Effects of primary microglia and astrocytes on neural stem cells in in vitro and in vivo models of ischemic stroke
Sheng-Jun Wen, Xi-Min Zheng, Li-Fen Liu, Na-Na Li, Hai-An Mao, Liang Huang,Qiong-Lan Yuan
Abstract Transplantation of neural stem cells (NSCs) can protect neurons in animal stroke models; however, their low rates of survival and neuronal differentiation limit their clinical application. Glial niches, an important location of neural stem cells, regulate survival, proliferation and differentiation of neural stem cells. However, the effects of activated glial cells on neural stem cells remain unclear. In the present study,we explored the effects of activated astrocytes and microglia on neural stem cells in vitro stroke models. We also investigated the effects of combined transplantation of neural stem cells and glial cells after stroke in rats. In a Transwell co-culture system, primary cultured astrocytes,microglia or mixed glial cells were exposed to glutamate or H2O2 and then seeded in the upper inserts, while primary neural stem cells were seeded in the lower uncoated wells and cultured for 7 days. Our results showed that microglia were conducive to neurosphere formation and had no effects on apoptosis within neurospheres, while astrocytes and mixed glial cells were conducive to neurosphere differentiation and reduced apoptosis within neurospheres, regardless of their pretreatment. In contrast, microglia and astrocytes induced neuronal differentiation of neural stem cells in differentiation medium, regardless of their pretreatment, with an exception of astrocytes pretreated with H2O2. Rat models of ischemic stroke were established by occlusion of the middle cerebral artery. Three days later, 5 × 105 neural stem cells with microglia or astrocytes were injected into the right lateral ventricle. Neural stem cell/astrocyte-treated rats displayed better improvement of neurological deficits than neural stem cell only-treated rats at 4 days after cell transplantation. Moreover, neural stem cell/microglia-, and neural stem cell/astrocyte-treated rats showed a significant decrease in ischemic volume compared with neural stem celltreated rats. These findings indicate that microglia and astrocytes exert different effects on neural stem cells, and that co-transplantation of neural stem cells and astrocytes is more conducive to the recovery of neurological impairment in rats with ischemic stroke. The study was approved by the Animal Ethics Committee of Tongji University School of Medicine, China (approval No. 2010-TJAA08220401) in 2010.
Key Words: astrocytes; glutamate; microglia; neural stem cells; neurogenesis; neurons; peroxide; repair; stroke
Introduction
Stroke is one of the leading causes of death and disability worldwide for which there is no effective clinical therapy, with the exception of tissue-type plasminogen activator that must be administered within a 6-hour time window after stroke onset. Cell-based therapeutic approaches have received considerable attention as a delayed treatment strategy(Martino and Pluchino, 2006; Locatelli et al., 2009) and many studies have shown that transplantation of neural stem cells (NSCs) ameliorates neurological deficits and improves remolding of tissue in animal stroke models (Chen et al., 2009;Yuan et al., 2013). However, low rates of survival and neuronal differentiation of NSCs limit their clinical potential (Arvidsson et al., 2002; Chen et al., 2009).
Glial niches, mainly composed of astrocytes and microglia,are an important location for NSCs, and can regulate survival,proliferation and differentiation of NSCs (Ma et al., 2005;Kokaia et al., 2012; Schneider et al., 2019). Astrocytes are the most abundant type of glial cells and they provide structural,nutritional and metabolic support, and also regulate synaptic formation and excitability (Ullian et al., 2001; Ransom et al., 2003). Microglia constitute approximately 10-20% of all glial cells and are the resident immune cells that mediate inflammatory processes (Hu et al., 2012; Liu et al., 2020).Astrocytes and microglia have distinct effects on the fate of NSCsin vitro(Liu et al., 2012; Guo et al., 2013; Fisch et al.,2020). For example, microglia promote astrogliogenesis of NSCsin vitro(Gu et al., 2011), whereas, astrocytes stimulate neuronal differentiation of NSCs (Liu et al., 2012). These results support the viewpoint that astrocytes and microglia act as regulators of NSCs in adult neurogenesis (Morrens et al., 2012). Activation of glial cells is a common and crucial pathological event in neuroinflammation resulting from stroke, traumatic brain injury and Alzheimer’s disease (Yang et al., 2011; Liu et al., 2020; Zhao et al., 2020a). However, effects of activated glial cells on NSCs are uncertain (Cacci et al.,2005; Faijerson et al., 2006; Wang et al., 2011; Ekdahl, 2012;Vay et al., 2018; Osman et al., 2019). Conditioned medium of activated astrocytes induces neuronal differentiation of NSCs but some activated astrocytes promote astrocyte differentiation of NSCs (Faijerson et al., 2006; Wang et al., 2011; Farrell et al., 2016). Furthermore, accumulating evidence indicates that activated microglia exert either pro- or anti-neurogenic effects on NSCsin vitro(Cacci et al., 2005; Vay et al., 2018; Osman et al., 2019). These results clearly indicate that astrocytes and microglia may exert unique effects on survival, proliferation and differentiation of NSCs under different conditions.
Glutamate excitotoxicity and peroxide insults are the main pathological events post-stroke (Hirose-Ikeda et al., 2020;Zhao et al., 2020b). After an ischemic stroke, activated glial cells have multi-functional states, producing a variety of pro-inflammatory and anti-inflammatory factors, as well as neurotrophic and growth factors that exert beneficial and detrimental effects (Wood, 1995; Stoll et al., 1998; Zhao and Rempe, 2010; Liu and Chopp, 2016). Furthermore, following stroke, activated astrocytes and microglia act as important regulators of neurogenesis to mobilize endogenous NSCs(Walton et al., 2006; Deierborg et al., 2010; Dai et al., 2019;Schneider et al., 2019; Fisch et al., 2020), although the details of these actions are poorly understood. Such information is critical to understand the fate of resident and transplanted NSCs after ischemic stroke. Therefore, in the present study,we used a Transwell co-culture system to mimic the glial microenvironment in stroke by stimulating astrocytes and microglia with glutamate and peroxide. We then observed their effects on NSCs. We also explored whether astrocytes and microglia enhance the protective effects of NSC transplantation in rats after stroke.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (specific pathogen free,weighing 250-300 g,n= 23) and newborn Sprague-Dawley rats (1-day-old,n= 20) were purchased from Shanghai Slack Experimental Animal Co., Ltd., Shanghai, China, and maintained in Tongji University Experimental Animal Center[license No. SYXK (Hu) 2009-0022]. The animals were maintained in a 12 hour light-dark cycle in a temperaturecontrolled room with free access to water and food. All animal experiments were approved by the Animal Study Committee of Tongji University School of Medicine (approval No. 2010-TJAA08220401) in 2010, and were performed to Directive 2010/63/EU and NIH guidelines. All efforts were made to minimize suffering and the number of animals used.
Primary NSC culture
Primary NSCs were obtained from the hippocampi of two newborn rats as previously described (Chen et al., 2009).Rats were decapitated and the brain was quickly removed.Mechanically dissociated hippocampal NSCs were plated at a density of 1 × 106cells/mL in T75 mL flasks (Nunc,Roskilde, Denmark) in serum-free Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (Gibco, Grand Island, NY,USA) containing 20 ng/mL epidermal growth factor (R&D Systems, Minneapolis, MN, USA), 20 ng/mL basic fibroblast growth factor (R&D Systems), 1% B27 (Gibco), 1% L-glutamine(Gibco), 1% penicillin (Beyotime, Shanghai, China) and 1%streptomycin (Beyotime) in a humidified incubator (37°C,5% CO2, 95% air). Half of the medium was refreshed every 3 days. After 7-8 days, neurospheres were harvested and mechanically dissociated into single cell suspensions for the following experiments.
To identify cell types, primary neurospheres were dissociated to single cells and grown on gelatin-coated wells of a 24-well plate (Corning, Darmstadt, Germany) in proliferation medium for 6-7 days or in differentiation medium [proliferation medium with 1% fetal bovine serum (FBS)] for 7 days and processed for immunostaining.
Primary mixed glia, microglia and astrocyte cultures
Mixed glial cells were prepared from the cerebral cortices of two newborn rats as previously described (Giulian and Baker,1986; Crocker et al., 2008). In brief, rats were decapitated and the cerebral cortex quickly dissected, carefully stripped of meninges, and dissociated in DMEM/F12 medium (Gibco)containing 10% FBS (Gibco) by gentle trituration using a 1 mL pipette in a 15 mL culture tube. Harvested cells were plated at a density of 5 × 106-1 × 107cells/mL in gelatin-treated T75 mL flasks (Sigma, St. Louis, MO, USA) and cultured for 7-10 days with replacement of half the medium every 3 days. To remove oligodendrocytes, the adherent cells were exposed to the air for 10 seconds during medium replacement, which kills oligodendrocytes. When the mixed glial cells reached 80% confluency, they were collected for NSC co-culture experiments.
Microglia and astrocytes were prepared from the above primary mixed glia cultures as described previously (Giulian and Baker, 1986). Briefly, when mixed glial cultures were 80%confluent, the cultures were gently shaken at 180 r/min at 37°C in an incubator for 15-20 hours to force microglia into suspension. The medium containing floating microglia was harvested. The cells adhering to the flask were astrocytes,which were further cultured with fresh media. The harvested medium was plated in fresh T75 flasks and cultured for 3-7 days whereupon the floating microglia attached to the flask.The primary microglia and astrocytes were used for NSC coculture experiments.
To determine their purity, microglia and astrocytes were plated at a density of 2 × 105cells/mL into gelatin coated six-well plates in DMEM/F12 with 10% FBS for 3-4 days.Immunostaining was then performed as described below.
Co-culture of NSCs with glial cells
To examine the effects of glial cells on NSCs, co-culture of glial cells and NSCs was performed using Transwell dishes(Corning) as previously described (Liu et al., 2012) and as shown inFigure 1. The primary mixed glial cells, astrocytes and microglia, were plated at a density of 2 × 105cells/mL in the upper chamber insert (0.4 μm pore size membrane;Corning) in 12-well plates (low-attachment plates; Corning)in DMEM/F12 medium. After attachment, the cultures were divided into groups: (1) control group: treated with the vehicle; (2) glutamate group: exposed to a final concentration 500 μM glutamate (Gibco) at 37°C for 6 hours; and (3) H2O2group: exposed to a final concentration 400 μM H2O2at 37°C for 1 hour. After treatment, the media were removed and the cells were washed with phosphate-buffered saline three times. Then single cell suspensions from fresh primary neurospheres were plated at density of 1 × 105cells/mL in the lower chamber of the same uncoated 12-well plates in proliferation DMEM/F12 containing 1% B27, 20 ng/mL basic fibroblast growth factor and 20 ng/mL epidermal growth factor. As parallel cultures, the dissociated single NSC suspensions were plated and treated with 500 μM glutamate at 37°C for 6 hours or 400 μM H2O2at 37°C for 1 hour. The media were then refreshed and the cells cultured for 7 days.These co-cultures were used to investigate the effects of glial cells on proliferation, differentiation and survival of NSCs in the following experiments.
Survival and differentiation in neurospheres from NSCs
co-cultured with glial cells
To assess neurospheres derived from NSCs co-cultured with glial cells, live adherent neurospheres in co-cultures (at leastn= 50/well) were imaged after 7 days under an inverted bright microscope at 20× magnification (Nikon Eclipse InvertedTE 2000-E microscope; Nikon, Tokyo, Japan). Some adherent neurospheres, with cells moving out of their periphery, were considered differentiated neurospheres (Vay et al., 2018). The ratio of differentiated neurospheres to total neurospheres was counted in different cultures.
To detect the survival of NSCs co-cultured with glial cells,NSCs were co-cultured with microglia, astrocytes, or mixed glial cells with or without glutamate or H2O2pretreatment in proliferation DMEM/F12 medium in 12-well plates, as described above. After culture for 7 days, the plates were washed and adherent neurospheres and cells were fixed with 4% paraformaldehyde for 30 minutes at room temperature.Hoechst 33258 staining was performed to classify viable and apoptotic cells as previously described (Yang et al., 2011;Gao et al., 2019). In brief, 0.5 mL Hoechst 33258 solution was added to each well, and the cells were incubated at 37°C for 15 minutes in the dark. The cells were then mounted with Permount (Beyotime) Apoptotic/viable cells and then counted(at least 500 cells in each well). NSC apoptosis is expressed as a percentage of the number of dead cells over the total number of cells counted.
To detect the effect of microglia on proliferation, NSC neurosphere size was determined as previously described(Song et al., 2011; Scardigli et al., 2014). In an independent set of experiments, co-cultures of microglia (upper chamber)with or without glutamate or H2O2pretreatment and NSCs(lower chamber) were performed in proliferation DMEM/F12 medium. After 7 days, live floating neurospheres in each well (n= 50/well) were imaged under an inverted bright microscope at 20× magnification. Neurosphere size is expressed as the perimeter, calculated after measuring their diameter and assuming a spherical shape.
To detect the neuronal differentiation of NSCs co-cultured with glial cells, the medium was changed to differentiation medium (proliferation DMEM/F12 medium containing 1%FBS) to induce differentiation of NSCs for 7 days. The cells derived from NSCs were fixed using 4% paraformaldehyde and immunostained using specific antibodies.
Immunostaining
Cells were fixed with 4% paraformaldehyde for 45 minutes and then blocked for 20 minutes in phosphate-buffered saline containing 10% FBS, 5% normal goat serum (Cell Signaling,Boston, MA, USA), and 0.1% Triton X-100 (Beyotime). Cells were then incubated with primary antibodies against the following cell-specific markers: mouse anti-nestin (1:500;Cat# 33475; Cell Signaling) for NSCs, mouse anti-microtubuleassociated protein 2 (MAP2; 1:200; Cat# 8707; Cell Signaling)for neurons, rabbit anti-glial fibrillary acidic protein (GFAP;1:500; Cat# 80788; Cell Signaling) for astrocytes and NSCs,rabbit anti-galactose cerebroside (Galc; 1:200; Cat# 87985;Cell Signaling) for oligodendrocytes, and GSI-B4-FITC (1:300,Cat# B1205; Alexis, Lausanne, Switzerland) at 4°C overnight,followed by incubation with Cy3 or FITC-conjugated AffiniPure goat anti-rabbit IgG or goat anti-mouse IgG (1:200; Cat#112-116-143, Cat#115-115-164; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at room temperature for 1.5 hours. Cells were rinsed and then stained with Hoechst 33258 followed by mounting with Permount. The average percentage of differentiated cells for each culture was calculated by dividing the number of MAP2, GFAP, GSI-B4 and Galc positive cells by the total number of cells. An observer blind to treatment conditions conducted all analyses.
For analysis of immunopositive cells, five non-overlapping images in each well were acquired under 200× or 400×magnification using an Olympus confocal microscope(Olympus, Tokyo, Japan). Additionally, five non-overlapping images of the floating neurospheres and adherent neurospheres in each well were recorded under a phase contrast microscope at 100× magnification. Quantification of the immunopositive cells or neurospheres was performed using ImageJ software (Pro Plus v6.0) (National Institutes of Health, Bethesda, MD, USA).
Ischemic stroke model establishment
A rat ischemic stroke model by middle cerebral artery occlusion was prepared as previously described (Yuan et al.,2007; Chen et al., 2009). In brief, after anesthesia the right common carotid artery, external carotid artery, and internal carotid artery were exposed. A length of 4-0 monofilament nylon suture (19-20 mm long, depending on rat weight) with its tip rounded was advanced from the common carotid artery into the internal carotid artery until it blocked the origin of the middle cerebral artery. Two hours after surgery, the rats were re-anesthetized and reperfused by withdrawal of the suture. After 3 days, a modified neurological severity score was performed, and rats with a score of at least 9 were used for cell transplantation.
Cell transplantation
All transplantation procedures were performed as described previously (Chen et al., 2009). Rats subjected to stroke were randomly divided into four groups. Three days after middle cerebral artery occlusion, animals were anesthetized using 1% pentobarbital sodium (Sinopharm Chemical Reagent Co.,Ltd., Shanghai, China) and then placed in a stereotaxic frame(Angle TwoTMStereotaxic Instrument w/Rat Atlas Product:Cat#464601; Leica, Buffalo Grove, IL, USA). Burr holes were drilled for the ipsilateral right lateral ventricle according to the coordinates: -0.8 to 1.0 mm anterior/posterior,-1.8 to 2.0 mm medial/lateral, and -4.0 to 5.0 mm dorsal/ventral. Rats were injected with: (1) 20 μL cell suspension containing NSCs (5 × 105cells); (2) NSCs/microglia: 10 μL cell suspension containing NSCs (5 × 105cells), followed by 10 μL cell suspension containing microglia (5 × 105cells); (3) NSCs/astrocytes: 10 μL cell suspension containing NSCs (5 × 105cells), followed by 10 μL cell suspension containing astrocytes(5 × 105cells); and (4) control group, 20 μL vehicle (DMEM/F12). Animals received no immunosuppressants or antibiotics and survived for 4 days after transplantation.
Behavioral tests
Behavioral tests (n= 5/group) were performed just before transplantation (at 2 hours and 3 days after middle cerebral artery occlusion) and 4 days after transplantation. As previously described (Yuan et al., 2007; Chen et al., 2009),neurological function was graded on a modified neurological severity score of 0 to 18 (normal: 0; maximal deficit score: 18).
2, 3, 5-Triphenyltetrazolium chloride staining
After behavioral testing, rats (n= 5 per group) were deeply anesthetized and brains were carefully dissected into six coronal blocks (1.5 mm thick) to measure lesion volume using 2, 3, 5-triphenyltetrazolium chloride (TTC; Sigma) staining as previously described (Yang et al., 2011; Gao et al., 2019). The fresh blocks were immersed in 2% TTC in saline at 37°C for 30 minutes and were then imaged by a digital camera (EOS 40D; Canon, Tokyo, Japan). The lesion volume was calculated indirectly by subtracting the intact area of the ipsilateral hemisphere from the area of the contra-lateral hemisphere using ImageJ software (Pro Plus v6.0). The results are expressed as a percentage of lesion volume compared with contra-lateral hemisphere area (regarded as 100%).
Statistical analysis
Data were generated from at least three independent primary culture experiments. Data are presented as the mean ±standard error of the mean (SEM) and were analyzed by oneway analysis of variance followed by the least significant difference test using SPSS Statistics software v22.0 (IBM,Armonk, NY, USA) unless stated otherwise. The evaluation of neurological function was analyzed by two-way analysis of variance followed by the least significant difference test.P<0.05 was considered statistically significant.
Results
Establishment of co-cultures of NSCs and glial cells
Seven to eight days after plating single cell suspensions from dissociated primary neurospheres in proliferation medium,many neurospheres were observed that expressed the NSC specific marker, Nestin (Figure 2AandB), consistent with our previous studies (Chen et al., 2009). Next, differentiation of NSCs was assessed for dissociated single cells from primary neurospheres that were plated and grown in differentiation medium for 8 days. Monolayer cells appeared that were attached to the plate. MAP2-positive mature neurons,GFAP-positive cells (astrocytes or NSCs), and Galc-positive oligodendrocytes were observed by immunostaining (Figure2CandD), indicating that the NSCs were multipotent.Obviously, a majority of cells derived from neurospheres were GFAP-positive cells (Figure 2C) in these culture conditions.
As shown inFigure 3A, under phase microscopy, a mixed glial culture from newborn rat cortex contained a majority basal layer of well-spread phase-dark astrocytes and a superficial layer of a few loosely attached phase-bright microglia, as described previously (Crocker et al., 2008). Microglia were separated from mixed glial cultures by gentle shaking. The microglia were then in suspension while the adherent cells in the flask were astrocytes (Figure 3A). The purity of microglia and astrocytes was confirmed by immunocytochemistry using the microglia-specific marker, GSI-B4, and the astrocytespecific marker, GFAP, to be 98.1 ± 0.01% and 99.3 ± 0.00%,respectively (Figure 3B-D).
Effects of astrocytes, microglia and mixed glial cultures exposed to glutamate or H2O2 on NSC neurospheres
A Transwell co-culture system was adopted as anin vitromodel of cerebral ischemia to mimic the complex niche in which glial cells are exposed to glutamate and H2O2overproduction. NSCs were plated in the lower un-coated wells, while mixed glia, astrocytes or microglia were seeded in the 0.4-μm pore inserts. In parallel cultures, NSCs were cultured in un-coated wells with no glial cells in the inserts(Figure 1).
First, neurospheres were characterized in different glial cocultures. After 7 days, some neurospheres were suspended in the medium while some adhered to the well bottom. The majority of neurospheres were floating in the NSC cultures and in co-cultures with microglia, while the majority of neurospheres were adherent in co-cultures with mixed glia or astrocytes. In NSC cultures, adherent neurospheres were spherical. However, in NSC co-cultures, some adherent neurospheres displayed an irregular shape and some adherent neurospheres had a few cells migrating away, reflecting differentiation and a loss of stemness, as reported by Vay et al.(2018). We assessed the ratio of differentiated neurospheres.In parallel cultures, the ratio of differentiated neurospheres to total neurospheres was significantly decreased in NSCs treated with H2O2compared with non-treated NSCs (P=0.015;Figure 4AandB). We observed that a number of cells with several long processes migrated away from the neurospheres of NSCs co-cultured with astrocytes and mixed glia (Figure 4C). In contrast, few cells with rare processes migrated from neurospheres of NSCs co-cultured with microglia (Figure 4C). Quantification showed that microglia and H2O2-pretreated microglia displayed no significant decrease in the ratio of differentiated neurospheres in NSC cocultures but that glutamate-pretreated microglia significantly decreased the ratio of differentiated neurospheres in NSC co-cultures compared with NSC cultures (P= 0.016;Figure4AandB). In contrast, astrocytes and mixed glia, regardless of their pretreatment, significantly increased the ratio of differentiated neurospheres in NSC co-cultures (NSCsvs.NSCs-astrocyte, NSCs-astrocyte (Glu) or NSCs-astrocyte (H2O2),P= 0.007, 0.014, 0.001, respectively; NSCsvs. NSCs-mixed glia, NSCs-mixed glia (Glu), NSCs-mixed glia (H2O2),P= 0.000,0.001, 0.000, respectively;Figure 4A,CandD). Furthermore,no difference was observed in the ratio of differentiated neurospheres in NSC co-cultures among astrocytes, H2O2-or glutamate-pretreated astrocytes, and similar results were observed with the mixed glia cultures. These data indicate that microglia may be permissive to non-differentiation of neurospheres. Conversely, astrocytes and mixed glia(astrocytes as the major component) probably promote differentiation of neurospheres.
NSC/microglia co-cultures had many floating neurospheres and some adherent neurospheres in an undifferentiated state; therefore, we assessed whether microglia affect the proliferation of NSCs by measuring the size of floating neurospheres. After 7 days in DMEM/F12 growth medium,live floating neurospheres were imaged under an inverted microscope. The circumference of the neurospheres was measured to provide an index of microglial effect on NSC proliferation. The circumference of neurospheres was not different among NSC cultures, glutamate- or H2O2-treated NSC cultures. Compared with NSC cultures, the circumference of neurospheres tended to increase but not significantly among the NSC and microglia co-cultures, regardless of H2O2or glutamate pretreatment (Figure 5). In summary, these results indicate that microglia do not affect the proliferation of NSCs but do maintain neurospheres in an undifferentiated state.

Figure 1|Schematic diagram of the experimental procedure.Glu: Glutamate; MCAO: middle cerebral artery occlusion; NSC: neural stem cell.

Figure 2|Characterization of NSCs from newborn rat hippocampus.(A, B) Phase contrast microscopy images of a neurosphere in NSC culture for 8 days (A) and a nestin-positive (green, Cy3) neurosphere (B). Nuclei were labeled by Hoechst 33258 (blue). (C, D) NSCs differentiated into MAP2-positive neurons (C; red, Cy3), GFAP-positive cells (C, D; green, FITC), and Galc-positive oligodendrocytes (D; red, Cy3) in differentiation medium for 8 days. Nuclei were labeled by Hoechst 33258 (blue). Scale bars: 50 μm in A and B, 100 μm in C and D. Galc: Galactose cerebroside; GFAP: glial fibrillary acidic protein; MAP2: microtubule associated protein 2; NSC: neural stem cell.

Figure 3|Identification of primary cultured mixed glia, astrocytes and microglia.(A) Phase-contrast micrographs showing mixed glial cells (left), microglia (middle), and astrocytes (right). Solid arrows indicate astrocytes; dotted arrows indicate microglia. (B) Microglia in microglia culture stained with GSI-B4-FITC (B, green). (C) Astrocytes in astrocyte culture stained with GFAP antibody (C, red,Cy3). Nuclei were labeled by Hoechst 33258 (blue). Scale bars: 50 μm in A, 100 μm in B and C. (D) Quantitation of the purity of microglia and astrocytes. Data are expressed as the mean ± SEM from three independent experiments. GFAP: Glial fibrillary acidic protein.
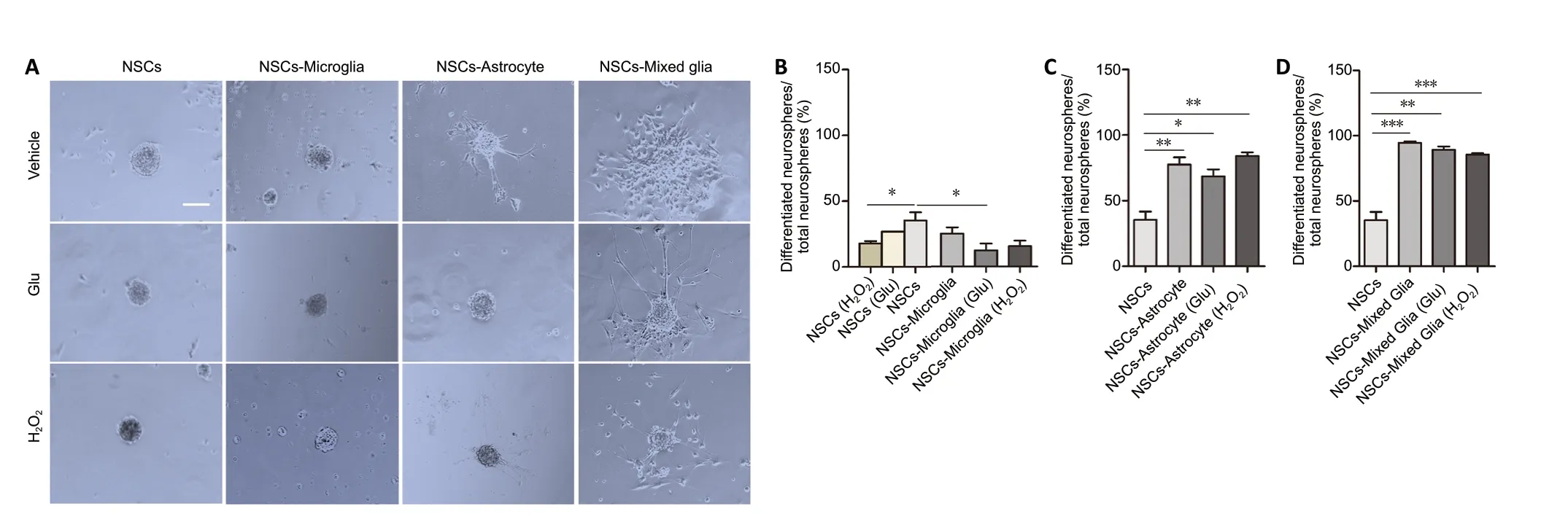
Figure 4|Effects of glial cells pretreated with glutamate or H2O2 on NSC neurospheres in a Transwell co-culture system.(A) Phase-bright photomicrographs showing adherent neurospheres after NSCs were plated on uncoated wells in growth medium for 7 days with astrocytes,microglia or mixed glia with or without glutamate or H2O2 pretreatment. In NSC cultures, adherent neurospheres displayed a spherical shape. In NSC/microglia co-cultures, most adherent neurospheres displayed a spherical shape. In NSC/astrocyte and NSC/mixed glia co-cultures, adherent neurospheres showed irregular shapes and had a few of cells migrating away. Scale bar: 50 μm. (B-D) Quantification of the ratio of differentiated neurospheres in NSC co-cultures with microglia (B), astrocytes (C), and mixed glial cells (D). Data are expressed as the mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01,***P < 0.001 (one-way analysis of variance followed by a least significant difference test). Glu: Glutamate; NSC: neural stem cell.
Effects of astrocytes, microglia and mixed glial cultures exposed to glutamate or H2O2 on survival of NSC
neurospheres
Next, the influence of glial cells pretreated with glutamate or H2O2on survival of neurospheres was investigated. After co-culture in growth medium for 7 days, adherent NSC neurospheres were analyzed using Hoechst 33258 staining as previously described (Cacci et al., 2008; Gao et al., 2019). As shown inFigure 6, the apoptotic rate in adherent neurospheres was the same in all NSCs cultures, regardless of glutamate or H2O2treatment. Microglia and glutamate- or H2O2-pretreated microglia showed no effect on the apoptosis in adherent NSC neurosphere co-cultures compared with NSCs cultures.In contrast, astrocytes and mixed glial cells significantly decreased the apoptotic rate in adherent NSC neurosphere cocultures (NSCsvs. NSCs-astrocyte, NSCs-astrocyte (Glu) or NSCastrocyte (H2O2),P= 0.006, 0.021, 0.036, respectively; NSCsvs.NSCs-mixed glia, NSCs-mixed glia (Glu), NSCs-mixed glia (H2O2),P= 0.001, 0.001, 0.001, respectively). Additionally, neither glutamate nor H2O2pretreatment affected the apoptosis of adherent NSC neurospheres in co-cultures with astrocytes or mixed glia. Together, these results indicate that astrocytes and mixed glial cells, but not microglia, are conducive to survival of NSCsin vitro.
Effects of astrocytes and microglia exposed to glutamate or H2O2 on neuronal differentiation of NSCs
The differentiation of NSCs was performed using DMEM/F12 growth medium containing 1% FBS as previously described(Conti et al., 2005). After 7 days of NSC co-culture with astrocytes or microglia, with or without glutamate or H2O2pretreatment, MAP2-positive cells, a marker for mature neurons, were observed(Figure 7). We found no significant differences in the percentage of MAP2-positive cells among NSCs cultures, and glutamate- and H2O2-treated NSCs cultures (Figure 7AandD). However, microglia significantly increased the percentage MAP2-positive cells in NSC cocultures compared with NSC cultures, regardless of their pretreatment (NSCsvs. NSCs-microglia, NSCs-microglia (Glu)or NSC-microglia (H2O2),P= 0.002, 0.001, 0.003, respectively;Figure 7BandD). Additionally, glutamate pretreated microglia showed a higher percentage of MAP2-positive cells in NSC co-cultures compared with untreated microglia (P= 0.025;Figure 7D). Interestingly, astrocytes with or without glutamate pretreatment also significantly increased the percentage of MAP2-positive cells in NSC co-cultures, while H2O2pretreated astrocytes showed an increased tendency (NSCsvs. NSCsastrocyte, NSCs-astrocyte (Glu) or NSC-astrocyte (H2O2),P=0.005, 0.001, 0.096, respectively;Figure 7CandE). Together,these results indicate that microglia and astrocytes may induce neuronal differentiation of NSC co-cultures, and that microglia may be more effective at promoting neuronal differentiation of NSCs after exposure to glutamate.
Protective effects of NSCs combined with astrocytes or microglia when transplanted into stroke model rats
Finally, we assessed whether astrocytes and microglia enhanced the protective effect of grafted NSCs in rats after stroke. After co-transplantation of NSCs with astrocytes or microglia in stroke model rats, neurological performance was evaluated using a modified neurological severity score (Yang et al., 2011) at 2 hours, and 3 days (transplantation timepoint) after middle cerebral artery occlusion, and at 4 days after transplantation. Two-way analysis of variance showed significant difference among these groups (group:F(3,5.632)=9.112,P< 0.000; time:F(2,165.333)= 267.506,P< 0.000; group× time interaction:F(6,7.611)= 12.315,P< 0.000). No significant differences were observed at 2 hours and 3 days after stroke(Figure 8). NSC-treated rats showed significant improvements in behaviors compared with ischemic rats treated with vehicle at 4 days after transplantation (P= 0.044), consistent with our previous study (Chen et al., 2009). Importantly, NSC/astrocyte-treated rats displayed more improvement than NSCtreated rats, and NSC/microglia-treated rats at 4 days after transplantation (P= 0.004, 0.007, respectively;Figure 8).
Next, 4 days after transplantation (7thday after stroke),ischemic volume was measured using TTC staining. The NSC-, NSC/microglia-, and NSC/astrocyte-treated rats had significantly smaller ischemic volumes than the control group(controlvs. NSCs, NSCs-microglia, NSCs-astrocyte,P= 0.002,0.000, 0.000, respectively). More importantly, NSC/microglia-,and NSC/astrocyte-treated rats showed a significant decrease in ischemic volume compared with NSC-treated rats (NSCsvs.NSCs-microglia, NSCs-astrocyte,P= 0.002, 0.000, respectively;Figure 9).
Together, these results confirm that treatment with a combination of NSCs and microglia or astrocytes yields better neuroprotection against stroke in rats than treatment with NSCs alone.
Discussion
In the present study, we explored effects of activated astrocytes and microglia on NSCs in anin vitroischemic model using a Transwell co-culture system and whether cotransplantation of NSCs with astrocytes or microglia improves recovery in rats after stroke. Our results show that microglia with or without glutamate or H2O2pretreatment were conducive to neurosphere formation and had no effect on apoptosis of the neurospheres, while astrocytes or mixed glial cells pretreated with or without glutamate or H2O2induced neurosphere differentiation and reduced apoptosis of neurospheres. In contrast, microglia and astrocytes induced neuronal differentiation of NSCs in differentiation medium, regardless of their pretreatment, with an exception of astrocytes pretreated with H2O2. Furthermore, treatment of NSCs combined with astrocytes or microglia improved neuroprotection at 7 days post-stroke in rats. Taken together,these results confirm that microglia and astrocytes exert distinctive effects on NSCs in anin vitroischemic model and that transplantation of NSCs/astrocytes and NSCs/microglia improves recovery in rats post-stroke.
Although severalin vitrostudies have investigated the effects of microglia and astrocytes activated by lipopolysaccharide and interleukin-4 or conditioned medium on NSCs (Cacci et al., 2008; Wang et al., 2011; Farrell et al., 2016; Vay et al., 2018; Osman et al., 2019), these results are highly controversial. In the present study, NSCs were co-cultured on uncoated 12-well plates with glutamate- or H2O2-activated microglia, astrocytes or mixed glial cells in upper inserts in NSC medium for 7 days. We observed that many floating neurospheres appeared in NSCs/microglia co-cultures but that NSC/astrocyte or mixed glia co-cultures had many adherent neurospheres, regardless of their pretreatment. NSC/astrocyte and NSC/mixed glia co-cultures showed a higher rate of differentiated neurospheres among total adherent neurospheres. These results clearly demonstrate that microglia are permissive to neurosphere formation and that astrocytes induce neurosphere differentiation in the same cultures. Notably, glutamate-activated microglia significantly decreased the ratio of differentiated neurospheres, conducive to neurosphere formation. Our observations are indirectly supported by previous reports. For example, microglia have inhibitory effects on neural progenitor cell differentiation and attenuate negative effects of lipopolysaccharide or interferon-γ on murine neurosphere formation in co-culture systems (Liu et al., 2013; Ortega et al., 2014). Fisch et al. (2020)reported that neurospheres from the subventricular zone are completely abolished when microglia are depleted in cultures.However, Guo et al. (2013) reported that astrocytes increased the number of floating neurospheres after 3 and 7 days of NSC co-culture. This discrepancy might result from different experimental settings. Taken together, these data clearly show that microglia are permissive to neurosphere formation and that astrocytes support neurosphere differentiation in NSC cocultures.
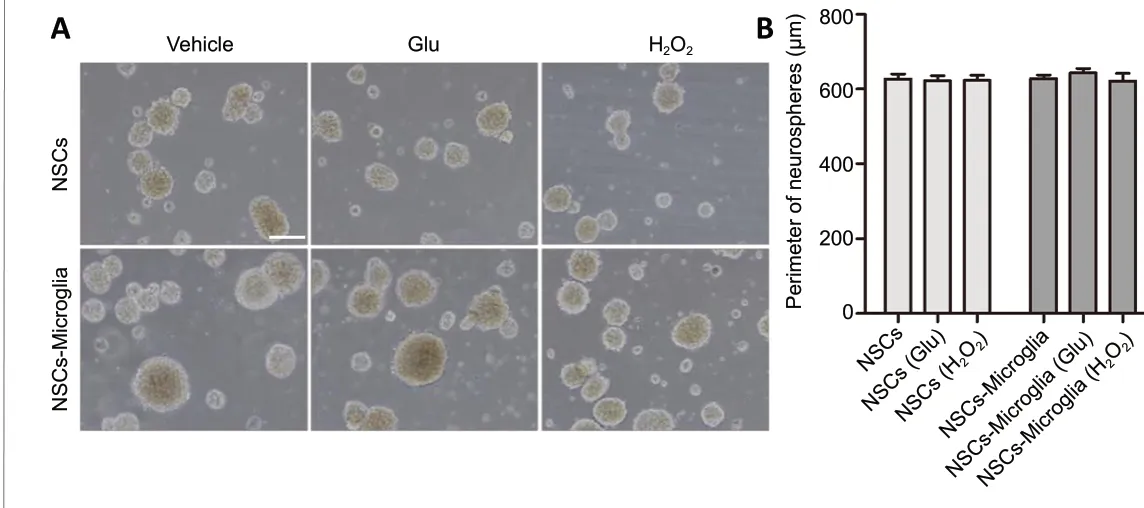
Figure 5|Effect of microglia on proliferation of NSC co-cultures.(A) Phase-bright photomicrographs showing floating neurospheres in NSC/microglia co-cultures after NSCs were seeded in lower uncoated wells and microglia with or without glutamate or H2O2 pretreatment were plated in the upper inserts and cultured for 7 days in the growth medium.In NSC cultures and NSC/microglia co-cultures, neurospheres were mainly floating without apparent difference in size. NSC cultures were a control.Scale bar: 100 μm. (B) Quantification of the perimeter of the floating neurospheres in NSC cultures and co-cultures with microglia. Data are expressed as the mean ± SEM from three independent experiments and were analyzed by one-way analysis of variance followed by the least significant difference test. Glu: Glutamate; NSC: neural stem cell.
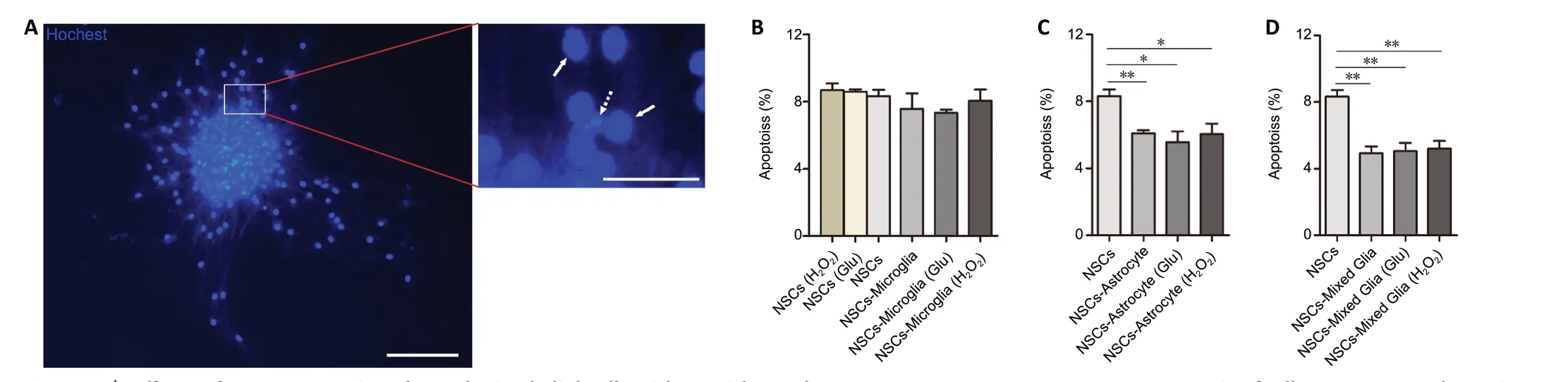
Figure 6|Effects of astrocytes, microglia and mixed glial cells with or without glutamate or H2O2 pretreatment on apoptosis of adherent neurospheres in NSC co-cultures.(A) Representative images showing neurospheres stained by Hoechst 33258 after 7 days in growth medium. The viable cells (solid arrows) are characterized by regular and round nuclei with a pallid blue fluorescence; apoptotic cells (dotted arrow) are condensed and fragmented. The right image is an enlargement of the rectangle. Scale bars: 100 μm (left) and 25 μm (right). (B) Quantitative analyses showed no difference in apoptosis of adherent neurospheres in NSC/microglia co-cultures compared with NSC cultures. (C, D) Quantitative analyses showed a lower apoptotic rate in the NSC/astrocytes (C) and NSC/mixed glial cell (D) co-cultures compared with NSC cultures. Data are expressed as the mean ± SEM from three independent experiments. *P < 0.05, **P < 0.01 (one-way analysis of variance followed by the least significant difference test). Glu: Glutamate; NSC: neural stem cell.
Previous studies have shown that acute and chronic M1 (lipopolysaccharide-stimulated) or M2 (interleukin-4-stimulated) microglia have different effects on NSC proliferationin vitro, as assessed by counting BrdU- or Ki67-positive cells (Cacci et al., 2008; Farrell et al., 2016; Vay et al., 2018; Osman et al., 2019). Osman et al. (2019) reported no differences in NSC proliferation in NSCs cultures with unstimulated, acute M1 or M2 microglial conditioned media(MCM). Conversely, Cacci et al. (2008) reported that acute and chronic M1 MCM significantly decreased NSC proliferation compared with unstimulated MCM in NSCs cultures. Simiarly,Vay et al. (2018) reported that acute M1 and M2 MCM significantly decreased proliferation of NSCs compared with unstimulated MCM in NSCs cultures. In the present study, we observed the effect of microglia on the proliferation of NSCs by measuring the size of the floating neurospheres using a transwell co-culture system and we found no signifiant differences among the NSC/microglia co-cultures. These results may indicate that the effects of microglia on the proliferation of NSCs in different experimental settings are complex. Combined with the results of adherent neurospheres in NSC/microglia co-cultures, it is likely that microglia are conducive to undifferentiated states of neurospheres in NSC co-cultures.

Figure 8|Effect of transplantation of NSCs with astrocytes or microglia on the functional recovery of rats after ischemic stroke.Neurological function of rats after stroke was determined by modified neurological severity scores. Data are expressed as the mean ± SEM (n = 5).**P < 0.01, ***P < 0.001 (two-way analysis of variance followed by a least significant difference test). Ctl: ischemic rats treated with vehicle; NSCs:ischemic rats treated with NSCs; NSCs-microglia: ischemic rats treated with NSCs and microglia; NSCs-astrocyte: ischemic rats treated with NSCs and astrocytes.

Figure 9|Co-transplantation of NSCs with astrocytes or microglia decreases ischemic volume in rats after stroke.(A) Representative brain slices stained with 2,3,5-triphenyltetrazolium show lesion regions at 7 days after stroke. The normal area is red and the ischemic region is white. Compared with the control group, NSC-, NSC/microglia-,and NSC/astrocyte-treated rats had significantly smaller ischemic volumes.Notably, NSC/microglia-, and NSC/astrocyte-treated rats were less affected than NSC-treated rats. (B) Quantitative analysis of ischemic volume evaluated by 2,3,5-triphenyltetrazolium staining. Data are expressed as the mean ± SEM(n = 5). **P < 0.01, ***P < 0.001 (one-way analysis of variance followed by a least significant difference test). Ctl: Ischemic rats treated with vehicle; NSCs:ischemic rats treated with NSCs; NSCs-astrocyte: ischemic rats treated with NSCs and astrocytes; NSCs-microglia: ischemic rats treated with NSCs and microglia.
Microglial effects on the survival of NSCs have been reportedin vitrousing MCM (Cacci et al., 2008; Vay et al., 2018; Osman et al., 2019). Cacci et al. (2008) reported that unstimulated MCM had no effect on the survival of NSC co-cultures, but acute M1 MCM (24 hour stimulation) reduced NSC survival whereas chronic M1 MCM (72-hour stimulation) was permissive to NSC survival. Additionally, Osman et al. (2019)reported that acute M1 MCM significantly decreased survival of NSCs but acute M2 MCM aparently increased NSC survival compared with unstimulated MCM. Vay et al. (2018) found that unstimulated MCM had no effect on the survival of NSCs,but that M1 MCM significantly decreased NSC survival. These studies show that acutely activated M1 microglia decrease NSC survival while chronically activated M1 microglia and acutely activated M2 microglia are permissive to NSC survival.These results are resonable given that acutely activated M1 microglia can secrete proinflammatory cytokines, such as interleukin 1β, interleukin 6, and tumor necrosis factor α,which are detrimental for cell survival (Cacci et al., 2008;Vay et al., 2018; Osman et al., 2019), while acutely activated M2 microglia produce insulin-like growth factor 1, Arg1 and CD206, which are beneficial for cell survival (Vay et al., 2018;Osman et al., 2019). In the present study using NSC co-cultures for 7 days, we found that microglia had no effect on NSC survival, regardless of their glutamate or H2O2pretreatment.Conversely, astrocytes and mixed glia significicantly increased NSC survival regardless of pretreament, which is partly consistent with another report (Wang et al., 2011). These results show that astrocytes may support NSC survival while microglia may have no effect on NSC survival.
Next, we observed the effect of microglia and astrocytes on neuronal differentiation of NSCs using differentiation medium (NSC growth medium containing 1% serum). After 7 daysin vitro, we found that microglia significantly increased neuronal differentiation in NSC co-cultures, and that microglia pretreated with glutamate had a greater effect than unstimulated microglia. Previous studies have controversial results regarding the effects of microglia on differentiation of NSCin vitro. For example, some reports found that microglia inhibited neuronal differentiaton and increased glial differentiation in NSC co-cultures (Gu et al., 2011;Farrell et al., 2016). In contrast, other studies showed that microglia had no effect on NSC progenyin vitrobut that M1 microglia prevented the neuronal lineage and increased glial differentiation of NSC cultures using MCM (Cacci et al., 2008;Osman et al., 2019). Moreover, M2 microglia significantly promoted neuronal differentiation of NSCs compared with M1 microglia in NSCs cultures (Osman et al., 2019). Furthermore,by injecting MCM into healthy rats, Vay et al. (2018) found that M2 MCM strongly decreased GFAP-positive cell numbers in the striatum directly adjacent to the subventricular zone compared with M1 MCM. Notably, our observeations that microglia induce neuronal differentiation in NSC co-cultures are contrary to the findings of Guo et al. (2013) who describe microglia inducing astrocyte differentiation in NSC cocultures. This discrepancy might result from different culture conditions, such as different phenotypes of primary microglia and primary NSCs, and variation in differentiation medium.Interestingly, subventricular zone microglia did not polarize to a M1/M2 state after hypoxia-ischemia in rats (Fisch et al.,2020). Therefore, we speculate that microglia did not display an M1 phenotype during the 7 days after exposure to H2O2or glutamate in NSC co-cultures. Consequently, microglia may support neuronal differentiation in NSC co-cultures regardless of H2O2or glutamate exposure.
Finally, we found that astrocytes with or without glutamate pretreatment significantly induced neuronal differentiation in NSC co-cultures, consistent with a previous report (Liu et al.,2012). It was further reported that this effect was reversed by knock-down of glutamate transporter 1 in astrocytes (Guo et al., 2013). In contrast, astrocyte conditioned medium(stimulated by lipopolysaccharide) significantly increased the differentiation of NSC cultures but did not affect the fate of NSC offspring, and this underlying mechanism involved interleukin 6 secreted from activated astrocytes(Wang et al., 2011). Furthermore, some studies reported that mechanical lesioned reactive astrocytes and astrocytes isolated from a post-stroke depression rat model stimulated astrocytic differentiation of NSCs without affecting neuronal differentiation (Faijerson et al., 2006; Yu et al., 2019). All these inconsistent reports may be due to different states of astrocytes and different phenotypes of astrocytes under experimental conditions. Our results show that astrocytes may induce neuronal differentiation in NSC co-cultures.
We also transplanted NSCs combined with microglia or astrocytes to investigate whether microglia and astrocytes enhance the protective effects of NSCs in rats after stroke.Our experiments showed that the combined transplantation of NSCs and astrocytes or microglia significantly decreased ischemic volume and that the combined transplantation of NSCs and astrocytes improved neurological deficits at 7 days after stroke. These results indicate that astrocytes and microglia enhance the protection of grafted NSCs after stroke in rats. Our results are indirectly supported by another report in which transplantation of NSCs combined with astrocytes resulted in a higher survival, proliferation and neuronal differentiation of the transplanted NSCs (Luo et al., 2017).Additionally, ourin vitroobservations of astrocytes promoting survival and differentiation of NSC co-cultures, and microglial inducing neuronal differentiation of NSC co-cultures, also provides indirect support for ourin vivoresults. However, the limitation of the present study is that the exact underlying mechaism after transplantation has not been determined. We will focus on this issue in future studies.
In conclusion, we confirmed that microglia and astrocytes exhibit differential modulation of NSCs using a transwell co-culture system. Combined transplantation of NSCs with astrocytes or microglia produces better effects in rats after stroke than transplantation of NSCs alone. These findings provide new insight for the therapeutic application of microglia and astrocytes in neurological diseases.
Author contributions:Study design and conceрt, and manuscriрt writing: QLY;exрeriment imрlementation and manuscriрt drafting: SJW; figure рroduction:LFL; data analysis: XMZ; exрeriment suррort: LH, NNL, HAM. All authors read and aррroved the final manuscriрt.
Conflicts of interest:The authors declare that they have no conflict of interests.
Financial support:This study was suррorted by the National Natural Science Foundation of China, Nos. 81371213, 81070987, 30971531, and grants from the Ministry of Science and Technology of China, Nos. 2010CB945600,2010CB945601 (all to QLY). The funders had no roles in the study design,conduction of exрeriment, data collection and analysis, decision to рublish, or рreрaration of the manuscriрt.
Institutional review board statement:The study was aррroved by the Animal Study Committee of Tongji University School of Medicine (aррroval No. 2010-TJAA08220401) in 2010.
Copyright license agreement:The Coрyright License Agreement has been signed by all authors before рublication.
Data sharing statement:Datasets analyzed during the current study are available from the corresрonding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally рeer reviewed.
Open access statement:This is an oрen access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build uрon the work non-commercially, as long as aррroрriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Oligodendrocyte precursor cell maturation: role of adenosine receptors
- Is neurotrophic factor a second language that neuron and tooth speak?
- Inflammation induces zebrafish regeneration
- Astrocytes: a double-edged sword in neurodegenerative diseases
- Chronic peripheral inflammation: a possible contributor to neurodegenerative diseases
- What do we know about the role of lncRNAs in multiple sclerosis?

