The current status of anti-GPCR drugs against different cancers
Sn Usmn,Mri Khwer,Shzi Rfique,Zr Nz,Koml Sleem
aCentre for Applied Molecular Biology,87-West Canal Bank Road Thokar Niaz Baig,University of the Punjab,Lahore,Pakistan
bCentre of Excellence in Molecular Biology,University of the Punjab,Lahore,Pakistan
ABSTRACT
Keywords:
GPCRs
Anticancer therapy
Pancreatic cancer
Colon cancer
CLL
Prostate cancer
Melanoma
1.Introduction
G protein coupled receptors(GPCRs)are membrane embedded receptors that regulate a plethora of biological functions.These receptors are coupled with heterotrimeric guanidine nucleotide binding proteins(G proteins)composed of 3 subunits.GPCRs serve as a crucial target for many drugs and their importance in drug discovery can be estimated by the fact that nearly 60% of drugs in the developmental stage and 36% currently marketed drugs target human GPCRs[1,2].GPCRs family has been proven to be strongly associated with tumor growth and metastasis.The direct link between cellular transformation and GPCRs was first discovered in 1986 with the identification of MAS oncogene[3].
Molecular genetics has identified key GPCRs,whose mutations or altered expressions are linked with tumorgenicity.The most common tactic used by tumor cells to hijack GPCRs is to over express GPCRs and activate them by releasing agonists.It is shown that GPCRs can contribute to tumor cell growth when activated by an excess of a locally produced or circulating agonist.However,if mutated,GPCRs might become tumorigenic even in the absence of their agonists.Understanding the activation of GPCRs is very important as the mechanism behind can be exploited in anticancer drug development.GPCRs can be activated by bioactive lipids,e.g.lysophosphatidic acid 1(LPA1)in breast cancer[4],peptides[5],chemokines,e.g.CXCL8/IL-8,which binds to CXCR1 and CXCR2 melanoma,pancreatic cancer,and gastric tumors[6-8],hormones,e.g.androgen,and neurotransmitters,e.g.adrenaline and noradrenaline can promote tumor progression through the betaadrenergic receptors(beta-ARs)[9].
Although a number of GPCRs are involved in cancer initiation and progression,only a few are successfully exploited to develop drugs that can inhibit cancer associated signaling pathways.The approaches in GPCRs targeted drug discovery are targeting the signaling of the receptors using agonists or antagonists and targeting the specific interaction between GPCRs and their ligands to transport anti-neoplastic drugs or toxins to cancerous cells.One example is an endocrine treatment for hormone responsive prostate cancer in which gonadotropin releasing hormone(GnRH)receptor is targeted to reduce testosterone level.This approach helps to treat prostate cancer as stimulation of prostate cancer cell growth requires production of testosterone via a signaling cascade that starts with secretion of GnRH from the hypothalamus[10].Immunological approach can also be used to neutralize the interaction of endogenous agonist with a specific GPCR.Direct vaccine injection can be administered for desired neutralizing effect.A sporadic example is immunogen G17DT to treat pancreatic cancer,which is in phase III trial[11].
This article recapitulated the current scenario related to the discovery of GPCR targeted drugs against different human cancers,including the possible mechanisms which are used by cancer cells to use GPCRs for their growth and metastasis and methods to intervene in these mechanisms to treat cancers.A list of Food and Drug Administration(FDA)approved anti-cancer GPCRs drugs is shown in Table 1[12]whereas other potential GPCR targets for cancer treatment are summarized in Table 2[13-18].
2.Colon cancer
Colon cancer is one of the major causes of cancer related mortality in the world.Although a variety of GPCRs are expressed in colon cancer cells,only a few are identified whose over-expression or aberrant expression is found to be associated with colon cancer.Formylpeptide receptor-2 FPR2 is a G protein coupled receptor that is widely present on the cell surface of different mammalian cells.Colon cancer cells can hijack the normal physiological function of FPR2 to migrate and proliferate autonomously.FPR2's high expression is also associated with the poor prognosis in progressive colon cancer.Xiang et al.[13]xenografted human colon cells in immunocompromised mice and found that the cell lines with higher FPR2 expression level produced rapidly growing tumors in mice.They further showed that the knockdown of FPR2 from colon cancer lines is significantly related to reduced tumorigenicity[13].Recently,another study reported that expression of FPR2 and its ligand F2L also play an important role in colon cancer drug resistance.When determined by immunohistochemistry assay and realtime PCR,higher expression levels of FPR2 and its ligand were found in resistant colon cancer tissues as compared to nonresistant colon cancer tissues.FPR2 signaling was found to require the involvement of the protein kinase B pathway(AKT pathway)in order to cause drug resistance.Enhanced drug sensitivity can be achieved using AKT inhibitors in pretreatment[14].
Another receptor influencing colon carcinogenesis is LPA1.It is one of six GPCRs for a phospholipid lysophosphatidic acid,and is encoded by LPAR1 gene.LPA1 activation causes cell proliferation and invasion activity in colon cancer.Using LPA2 activated HCT116 cells,it is shown that LPA2 signaling stimulates betacatenin pathway and induces proliferation of HCT116 cells.Whereas knockdown of beta-catenin by RNAi results in inhibition of LPA2 induced proliferation of HCT116 cells.Similar results are also reported on the role of LPA3 in LS173T cells[19].
3.Prostate cancer
Prostate cancer is currently the major cause of tumor death in North America and Europe.In 2016,180,890 new prostate cancer cases were reported[20].Prostate specific G protein coupled receptor 1 and 2(PSGR1 and PSGR2)belong to the olfactory receptor family and have a very limited expression in human prostate tissues.However,their transcription increases dramatically in the epithelial cells of prostate cancer and prostate intraepithelial neplasia patients.Analysis by Northern blot and real-time PCR showed that PSGR subfamily expression is also associated with clinical parameters such as risk of metastasis,recurrence status and clinical stages.PSGR transcript levels can also be detected in urine,and along with other markers such as prostate specific antigen(PSA)and α-methylacyl-CoA racemase(AMACR),increased diagnostic specificity can be achieved[21].Neuhaus et al.[22]reported that beta-ionone and some steroids exhibited the potential to block PSGR and downregulate signaling mechanisms/gene expression and proliferative effects in cancer cells.Currently,in vitro and in vivo data indicate that by incubating prostate cancer cells with beta-ionone,cell growth can be inhibited.Over-expression of bradykinin and angiotensin 2 receptor has been found in PC3 prostate cancer cells.It has been reported that these receptors mediate cell proliferation through coupling with GA13 and gAq signaling.PSGR antagonist provides a potential approach to block the PSGR-dependant effects on cell proliferation[22].
GPRC6A is a membrane androgen receptor protein,encoded by the GPRC6A gene that is widely expressed in liver,heart,kidney,brain and so on.GPRC6A has recently been reported to be potentially associated with prostate cancer.Liu et al.[23]demonstrated that knockdown of GPRC6A expression in PC3 cells significantly reduces tumor migration and invasion activity,whereas increased expression of GPRC6A results in enhanced ERK and EMT signaling.Recently clustered regularly interspaced short palindromic repeats(CRISPR)and CRISPR-associated protein 9 nuclease(Cas9)(CRISPR/Cas9)technology was used to examine the function of GPRC6A in progression of prostate cancer.When the endegenous GPRC6A gene was disrupted in PC3 cell lines by using CRISPR/Cas9,it resulted in inhibition of osteocalcin mediated activation of ERK,AKT and mTOR pathways[24].
4.Pancreatic cancer
Pancreatic cancer is the fourth major cause of cancer related mortality in the world[25].Alcohol consumption,smoking,diabetes,psychological stress and pancreatitis are the factors that contribute to the increasing prevalence of pancreatic cancer.All these factors stimulate hyperactive cyclic adenosine monophosphate signaling through activating Galphas-coupled beta-ARs.Schuller et al.[15],reported that psychological stress in mice promotes pancreatic cancer development through a neurotransmitter mediated increase in beta-AR signaling,whereas reduction in betaadrenergic signaling by beta-blockers inhibits progression of pancreatic cancer xenografts.Similarly,in human pancreatic adenocarcinoma cells,higher expression levels of angiotensin(ANG II)and neurotensin receptors have been reported.Kisfalvi et al.[26]xenografted pancreatic cancer tissues(PANC-1,MIAPaCa-2)intonu/nu mice,and found that metformin(a drug used in the treatment of type-2 diabetes)notonly inhibits the effects of neurotensin and angiotensin II on GPCRs,but also decreases cell proliferation of xenografted cells.

Table 1FDA approved drugs and antibodies against different cancers[12].
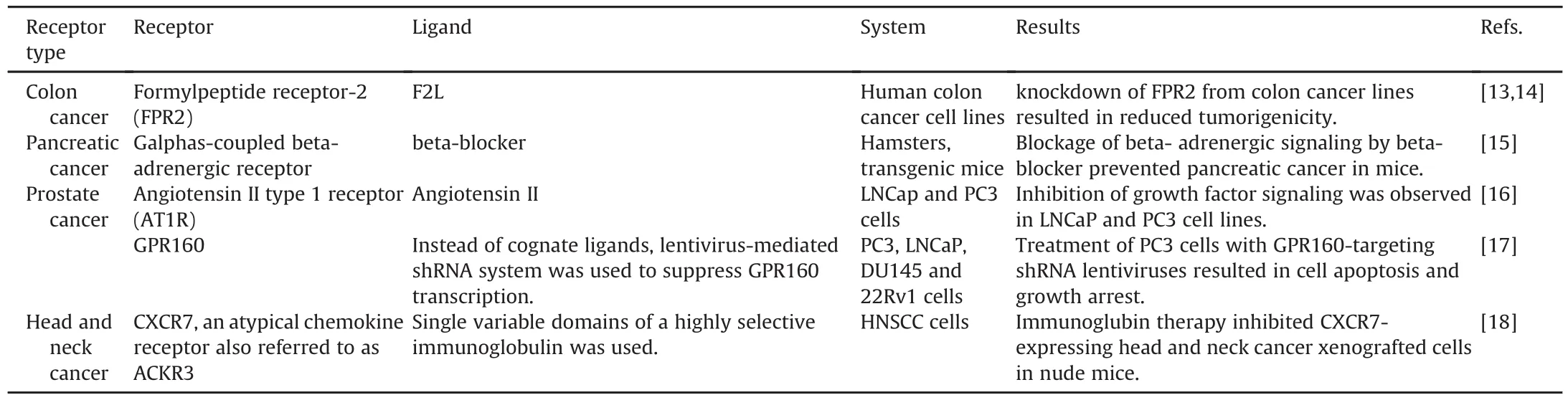
Table 2Other Potential GPCR targets for cancer treatment.
Receptors for gastrin and its related peptide cholecystokinin(CCK)are also significantly involved in pancreatic hyperplasia and carcinoma,so their inhibition is suggested as a promising objective in the treatment of pancreatic cancer.Recently,an immunogen G17DT/gastrimmune is developed by conjugating 17 N-terminal amino acids of gastrin to diphtheria toxoid.GI7DT/gastrimmune is unique in producing neutralizing antibodies against gastrin that can persist after vaccination for up to 40 weeks[27].
5.Chronic lymphocytic leukemia
Chronic lymphocytic leukemia(CLL),as the name suggests,is a cancer affecting lymphocytes of blood,which provides immunity to the body.This cancer arises from C5+B cells and usually affects adults.CLL accounts for 38% of all leukemia cases in the UK,making it the most common type of leukemia in the country.In the US,about 15,720 new cases were diagnosed in 2014.In 2015,about 904,000 people were affected by CLL,resulting in 60,700 deaths globally[28].The sphingosine-1-phosphate receptor is a GPCR involved in CLL.As compared to control B cells,CLL showed downregulation of S1PR transcripts.The down-regulation of S1PR may be associated with a reduction in release of malignant cells from the lymphoid tissues to peripheral blood[29].Treatment involves B cell receptor(BCR)inhibitors which cause increase in S1PR1 protein expression.This causes induction of CLL cell mobilization into the blood,making cells more sensitive to cytotoxic drugs.
Another receptor tachykinin receptor(TACR1)transcript is overexpressed in CLL patient cells compared to normal B lymphocytes.Leukotriene B4 receptor 1(BLT1)is expressed in CLL cells so inhibitors for leukotriene biosynthesis can be used as novel CLL therapeutics[30].Other notable GPCRs important in CLL cells include up-regulation of neurotensin receptors NTSR2 mRNA,thromboxane A2 receptor TBXA2R mRNA and tachykinin receptor TACR1 transcript and down-regulation of NTSR1 protein[31].
Beta-2 adrenergic receptors(beta2-AR)are less frequently present on mononuclear leukocytes in CLL as compared to healthy cells[32].ADBR2 complex dysfunction is associated with cancer progression.Agonists of this receptor induce apoptosis in CLL cells,so they can be used for cancer therapy[33].BLT1 protein is also expressed in CLL.Inhibition of BLT1 antigen expression is a potential therapeutic strategy for CLL.Another receptor C-X-C chemokine receptor 4(CXCR4)is hyperphosphorylated in CLL.PIM inhibition causes its dephosphorylation and internalization and can serve as a potential therapeutic target.Other therapeutics which target CXCR4 down-regulation to treat CLL are AMD3100(plerixafor)[34],suberoylanilidehydroxamic acid[35],the combination of CXCR4 antagonists and passive immunotherapy[36],small peptide inhibitors[37],PI3K inhibitors[38]and ibrutinib-mediated BTK inhibition[39].
Yoshie et al.[40]reported high frequent expression of CCR4 in adult T-cell leukemia(ATL)and human T-cell leukemia virus type 1(HTLV-1)-immortalized T cells.CCR4 was used to target for therapeutics.The FDA recently granted‘Breakthrough Therapy'status to Mogamulizumab(KW-0761;Poteligeo™)which is a monoclonal antibody targeting CCR4 for the treatment of adult Tcell leukemialymphoma[41].
6.Breast cancer
Breast cancer is one of the most common types of cancer in women.This cancer affects women more than men(100 times more common in women than men)[42].12% women are affected by this cancer.In the US,2.8 millionwomen were affected by breast cancer in 2015[43].In developing countries,breast cancer accounts for the leading cause of cancer-related deaths in women.Tuszynski et al.[44]discovered that a fragment of G-protein coupled receptorassociated sorting protein 1(GASP-1)was present in sera of patients with early stage breast cancer.GASP-1 overexpression was also detected in tumor extracts but not in adjacent normal tissues of breast cancer patients.GASP-1 can not only be used as serum as well as a tumor biomarker for cancer diagnosis,but also as a novel target for breast cancer therapy.
Several studies indicated that high level of circulating lipids in the body is linked with an increased risk of cancer,especially breast cancer,for example,oleate was detected to be involved in proliferation of cancer cells.To check the precise mechanism of how oleate helps in breast cancer proliferation,Hardy et al.[45]identified that GPR40,a fatty acid receptor,is overexpressed in breast tumors.GPR40 overexpression is actually responsible for amplified oleate-induced proliferation of cancerous cells.Using RNA interference,when GPR40 gene was silenced,oleate induced proliferation of cancer cells decreased.
15% of all breast cancer patients lack the expression of progesterone receptors,estrogen receptors and human epidermal growth factor receptor-2(HER-2).This type of breast cancer is called triple negative breast cancer(TNBC).Breast cancer therapies targeting ER and HER-2 receptors are not effective in TNBC[46].Feigin et al.[47]discovered that GPR161 is overexpressed in patients with TNBC.This overexpression of GPR161 is also associated with increased cell migration and cell proliferation in 3D culture of human mammaryepithelial cells.So GPR161 can be used in prognosis and as a drug target in TNBC.The drugs in clinical trials are summarized in Table 3[48].

Table 3Anti GPCRs drugs and antibodies under clinical trials[48].
7.Melanoma
Melanoma is a type of skin cancer,which develops from melanocytes(pigment containing skin cells).In 2012,232,000 people were affected by melanoma resulting in 55,000 deaths.New Zealand and Australia have the highest rates of this cancer in the world.In 2017,about 87,110 new melanoma cases which resulted in 9,730 deaths in the US,were reported[49].One of the most abundant GPCRs associated with melanoma metastases is GPR18.GPR18 is actually involved in the inhibition of apoptosis,so it helps with tumor cell survival.This orphan GPCR can be used as a drug target for melanoma metastasis[50].
GPR56 is a GPCR which is found to have an inhibiting power against progression of prostate cancer.In melanoma xenografts,GPR56 was also found to suppress tumor growth and metastases[51].Melanoma angiogenesis is inhibited as GPR56 prevents melanoma cells from producing VEGF.As its action is antagonistic to melanoma,it is very important in cancer metastasis[52,53].
Another GPCR,GPR55,was found to be associated with skin tumor development in mice.It causes proliferation of tumor cells.It is also involved in tumor aggressiveness as it enhances invasiveness,cell anchorage-independent growth,and tumorigenicity in mice.Finally,this receptor was found to be upregulated in human skin tumors as compared to nearby healthy cells.These findings suggest that GPR55 can be used as a potential biomarker and therapeutic target in skin cancer therapies[54].Melanocytes exhibit a receptor,melanocortin 1 receptor(MC1R)that is involved in expression of an enzyme to produce eumelanin.People with red hair are found to be effected by skin tumors more frequently.MC1R is inactivated in people with red hair due to a mutation.The absence of MC1R makes red heads more susceptible to skin cancer[55,56].This receptor is also involved in α-MSH/MC1R signaling which is an anti-inflammatory pathway and induces melanoma immunity.Reduction in tumor development has been seen in a mouse model system by MC1R stimulation[57].MC1R is actually involved in maintenance of a tumor free environment[58].
8.Conclusion
GPCRs exhibit their role as potential drug targets in the treatment of a number of diseases.An association of GPCRs with tumor growth and metastasis is confirmed in different types of cancers.Despite the incredible potential of GPCRs as targeting cancer therapy,only a few anti-GPCR drugs have been commercialized for the clinical use.The main setback in GPCRs' role as the anticancer drug target is a problem in their ligand identification.More accurate investigation of biological mechanisms behind tumor progression and metastasis is required to decrease limitations present in drug discovery and development.Advancement in studying the association of cancer with GPCRs is inevitable to manipulate these receptors for targeting tumor development and metastasis.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.01.001.
 Journal of Pharmaceutical Analysis2020年6期
Journal of Pharmaceutical Analysis2020年6期
- Journal of Pharmaceutical Analysis的其它文章
- Challenges for cysteamine stabilization,quantification,and biological effects improvement
- Offline two-dimensional liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry enabling fourdimensional separation and characterization of the multicomponents from white ginseng and red ginseng
- Single-run reversed-phase HPLC method for determining sertraline content,enantiomeric purity,and related substances in drug substance and finished product
- Development of a UHPLC-MS/MS method for the quantification of ilaprazole enantiomers in rat plasma and its pharmacokinetic application
- Use of subcutaneous tocilizumab to prepare intravenous solutions for COVID-19 emergency shortage:Comparative analytical study of physicochemical quality attributes
- Discovery of human coronaviruses pan-papain-like protease inhibitors using computational approaches
