature for 24 h.Removed formulation from petridish.Cut bio-flexy film so obtained in 1 cm2size,store in well closed air tight container.Similarly,six different formulations of nanosized Tiagabine:standard sodium carboxyl methyl cellulose polymer in ratios of 1:0.5,1:1,1:3,1:5,1:6 and 1:10 were prepared (Table 1,Table 2).

Fig.2 Modified M.S.in-vitro diffusion apparatus
Evaluation of formulated bio-flexy films [12]
ThicknessThe average thickness of formulations was determined using standard digital micrometer and reported with appropriate standard deviation.
Surface pH studyThe formulations were allowed to swell by keeping in contact with 1 ml of distilled water for 1 h at room temperature.Measured the pH by bringing the electrode in contact with the surface of formulation and equilibrating for 1 min.Performed the experiments in triplicate and reported the avg.values.It is essential for formulation (rather than polymeric solution) to be neutral to mucosal surface and compatible with soft palatal pH.
Ex-vivo mucoadhesion study of formulations by rotating cylinder methodMucoadhesivity of formulated films was evaluated on intestinal mucosa of Capra aegagrus (i.e.,goat).Cut the formulations of area 1 cm2of each ratio.Goat intestinal mucosa was tied over the rotating basket of I-dissolution apparatus.900 mL of buffer(pH 7.4) was used as dissolution media,maintained temperature at 37 °C and rotation at 50 rpm.Applied formulations over the inner surface of goat intestinal mucosa till dislodgement.Observed and reported the dislodgement and detachment of formulations from mucosal surface at regular intervals.
Ex-vivo mucoretention study of formulationsCut the formulations of area 1 cm2of each ratio.Capra aegagrus(goat) intestinal mucosa was tied over slanting condenser.Buffer was allowed to flow over it from a burette.Formulations were applied over the inner surface of goat intestinal mucosa until dislodgement.Observed and reported the dislodgement and detachment of formulations from mucosal surface at regular intervals.
Weight uniformity of formulated nanosized drugs loaded bio-flexy filmsWeighed formulations of 1 cm2size of each ratio in triplicate.Calculated and reported average weight.
Drug content uniformity of formulated nanosized drugs loaded bio-flexy filmsFormulations were dissolved in 100 mL phosphate buffer (pH 7.4) with occasional shaking for 24 h.Five mL of solution was diluted with phosphate buffer pH 7.4 up to 20 mL and filtered Determined drug content by U.V.analysis at λmax257 nm.
Folding endurance of formulated nanosized drugs loaded bio-flexy filmsThe number of times of film could be folded at the same place without breaking will give the value of the folding endurance.This test was performed on three formulations of each ratio.
Swelling percentage study of formulated nanosized drugs loaded bio-flexy films1 cm2sized films were weighed,transferred in petridish and added 10 mL of distilled water.Reweighed the formulations after 1 h water absorption and swelling of bio-flexy films caused increased in their weights.Performed study for 24 h.% swelling index was calculated and reported.
Percentage moisture uptake (PMU) of formulated nanosized drugs loaded bio-flexy filmsPercentage moisture uptake of drug loaded bio-flexy films was determined to check their physical stability in high moist conditions.1 cm diameter formulations were placed chloride in desiccators in saturated solution of aluminum at 79.5% humidity.Formulations were removed after 3 d and weighed.Percentage moisture absorption was calculated and reported.

In-vitro drug release study of bio-flexy films formulations using modified M.S.in-vitro diffusion apparatusFilled buffer pH 7.4 was in 36 vials (receiver compartment).Placed in thermostatically controlled compartment.Egg membranes were tied to donor compartment which contained formulations.Inserted donor compartments into receiver compartments.Maintained temperature at 37 °C with orbital shaker incubator.Samples were taken at regular intervals from 10 min to 48 h.Replaced buffer completely after each sampling and analyzed every sample using U.V.apparatus (Figure 2).
Stability studies of prepared films as per International Conference on Harmonization (ICH)guidelines (Q1B)Performed stability studies of prepared formulations as per ICH guidelines Q1B.Stability testing of pharmaceutical product is conducted assure the safety,efficacy and quality of dosage forms and shelf life.The stability studies of the formulations were performed at 40 ±2 °C with 45 ± 5 % relative humidity (RH),at 25 ± 2 °C with 60 ± 5% RH and at 2 ± 5 °C conditions of temperature and relative humidity for 3 months.Observed for change in pH,folding endurance,in-vitro drug release of formulations at room temperature and after stability study(25 ± 2°C with 60 ± 5% RH).
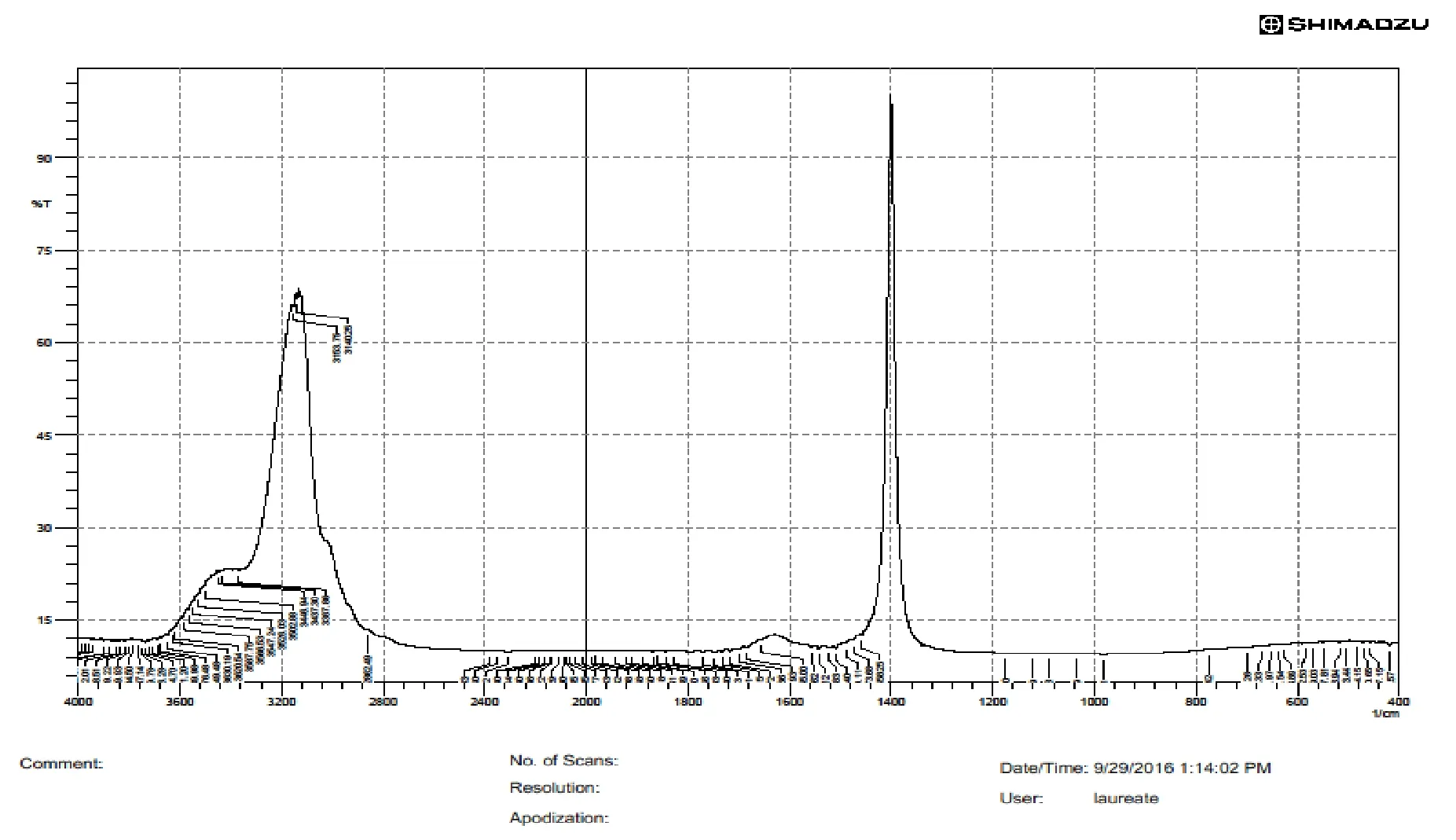
Fig.3 IR spectra of Cocos nucifera biopolymer
RESULTS AND DISCUSSION
In this study,trans oro-soft palatal mucosa was explored for its potentiality as a novel drug delivery platform for epilepsy treatment.In this research work,nanosized Tiagabine loaded bio-flexy films were formulated and evaluated.The aim of research work is brain targeting of drug via oro-soft palatal mucosa to obtain systemic drug delivery.The rationale of study is to explore the potentialities of soft palatal mucosa as a drug delivery platform.Biopolymer was isolated from Cocos nucifera kernels.Biopolymer was used to prepare flexy films because of its biodegradability,biocompatibility,non-toxic,non- irritant nature and no reaction on soft palatal surface.Biomaterial possessed in-built film forming properties along with mucoadhesive and mucoretentive properties.Thus,biomaterial was found to be suitable as bio-excipient for preparing bio-flexy films for trans oro-soft palatal delivery.
Yield of isolated biopolymer
Biopolymer was isolated from natural edible source of Cocos nucifera by simplified economic process.The isolation process was optimized six times and calculated the % yield.The results obtained were found to be reproducible with insignificant variation.Thus,this method can be adopted for scaling up in bulk.The % yield of Cocos nucifera biopolymer was found to be 10.2 ± 0.04%.
Physicochemical properties of isolated biomaterial
The biomaterial obtained from kernels of Cocos nucifera and showed following characteristics:
(a) Texture:powder.
(b) Color:brow
(c) Odor:odorless.
(d) Solubility:partially soluble in water.
(e) Color changing point:203 ± 1 °C.
(f) Molisch reagent test for carbohydrates:appearance of purple color at interface of the two layers due to formation of 5-hydroxy methyl furfural.This indicated presence of carbohydrates.
(g) Biuret test for proteins:color change was observed.Cu (II) ions form a violet-colored chelate complex that absorb light at 540 nm.This indicated presence of proteins.
(h) Test for starch:intense blue-black color did not appear confirmed the absence of starch in isolated biomaterial.
(i) Test for reducing sugar:brick red precipitate appeared indicating the presence of reducing sugar.It was due to formation of insoluble copper oxide.
Spectral studies of isolated biopolymer
IR spectroscopyPerformed for the isolated biomaterial in order to determine the presence of functional groups.Data revealed presence of carboxyl (-COOH),hydroxyl (-OH) groups which depicted that biopolymer possessed inbuilt mucoadhesivity.IR peaks of Cocos nucifera biopolymer were obtained at 3904 cm-1,3126 cm-1,1287 cm-1,1076 cm-1which indicated inbuilt functional groups RCH2OH,C=C-COOH,RCOOH,RNH2(Figure 3).
Differential scanning calorimetery (DSC)DSC peak of Cocos nucifera biopolymer was obtained at 86.54 °C,peak height was 3.8290 mW,delta H was 184.9613 J/g,onset depicted boiling point at 44.79 °C and glass transition temperature was 117.19 °C (Figure 4).
Nuclear magnetic resonance spectroscopy (NMR)1HNMR spectra of Cocos nucifera biopolymer confirmed the presence of carbohydrates residue within the biopolymer extracted as shift of carbohydrate protons were 3-6 ppm and the spectra when compared reflected the peak at 3.5513 ppm.δ 3.983 t(CH2) (Figure 5).
Scanning electron microscopy (SEM) of isolated biopolymerSEM image of Cocos nucifera biopolymer showed size range of 100 μm,flakes type irregular structure with smooth texture (Figure 6).
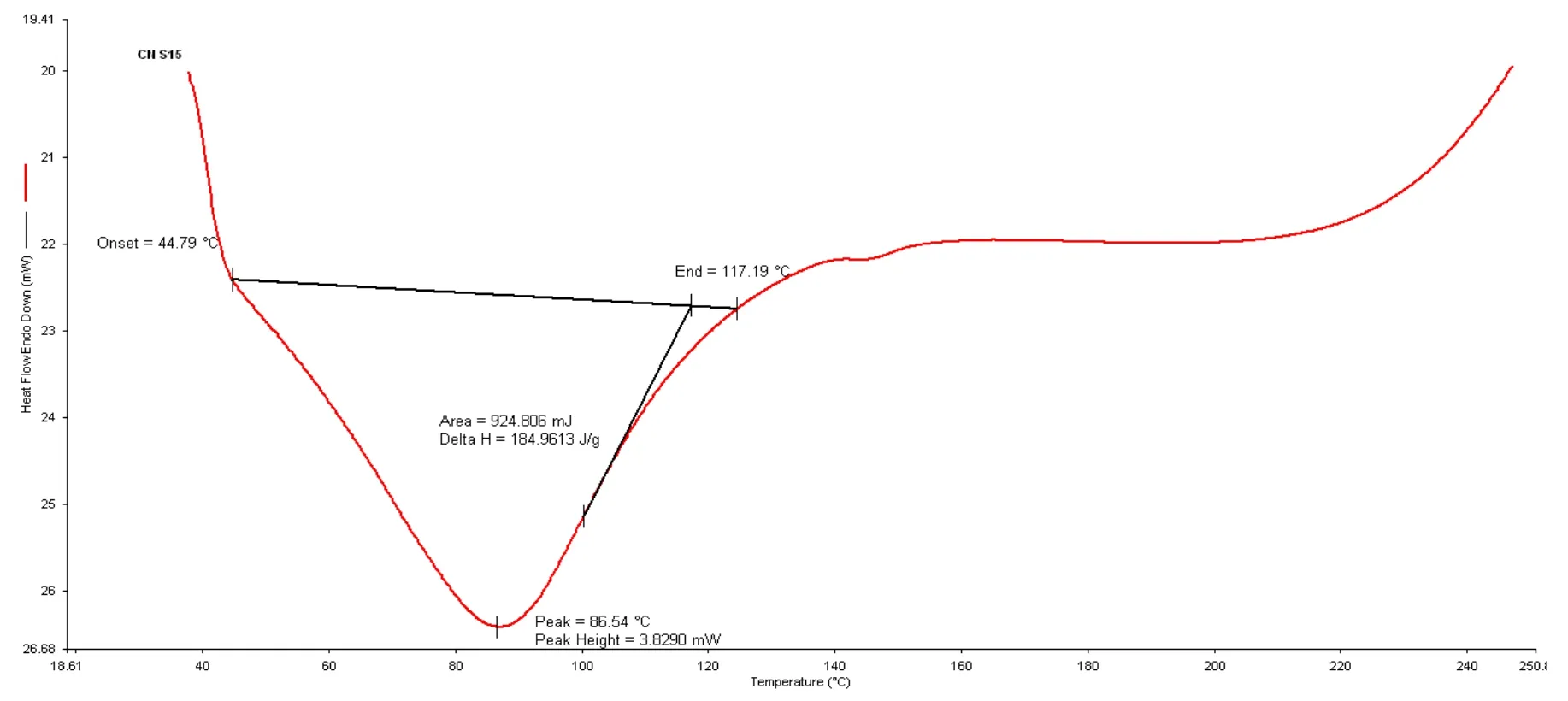
Fig.4 DSC spectra of Cocos nucifera biopolymer
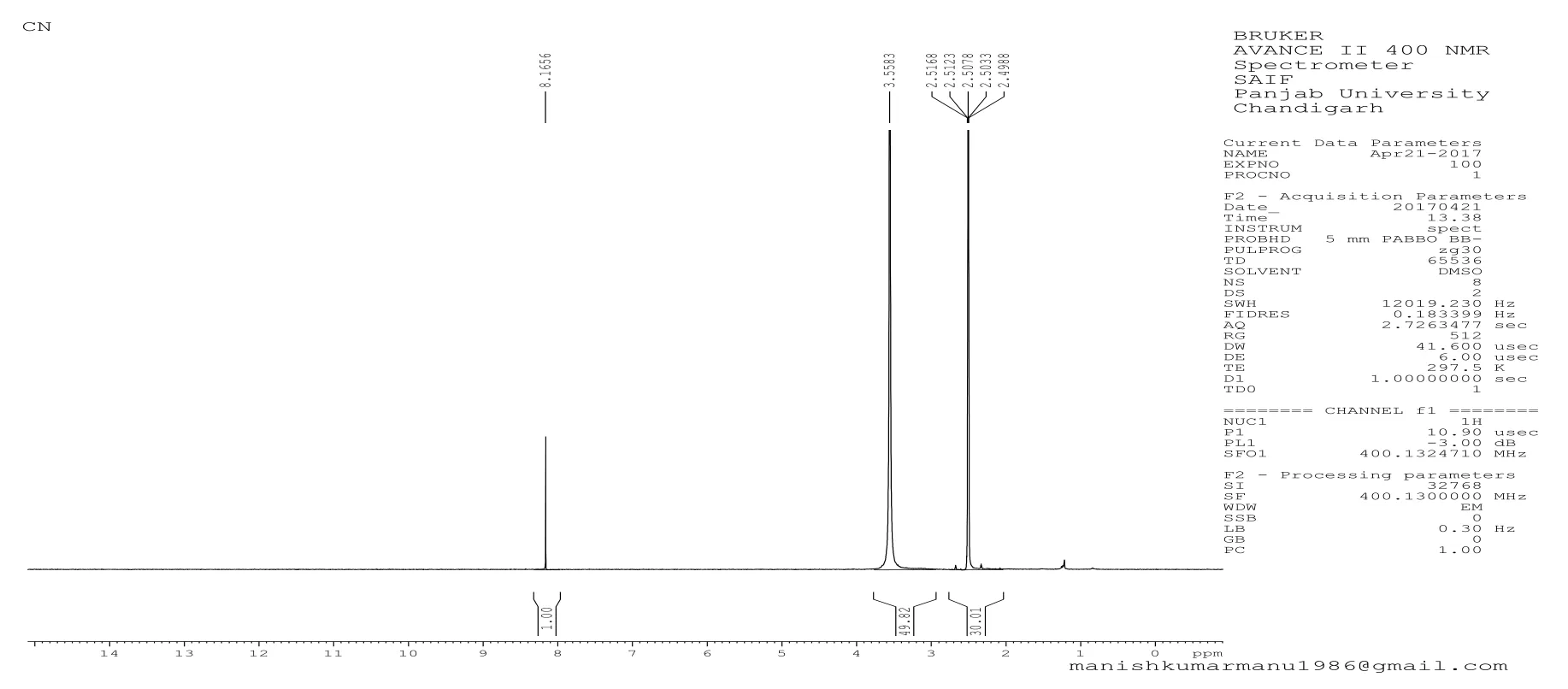
Fig.5 NMR spectra of Cocos nucifera biopolymer
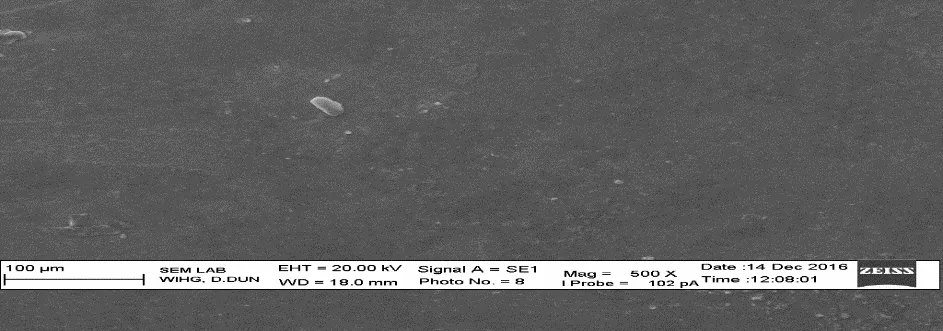
Fig.6 SEM of Cocos nucifera biopolymer

Table 3 In-vitro mucoadhesivity of Cocos nucifera biopolymer by shear stress method
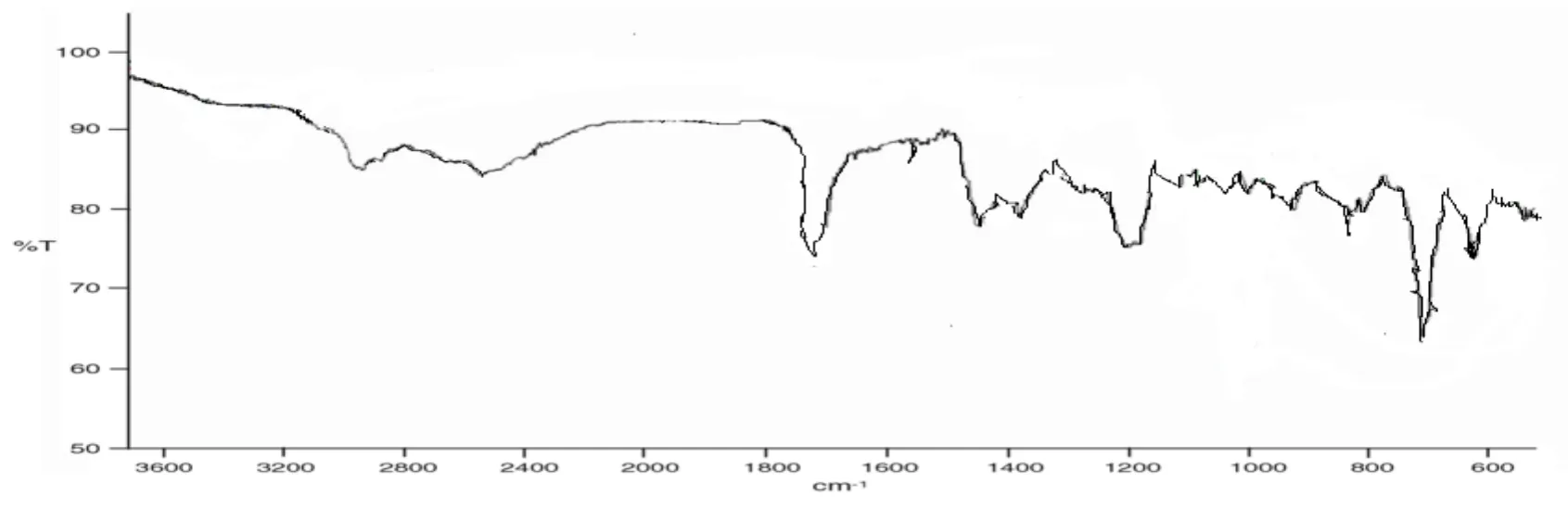
Fig.7 IR spectra of pure Tiagabine
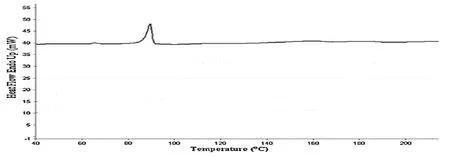
Fig.8 DSC spectra of pure Tiagabine
In-vitro mucoadhesivity of isolated biopolymers by shear stress methodIn-vitro mucoadhesion study data of Cocos nucifera biopolymer revealed that 1%,2% and 4% biopolymer concentrations showed significant results with P value < 0.05 when compared to 10% biopolymer concentration and also with 1% sodium carboxyl methyl cellulose standard polymer.Order of mucoadhesivity of all concentrations of Cocos nucifera biopolymer was 10% Cocos nucifera biopolymer > 8% Cocos nucifera biopolymer > 6% Cocos nucifera biopolymer > 4% Cocos nucifera biopolymer > 2% Cocos nucifera biopolymer >1% Cocos nucifera biopolymer (Table 3).
Spectral studies of pure Tiagabine
IR spectra of TiagabineIR peaks of Tiagabine were obtained at 1700 cm-1,1580 cm-1,1049 cm-1,1300 cm-1,1220 cm-1,which indicated functional groups at RCH2OH,N=O,S=O,RCOOH,RNH2respectively (Figure 7).
DSC spectra of Tiagabine:DSC peak of Tiagabine was obtained at 90 °C,Tf at 98 °C,Tg at 68 °C,onset at 64 °C(Figure 8).
Standard graphs of Tiagabine
Standard graph of Tiagabine in distilled waterPrepared calibration curve of tiagabine in distilled water by plotting graph of concentration versus absorbance.Linearity was obtained at a λmaxof 257 nm.R2value was 0.9997.(Figure 9a)
Standard graph of Tiagabine in phosphate buffer pH 7.4prepared calibration curve of tiagabine in phosphate buffer pH 7.4.by plotting graph of concentration versus absorbance.Linearity was obtained at a λmaxof 396 nm.R2value was 0.9967.(Figure 9b).
Drug-excipient interaction study
Drug-polymer interaction study of the isolated biopolymers was carried out using U.V.techniques using wet and dry methods.
Wet methodλmaxobtained at 260 nm,showed no significant difference from pure Tiagabine at 257 nm.Thus,no drug-excipient interaction occured.
Dry methodλmaxobtained at 260 nm,showed no significant difference from pure Tiagabine at 257 nm.Thus,no drug-excipient interaction occured.
ColorimetryTen mg of drug taken and mixed with biopolymers in ratio of drug:polymer 1:1 on glass plate.Added 10 μL of 1% solution of potassium permanganate.Observed change in color,diluted using distilled water,performed U.V.analysis.Scanned in 200-800 nm range and determined absorbance.Similarly performed with drug:potassium permanganate and drug:distilled water.Drug showed change in color from pink to brown with potassium permanganate whereas polymer showed no color change.No significant difference in shift of λmaxthan that of pure drug observed.
Nanosizing of Tiagabine
Graph was plotted between transmittance and λmaxof nanosized Tiagabine (by novel sonication and standard solvent evaporation methods) and it showed novel sonication method showed better result than standard solvent evaporation method (Figure 10).
Permeation study of Tiagabine
Permeation study of pure and nanosized Tiagabine using M.S.Apparatus revealed that nanosized Tiagabine permeated more through egg membrane than pure Tiagabine (Figure 11).
Evaluation parameters of formulations
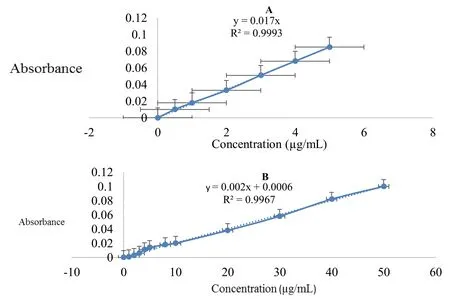
Fig.9 A:Standard graph of Tiagabine in distilled water B:Standard graph of Tiagabine in phosphate buffer pH 7.4

Fig.10 Comparative graph between % transmittance and λ max of nanosized Tiagabine

Fig.12 Permeation study of Tiagabine using M.S.apparatus
Thickness of formulated bio-flexy films using digital micrometerAs polymer concentration increased,thickness of bio-flexy films also increased proportionately.The thickness of nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer (1:0.5 referred as formulation:FCT1),(1:1 referred as formulation:FCT2),(1:3 referred as formulation:FCT3),(1:5 referred as formulation:FCT4),(1:6 referred as formulation:FCT5)and (1:10 referred as formulation:FCT6) was found to be in range of 0.026 ± 0.004 mm to 0.040 ± 0.02 mm.
Surface pH of formulated bio-flexy filmsPrepared formulations were found to be suitable for soft palatal delivery because they are in the range of physiological pH.The surface pH of nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer (FCT1-FCT6)was found to be in range of 7.01 ± 0.04 to 7.01 ± 0.02.

Figure 12 In-vitro drug release graph of nanosized Tiagabine loaded bio-flexy films using Cocos nucifera biopolymer by modified M.S.diffusion apparatus

Table 4:Kinetics release of Tiagabine-Cocos nucifera polymer bio-flexy films
Ex-vivo mucoadhesion study of formulated bio-flexy films using Capra aegagrus (goat) intestinal mucosa by rotating cylinder methodEx-vivo mucoadhesion study by rotating cylinder method revealed that nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer (FCT1-FCT6) showed mucoadhesivity on Capra aegagrus mucosal surface for time period of 20-90 min.
Ex-vivo mucoretention study of formulated bio-flexy films using Capra aegagrus (goat) intestinal mucosaExvivo mucoretention study revealed that nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer (FCT1-FCT6) were mucoretentive on Capra aegagrus mucosal surface for time period of 60-180 min.
Weight uniformity of formulated bio-flexy filmsAs polymer concentration increased,the weight uniformity of bio-flexy films also increased proportionately.The weight uniformity of nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer (FCT1-FCT6) was found to be in range of 0.012 ± 0.04 mg to 0.020 ± 0.02 mg.
Drug content uniformity of formulated bio-flexy filmsAs polymer concentration increased,the drug content uniformity of bio-flexy films also increased proportionately.The drug content uniformity of nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer (FCT1-FCT6) was found to be in range of 69.5 ± 0.35% to 72.9 ± 0.26%.
Folding endurance of formulated bio-flexy filmsAs polymer concentration increased,the flexibility of bioflexy films also increased proportionately.The formulations were devoid of brittleness,showed significant folding endurance because of presence of fructose and dextrose as excipients in optimized quantity.The folding endurance of nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer (FCT1-FCT6) was found to be in range of 84-107.
Swelling percentage of formulated bio-flexy filmsThe swelling percentage of nanosized Tiagabine loaded bioflexy films containing Cocos nucifera biopolymer (FCT1-FCT6) was found to be in range of 65 ± 0.5% to 73 ± 0.2%.
Percentage moisture uptake of formulated bio-flexy filmsThe percentage moisture uptake of nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer (FCT1-FCT6) was found to be in range of 2.0 ± 0.14% to 2.8 ± 0.12%.
In-vitro release study of formulated bio-flexy films by modified m.s.diffusion apparatusPerformed for 48 h.The in-vitro drug release pattern for all the prepared bioflexy films was compared.Calculated T50%(time during which 50% drug is released) and T80%(time during which 50% drug is released) values along with the kinetics data from BITS software by comparing R2values.Arranged formulations based upon the above parameters in descending order.The drug release pattern for formulations FCT1-FCT6 containing Cocos nucifera biopolymer based on the T50%and T80%was found to be FCT1 (1:0.5) > FCT5(1:6) > FCT3 (1:3) > FCT2 (1:1) > FCT4 (1:5) > FCT6(1:10).Based on all above-mentioned evaluation parameters,FCT1 (containing Tiagabine:Cocos nucifera biopolymer (1:0.5)) bio-flexy film was selected as the best formulation as it showed significant values of T50%:27 h.,T80%:31 h and having R2= 0.9221,Higuchi matrix as best fit model,follows anomalous transport release mechanism in comparison to other formulations of same biopolymer(Figure 12,Table 4).
Formulations (1:0.5 referred as formulation:FET1),(1:1 referred as formulation:FET2),(1:3 referred as formulation:FET3),(1:5 referred as formulation:FET4),(1:6 referred as formulation:FET5) and (1:10 referred as formulation:FET6) containing sodium carboxyl methyl cellulose standard polymer showed drug release pattern on basis of T50%and T80%of FET5 (1:6) > FET1 (1:0.5) >FET2 (1:1) > FET3 (1:3) > FET4 (1:5) > FET6 (1:10).Based on all evaluation parameters,FET5 (containing Tiagabine:sodium carboxyl methyl cellulose standard polymer (1:6)) Flexy film was found to be best formulation because it showed significant values of T50%:40.66 h,T80%:43.79 h and having R2= 0.9301,Higuchi matrix as best fit model,follows fickian diffusion (Higuchi matrix) release mechanism (Figure 13,Table 5).
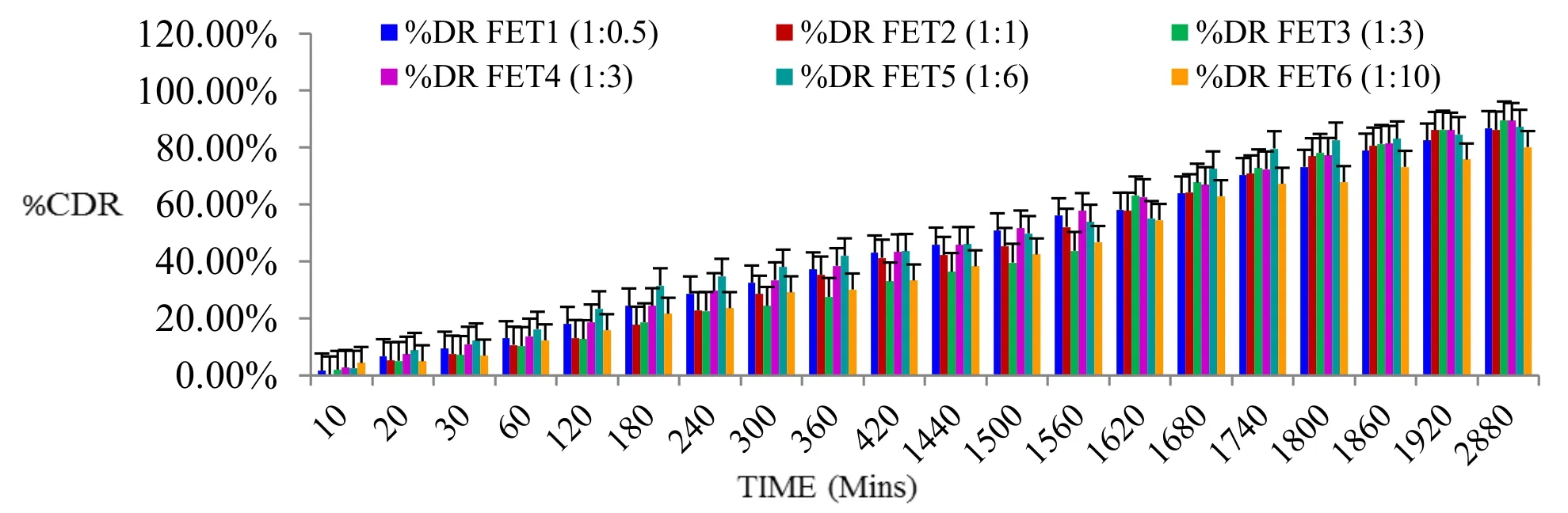
Figure 12 In-vitro drug release graph of nanosized Tiagabine loaded bio-flexy films using sodium carboxyl methyl cellulose standard polymer by modified M.S.diffusion apparatus
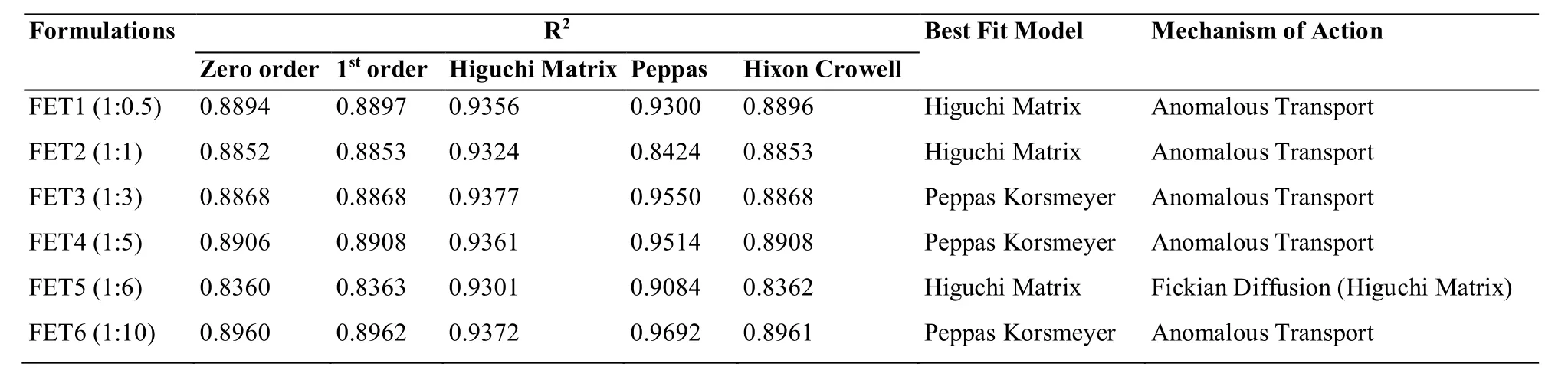
Table 4:Kinetics release of Tiagabine-Cocos nucifera polymer bio-flexy films
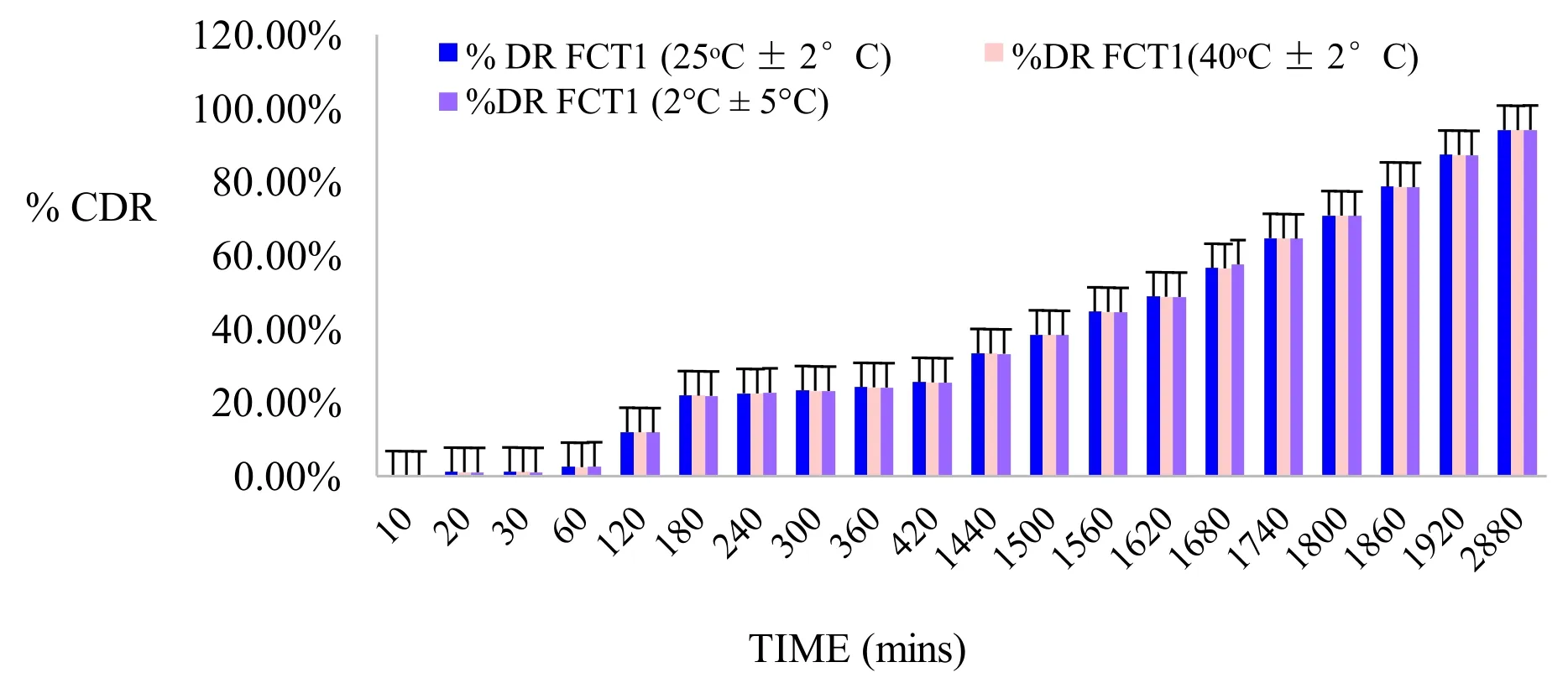
Figure 15:Stability study graph of best formulations of nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer
Stability studies of formulated nanosized drugs loaded bio-flexy films as per ICH guidelines Q1BStability studies were conducted as per ICH guidelines Q1B.Stability testing of pharmaceutical product is performed for assurance of safety,efficacy of formulations along with their shelf-life determination.The stability studies of the formulations were conducted at 40 ± 2oC and 45 ± 5% RH,25 ± 2oC and 60 ± 5% RH and 2 ± 5oC temperature and RH values respectively period of three months.Surface pH,folding endurance and in-vitro drug release of formulations were determined at varying conditions i.e.,room temperature and after stability study(25 ± 2oC and 60 ± 5% RH).Formulations were stable(Figure 14).
CONCLUSION
The conventional oral therapy for epilepsy leads to significant drug accumulation and economic burden in patients.This research explores trans oro-soft palatal route as a novelistic drug delivery platform for site specific treatment of epilepsy.The purpose of study is to formulate and evaluate nanosized Tiagabine loaded bio-flexy films consisting of novel biopolymer isolated from Cocos nucifera kernels.Biopolymer was biodegradable,inert,showed filmability,mucoadhesivity,mucoretentivity properties.The biopolymer which was isolated from Cocos nucifera showed percentage yield of 10.2 ± 0.04%.The biopolymer was brown in color,odourless,partially soluble in water,partially soluble in water.Its colour changing point was found to be 203 ± 1 °C.It was tested positive for proteins and carbohydrates; amino acids were not present.Ratios were chosen at six levels for drug:biopolymer(1:0.5 to 1:10) and six levels for drug:sodium carboxyl methyl cellulose (1:0.5 to 1:10) for formulating flexy-films.Anticonvulsant Tiagabine unidirectional bio-flexy films were formulated by solvent casting method.thickness of nanosized tiagabine loaded bio-flexy films containing cocos nucifera biopolymer (FCT1-FCT6) was ranging from 0.026 ± 0.04 mm to 0.040 ± 0.02 mm,folding endurance:84-107,surface pH:7.01 ± 0.04 to 7.01 ± 0.02,weight uniformity:0.012 ± 0.04 to 0.020 ± 0.02,drug content uniformity:69.5 ± 0.35% to 72.9 ± 0.26%,swelling percentage:65 ± 0.5% to 73 ± 0.2%,percentage moisture uptake (PTU):2.0 ± 0.14% to 2.8 ± 0.12%.Mucoadhesion study by dynamic method revealed that nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer were mucoadhesive for time period of 20-90 min.Mucoretentive study revealed that nanosized Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer were mucoretentive for time period of 60-180 min.The drug release pattern for formulations FCT1-FCT6 containing Cocos nucifera biopolymer based on the T50%and T80%was found to be FCT1 (1:0.5) > FCT5(1:6) > FCT3 (1:3) > FCT2 (1:1) > FCT4 (1:5) > FCT6(1:10).In-vitro drug release was performed for all the formulations and the data indicate that drug loaded formulations show the sustained release behavior.Graph was plotted between % cumulative drug release (CDR) and time,the R2value,T50%and T80%were calculated from graph.Formulation FCT1 (containing Tiagabine:Cocos nucifera biopolymer (1:0.5)) bio-flexy film having R2=0.9221,Higuchi matrix as best fit model,follows anomalous transport release mechanism,T50%:27 h.,T80%:31 h.using BITS Software 1.12.Stability study revealed stable bio-flexy films with no significant change in physical appearance and stable pH.Prepared formulations of Tiagabine loaded bio-flexy films containing Cocos nucifera biopolymer were suitable for soft palatal delivery.
This research is an approach to deliver antiepileptic molecules to brain at lesser dose than oral dose to the patients by completely bypassing oral therapy.
ACKNOWLEDGMENT
We wish to acknowledge Mr.Anuj Aggarwal (Chairman,DIT University),Prof.K.K.Raina (Vice Chancellor,DIT University) for providing platform for conducting the research work.
 Precision Medicine Research2021年4期
Precision Medicine Research2021年4期
- Precision Medicine Research的其它文章
- Targeting SARS-CoV-2 with therapeutic monoclonal antibodies
- 18F-Deoxyglucose Positron Emission Tomography/Computed Tomography in Multiple Myeloma
- Protective effects of modified Wendan decoction on hippocampal neurons in a rat model of depression
- Mini-review on personalized medicine:a revolution in health care
