Evolution from small molecule to nano-drug delivery systems:An emerging approach for cancer therapy of ursolic acid
Jingwei Sho,b,1,∗,Yifn Fng,Ruirui Zho,Fngmin Chen,Mingyue Yng,Jili Jing,Zixun Chen,Xiotin Yun,Lee Ji,b
aCancer Metastasis Alert and Prevention Center,and Fujian Provincial Key Laboratory of Cancer Metastasis Chemoprevention and Chemotherapy,College of Chemistry,Fuzhou University,Fuzhou 350116,China
bMarine Drug R&D Center,Institute of Oceanography,Minjiang University,Fuzhou 350108,China
Keywords:Ursolic acid Nanosytems Carrier-free Photosensitizer Anticancer
ABSTRACT Ursolic acid (UA),a natural pentacyclic triterpenoid,possesses widespread biological and pharmacological activities.However,drawbacks such as low bioavailability,poor targeting and rapid metabolism greatly hinder its further clinical application.Recently,with the development of nanotechnology,various UA nanosystems have emerged as promising strategies for effective cancer therapy.This article reviews various types of UA-based nanodelivery systems,primarily with emphasis placed on novel UA-based carrier-free nanodrugs,which are considered to be innovative methods for cancer therapy.Moreover,this review presents carrier-free nano-drugs that co-assembled of UA and photosensitizers that displayed synergistic antitumor performance.Finally,the article also describes the development and challenges of UA nanosystems for future research in this field.Overall,the information presented in this review will provide new insight into the rational utilization of nano-drugs in cancer therapy.
1.Introduction
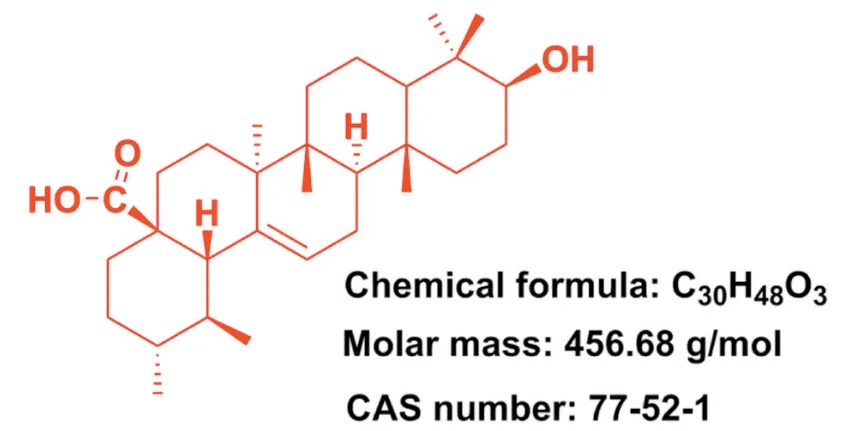
Fig.1-Structure of ursolic acid(PubChem CID:64,945).
Cancer is a devastating disease that exerts influential public health trouble in the world [1,2].Current treatment options for cancer include surgery,radiotherapy,chemotherapy,hormonal therapy,and immunotherapy or combinational chemotherapy,etc.Among them,surgical resection remains a preferred treatment for patients with solid tumors,such as breast cancer,colon cancer,rectum cancer,lung cancer and so on[3].However,most surgical approaches exist limitations of low resection rates and high recurrence rates[4].The majority of patients also received adjuvant chemotherapy after surgical resection to ameliorate the risk of recurrence.Therefore,chemotherapy becomes the first treatment option for metastatic or advanced tumors to improve the survival rate of cancer patients [4].Current anticancer drugs available in the market are mainly consist of conventional chemotherapeutic drugs,molecular-targeted drugs,immune checkpoint antagonists,and hormonal therapy drugs,such as paclitaxel,sorafenib,tremelimumab,avelumab,dexamethasone and so on [5-8].Despite the fact that encouraging progress has been achieved in clinical cancer therapies,current treatments have been identified with some disadvantages(e.g.,multidrug resistance (MDR),poor sensitivity and serious side effects)that failed to eradicate tumors or prevent their recurrence[9-11].Therefore,it is in urgent need to overcome the shortcomings of current cancer therapies and further explore novel anticancer drugs with superior efficacy and low toxicity for the treatment of neoplastic disease.
Pentacyclic triterpenoids is an important class of bioactive natural compounds and which has gained much attention because of its superior therapeutic actions and fewer adverse effects in cancer therapy [12-14].Ursolic acid(UA) (3b-hydroxy-urs-12-en-28-oic acid,Fig.1),one of the pentacyclic triterpene carboxylic acids,can be extracted from various plants (e.g.,Sanguisorba Officinalis,Rosmarinus Officinalis,Salvia officinalis,etc.) [15-17].UA was confirmed to possess multiple pharmacological properties including antidiabetic [18],anti-inflammatory [19],antioxidant [20],antimicrobial[21],anti-hyperlipidaemia,etc[22].In particular,UA and its derivatives were also found to effectively inhibit a series of cancer cell growth [23-26],indicating their great potential to be served as promising chemotherapeutic agents for the treatment of human cancers.However,the poor water solubility and permeability of UA greatly hindered its preclinical or clinical applications.Various preclinical trials have revealed that oral administration of UA performed poor intestinal absorption and UA was rapidly eliminated by gut wall/liver metabolism,ultimately resulting in low bioavailability of UA [27,28].For instance,when administered orally,UA only displayed a half-life of 0.71±0.09 h andCmax(peak concentration) of 1.10±0.31 μg/ml in rats [27].When administered intravenously,low molecular weight UA would easily diffuse throughout the body and further resulted in nonspecific distribution [29].Notably,these disadvantages seriously hindered the clinical application of UA.In recent decades,increasing evidence suggested that constructing nano-drug delivery systems were able to effectively improve bioavailability and enhance the targeting efficiency of agents.
The nano-drug delivery systems are widely applied in delivering various agents like drugs,photosensitizers,phytochemicals,biomolecules,and other compounds[30-33].Nanoparticles can efficiently permeate into tumor tissues due to the enhanced permeability and retention (EPR)effect of tumor blood vessels.In contrast,normal blood vessels will prevent nanoparticles from permeating [34].Therefore,nano-drug delivery systems can not only improve the targeted accumulation of therapeutic agents at the tumor sites but also reduce their biodistribution in normal tissues [35].It has also been reported that MDR and efflux transporters could be overcome through nanoparticles-mediated internalization [36].Additionally,some investigations suggested that UA-based nano-delivery systems displayed great potential for ameliorating or overcoming the shortcomings of UA[37].
Therefore,this review summarizes the status of preclinical and clinical trials of UA nanosystems in cancer therapy,including traditional nanocarrier-based delivery systems and novel carrier-free delivery systems (Fig.2).The various UA nanosystems with the properties of improved bioavailability and pharmacokinetic are exhaustively summarized in this review.Besides,this review also discusses the clinical trials of UA liposomes for the treatment of human cancers.The purpose of this review is to offer useful insight into the prospect and challenge of discovering ideal UA nanosystems for clinical cancer therapy.
2.Anti-cancer activities of ursolic acid
Recently,anticancer property of UA has been extensively investigated in area of oncology,especially in breast cancer[38],hepatocellular carcinomas[39],cervical cancer[40],lung cancer [41],melanoma [42],gall bladder carcinomas [43]and prostate cancer[44].Epidermal growth factor receptor(EGFR),an over-expressed protein in cancer cells,has been identified as a therapeutic target in cancer therapy [45].Of note,mitogen-activated protein kinases (MAPK) is a downstream effector of EGFR.Studies demonstrated that UA could attenuate the phosphorylation of ERK 1/2,EGFR,p38 MAPK,and c-Jun N-terminal kinase (JNK) so as to induce cancer apoptosis and inhibit cancer cell proliferation and tumor angiogenesis [46].The phosphoinositide 3-kinase (PI3K)/protein kinase B(AKT),Janus kinase 2(JAK2)/signal transducer and activator of transcription 3 (STAT3) pathways are three crucial intrinsic pathways (or mitochondrial pathway)involved in apoptosis.Moreover,UA inhibits these cellular signaling pathways by modulating apoptosis-related genes(e.g.,Bax,Bad,Bcl-2,Bcl-xL,BID),apoptosis-related protease(e.g.,Caspase-3,Caspase-9,Caspase-8,PARP),protein kinases(e.g.,JAK2,mTOR,PI3K,AKT),transcription factors STAT3 and nuclear factor (NF)-κB,thus suppressing the development of cancer cell and inducing cancer cell apoptosis [47,48].Additionally,UA was also found to suppress the expression of pro-inflammatory cytokines,such as tumor necrosis factor(TNF)-α,TNF-γ,interleukin (IL)-2,IL-6,IL-18,which,in turn,influence the inflammatory response [38].Additionally,some studies revealed that UA significantly down-regulated the expression levels of matrix metalloproteinases (MMP)(e.g.,MMP-9,MMP-2) and cell surface adhesive molecules(e.g.,ICAM-1,VCAM-1,E-selectin and P-selectin),thereby suppressing tumor invasion and metastasis.The primary mechanisms of action of UA are summarized in Fig.3.The mechanistic elucidations and signaling pathways will be helpful in selecting proper therapeutic targets and designing ideal UA nanoformulations for cancer therapy.
3.Traditional nanocarrier-based drug delivery systems of ursolic acid
In the past decades,nanotechnology has been extensively applied in pharmaceutical research and a variety of nanocarriers have been developed in order to facilitate drug delivery.The advantages of most nanocarriers are presented below.(i) Improving bioavailability of drugs by enhancing drug solubility,stabilization and loading capacity.(ii) Easily to be modified by surface functionalization due to the large surface-to-volume ratio in nanosized materials.(iii)Controlled or targeted release of the payloads in demand,and(iv)equipped with great biocompatibility and biodegradability.Considerable nanocarriers such as liposomes,chitosan,polymer,dendrimer,mesoporous silica and nanostructured lipid carriers have been developed for optimizing UA-based nanoformulation design(Table 1).
3.1.Liposome
Liposome is the most well-studied nanocarrier which composed of phospholipids and cholesterol molecules[49,69].In comparison with free drug,the liposomal drug possesses several attractive features such as controlled release,reduced toxicity,excellent stability,targeted delivery,increased solubility,higher cellular uptake,and multiple routes of administration [70].De Araújo Lopes et al.developed UA-loaded liposomes which composed of dioleoylphosphatidylethanolamine(DOPE),cholesteryl hemisuccinate (CHEMS),and distearoylphosphatidylethanolamine-polyethylene glycol(DSPE-PEG) 2000.The obtained liposome with an average diameter of 191.1 nm,was benefit for drug accumulation into tumor tissues.Additionally,due to the strong interaction between UA and phospholipids in the liposome bilayer,the characteristics of liposomes remained unchanged for 60 d[49].In another study performed by Zhao et al.,PEG-modified UA liposomes released 53% of the loaded UA in 72 h.The inhibitory cell rates of PEG-UA NPs were found to be 68.27%in vitro,while the inhibition rate of free UA at the same concentration (250 μg/ml) was only 57.03%.Overall,surfacemodifications of liposomes with PEG polymer are able to enhance stability of conventional liposomes[50].
Recently,chitosan-modified UA liposomes (CS-UA-L) has been successfully prepared with high tumor targeting,drug controlled release and low side effects[51].In vitrodrug release studies showed that 35.7% of the loaded UA was released within 72 h at pH 7.4,while 100% was released at pH 5.5,indicating a pH-responsive sustained drug release manner.Furthermore,it was also found that the UA accumulation amount in tumor tissues of CS-UA-L-administrated mice was 4.2-fold more than free UA administrated groups[51].
Ligand-targeted UA liposomes have also been designed to enhance therapeutic efficacy but minimize side effects as much as possible [71].Yang et al.developed UA-loaded FA receptor-targeted liposomes (FTL-UA) with an IC50against KB cell lines of 22.05 μM,whereas the IC50of FA receptorblocking group was 65.66 μM.It was shown that FTL-UA effectively targeted KB cells through the FA receptor-mediated pathway [52].Overall,surface-functionalized liposomes not only facilitate the stability and bioavailability of UA,but also improve its targetability and therapeutic efficacyin vivo.
3.2.Polymeric nanoparticles
A variety of polymeric nanocarriers including chitosan [72],polybutylcyanoacrylate (PBCA),polylactic acid (PLA) and polylactic acid-glycolic acid copolymer (PLGA) [73]have been widely used for the drug delivery.Polymeric nanoparticles show many advantages surpass other nanocarriers such as the simplest preparation method and excellent stability.Zhang et al.[29]proposed that UA-loaded amphiphilic methoxy poly(ethylene glycol)-polycaprolactone (mPEG-PCL) nanoparticles presented a slightly better cell killing activity than free UA (IC50:46.0±2.8 vs.92.5±3.1 μM).The higher anticancer efficiency of mPEG-PCL nanoparticles were achieved due to the enhanced suppression of COX-2 and activation of Caspase 3.The conjugations of PEG or PEGylated nanoparticles were found to extend blood circulation half-life by reducing the recognition and uptake in the mononuclear phagocyte system(MPS).Subsequently,another UA-loaded amphiphilic molecule poly(N-vinylpyrrolidone)-block-poly(ε-caprolactone) (PVP-b-PCL) nanoparticles were developed and with an IC50of 32.89±3.23 μM (free UA,IC50at 59.84±4.12 μM) [53].Zhang et al.demonstrated that the PVP-b-PCL nanoparticles exhibited longer blood circulation in comparison with mPEG-PCL nanoparticles,which might be ascribed to their evasion of reticuloendothelial system (RES)[53].

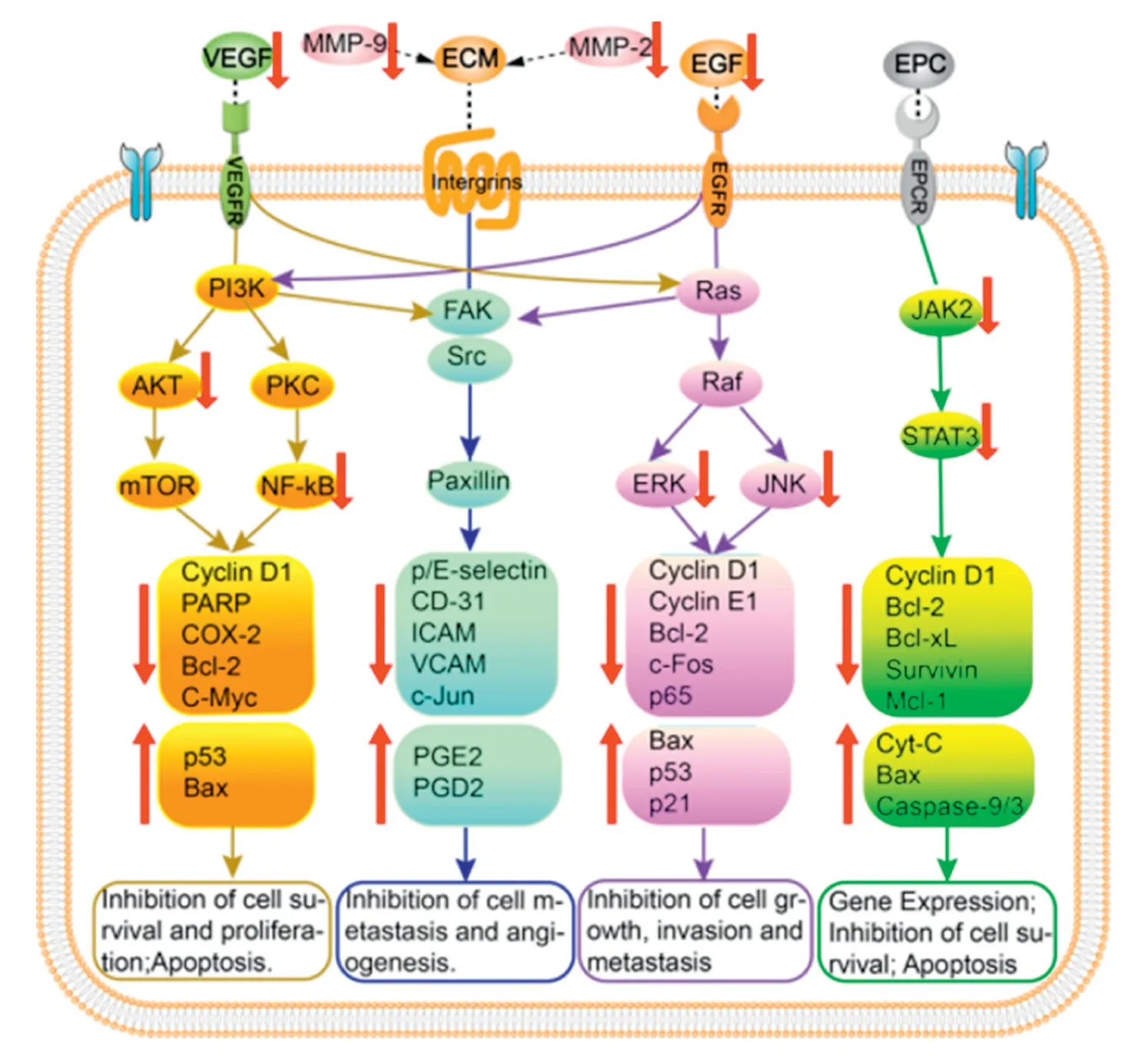
Fig.3-The primary mechanism of anticancer activities of UA.
In another formulation,UA-loaded PLA nanoparticles have been developed by the emulsification-solvent evaporation technique[54].30%of the loaded UA was released from these nanoparticles in 8 h,characterized as a burst release,and 60%was released within 120 h.These PLA nanoparticles showed negligible hemolysis on erythrocytes and lower cytotoxicity against cancer cells than free UA [54].In another study,a novel UA delivery system using PLGA as nanocarrier (PLGAUA NPs)was prepared and evaluated with higher cytotoxicity against melanoma cell lines.The nanoparticles showed an initial burst release of 30% loaded UA but followed by a sustained drug release manner for 15 d.Simultaneously,the nanoparticles showed a biphasic clear manner,with 50% of the dosage rapidly cleared in 1 h and the rest was slowly cleared in the blood circulation[55].Wang et al.reported that gold-UA-loaded PLGA nanoparticles exhibited great cellular uptake and effectively suppressed proliferation,invasion and migration of cervical cancer cells[74].Oprean et al.developed UA-loaded polyurethane nanoparticles and assessed for their anti-proliferative against breast cancer cells [56].It was found that these nanoparticles displayed remarkable antiproliferative activitiesin vitro.The improved drug loading efficiency could be contributed to the great affinity between the hydrophobic UA and polymeric molecules.Accumulating evidence have supported the prolonged blood circulation and enhanced retention concentration of UA at the tumor sites after incorporated into polymeric nanoparticles.In addition,most polymers are biodegradable and biocompatible molecules,indicating their great potential to exhibit minimal toxicityin vivo.
3.3.Polymer-drug conjugates
Growing interests have been focused on polymer-drug conjugates as they present numerous benefits when comparing with individual drug molecules:(i) Improving water solubility of hydrophobic drugs.(ii) Enabling sustained drug release by controlling the breaking of covalent bonds.(iii)Reducing toxicity side effects of drugs with narrow windows for treatment,thereby augmenting their clinical application prospects [75].Jin et al.reported that the conjugation of UA and chitosan (CH-UA-NPs) could be achieved via covalently linking the carboxyl group of UA to the amino group of chitosan.The CH-UA-NPs significantly alleviated the adverse effects by saving approximately 10-fold of the dosage of free UA [57].In another study,UA chitosan nanoparticles were further modified by folic acid (FA-UA-CS-NPs) in order to improve its targeted efficiency.Cellular uptake experiments showed that the FA-UA-CS-NPs could selectively target MCF-7 cancer cells through FA receptor-mediated endocytosis[58].

Fig.4-Covalent conjugates of UA to polymeric carriers[57,60-62].
Dendrimers with highly-branched structures and biodegradable properties have been widely used in the preparation of nanoparticles.In a study,UA polymeric conjugations were designed and synthesized by conjugating of polyamidoamine dendrimers (G3/G5) with UA and FA(FA-G3/5-UA) [59].The results marked that the FA-G3/5-UA significantly enhanced cellular uptake in Hela cells (FA receptor over-expressing cell line) due to the electrostatic absorptive and FA receptor-mediated endocytosis.The IC50(μM) of FA-G5-UA and free UA was 20.24 and 44.35,respectively.Our group further modified the surface of low-PAMAM (G0/G1) dendrimers with lactobionic acid (LA)in order to develop a novel targeted nanocarrier for UA delivery (UA2-G0-LA NPs) [60].Thein vitrostudies showed that the UA2-G0-LA NPs had much higher cytotoxicity against ASGPR-overexpressing cell lines than other groups.
In a recent study,UA was covalently linked to eightarm-PEG and pectin to form an amphiphilic pro-drug(8armPEG-UA).The 8armPEG-UA and another hydrophobic drug hydroxycamptothecin (HCPT) were self-assembled into nanoparticles (Pec-8PUH NPs) [61].The hydrophobic drug UA served as the core while the hydrophilic pectin and 8armPEG served as the surface shell,which ensured the enhancement of UA stability and solubility in the neutral aqueous environment [61].Furthermore,pharmacokinetic experiments revealed that the Pec-8PUH NPs could exhibit prolonged clearance and blood circulation half-life as compared to free drug.Liu et al.reported the nanoparticles(CMC-UA/HCPT NPs) which self-assembled from UA,carboxymethylcellulose (CMC) and HCPT [62].The blood circulation half-life of CMC-UA/HCPT NPs was 7.3-fold higher than that of free UA [62].Overall,conjugation of UA with hydrophilic polymers (Summarized in Fig.4) could improve the solubility of UA and also impart some unique pharmacokinetic properties.
3.4.Solid lipid nanoparticles(SLNs)and nanostructured lipid carriers(NLCs)
Lipid based nanocarriers are able to increase cell membrane permeability and then enhance the cellular uptake of drugs [76].Based on the diverse internal structures,lipid nanoparticles are typically classified into solid lipid nanoparticles(SLNs)and nanostructured lipid carriers(NLCs)[77].The SLNs are only comprised of solid lipid,whereas the NLCs are made up of a mixture of blended solid and liquid lipid [78].In a study,UA-loaded NLCs which composed of trierucin,hydrogenated soy phosphatidylcholine and oleic acid was prepared by the hot homogenization-ultrasonication method [63].It was found that the UA NLCs exhibited higher cytotoxicity on K562 leukemic cells and B16 melanoma cells in comparison with free UA [63].Zhou et al.designed UA loaded phospholipid nanoparticles (UA-PL-NP) by the solvent emulsification-evaporation and ultrasonic dispersion technology and further evaluated their targetability [64].TheAUC0-12ratio of UA-PL-NP in the liver was 8.6-fold higher than free UA,indicating its great targetability to liver tissues.Then another group optimized the phospholipid nanoparticles by a novel method(Response Surface Methodology)to improve its bioavailability and pharmacokinetic properties.It was shown that the elimination half-life(T1/2el,8.28±1.98 h)of optimized nanoparticles was higher than free UA (0.69±1.76 h) [65].To date,UA-loaded solid lipid nanoparticles (UA SLNs) has not been reported in the literature.In summary,UA NLCs not only significantly enhanced bioavailability of UA but also showed superior anti-tumor activities in pre-clinical studies.
3.5.Mesoporous silica nanoparticles
Mesoporous silica nanoparticles (MSNs) is a promising drug delivery system due to its high surface area,narrow pore size distribution,superior chemical property,mechanical stability,and facile surface functionalization [79-83].UA-loaded MSNs(UA@MSN-UA)with particle size of 100 nm displayed a higher cytotoxic effect against HepG2 cells than free UA [66].The nanoparticles released 76%of the loaded UA in 96 h at pH 5.5,whereas 53% was released at pH 7.4,indicating its sustained and pH-responsive release properties.In another study,our group developed active targeting ligand FA and chitosan comodified UA-loaded MSNs (UA@M-CS-FA) and evaluated the targeted delivery efficiency of UA to FA-receptor positive cancer cells [67].Mechanistically,UA@M-CS-FA exhibited remarkable inhibition on the migration of cancer cells through regulating P53/MMP-9/PTEN/CD44 proteins.Also,in vivoexperiments revealed that UA@M-CS-FA significantly inhibited the tumor proliferation and migration[67].Similarly,another mesoporous silica nanocarrier(MSN-CS-LA)modified with pH-responsive chitosan and LA was developed for codelivering UA and sorafenib.This nanoparticle could improve the cellular uptake and internalization of drugs against ASGPR over-expressing cell lines[68].In conclusion,the development of multifunctional MSNs provides a new way for efficiently delivering UA into tumor tissues.
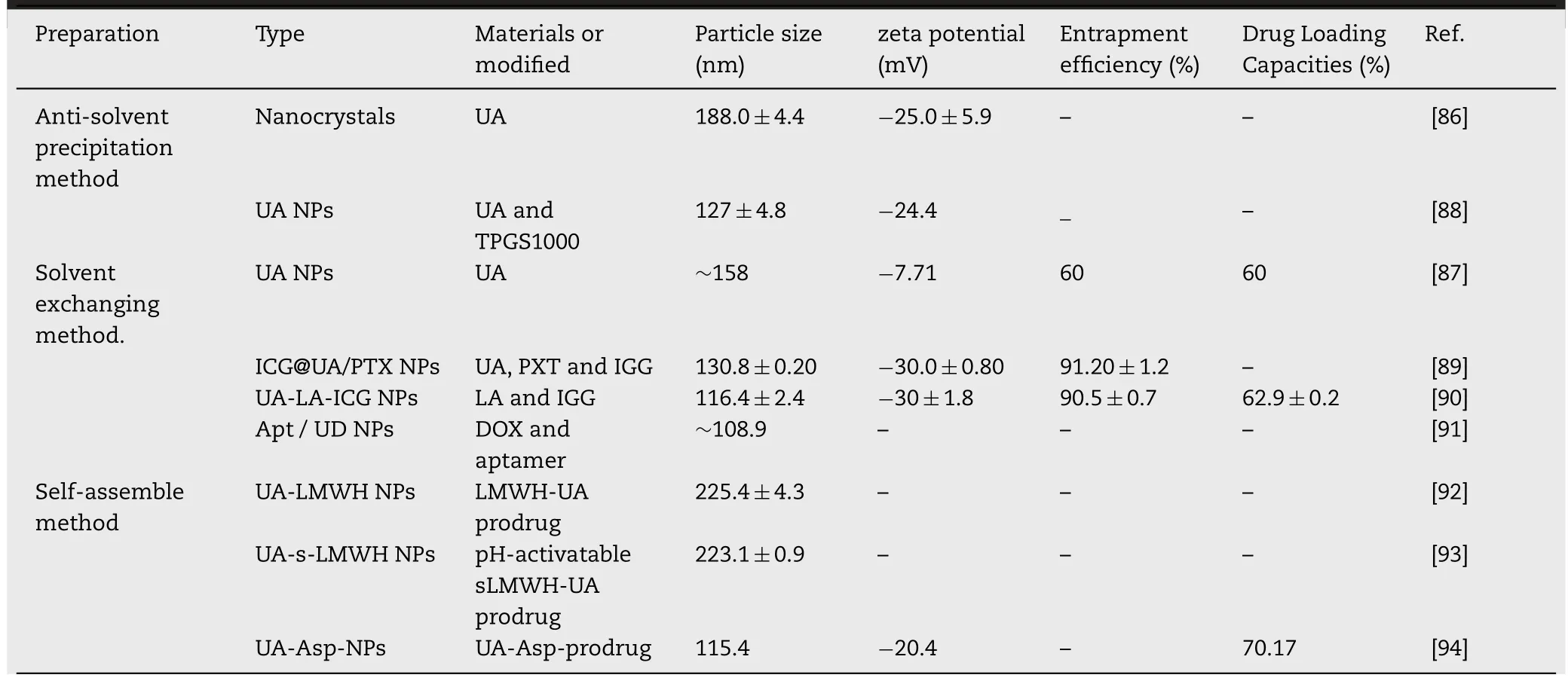
Table 2-The preparation and characteristics of novel carrier-free based nano-drug delivery systems of UA.
4.The novel carrier-free nano-drug delivery systems of ursolic acid
The applications of various nanocarriers provide some useful strategies for delivering UA into tumor tissues as well as enhancing its cellular uptake and internalization.Nevertheless,the clinical applications of nanocarriers have been limited due to their potential toxicity,indistinct metabolism and sophisticated preparation process [84,85].Hence,an increasing attention has been paid to develop novel carrier-free nano-drugs (Table 2) in order to achieve better anti-cancer efficacy as well as reduce the side effects.
4.1.Pure nano-drug
Nanocrystals,one of the broadly used carrier-free delivery systems,was mainly composed of insoluble drugs and certain surfactants [95].Many clinical trials revealed that nanocrystals could efficiently improve the bioavailability of drugs and ameliorate individual therapeutic differences [96].In a study by Song et al.,the UA nanocrystals were formulated by the anti-solvent precipitation method [86].It was shown that UA nanocrystals had good aqueous dispensability and could be completely dissolved in 0.5%sodium dodecyl sulfate solution within 120 min.Moreover,the characteristics of nanocrystals remained unchanged for 49 d,which indicated its great stability.Pi et al.evaluated the relative bioavailability of UA nanocrystal and found it was 2.56-fold higher than that of free UA [97].The nanocrystals may represent a promising delivery system for improving dissolution velocity and anticancer efficiency of UA.
In addition to nanocrystals,there are many different forms of pure nano-drugs.For instance,Wang et al.developed UA nanosuspensions by the anti-solvent precipitation method using a four-stream multi-inlet vortex mixer.In this study,two types of UA nanosuspensions with different particle sizes were evaluated for their anticancer activity[98].It was shown that the UA nanosuspensions(300 nm)significantly inhibited the growth of the MCF-7 cells,which may be due to the enhanced adhesion of nanoparticles to cell surface.Inspired by the electrostatic and hydrophobic interactions between UA and the nanocarriers,our group has recently developed novel UA nanoparticles by a solvent exchanging method[87].The UA NPs with particle size of approximately 150 nm was found to effectively permeate into tumor tissues by the EPR effect.The cell viability test showed that the IC50of UA NPs and free UA were 48.12 and 39.74 μM,respectively [87].Also,the UA NPs showed less toxicity to normal tissues,indicating its great potential in future clinical applications.In a similar study,in order to improve the bioavailability of UA,a novel UA nanoemulsion which stabilized with TPGS1000 was developed by Qiao et al.[88].AUC0→12andCmaxof UA nanoemulsion were found to be approximately 27.5-and 9-fold higher than free UA,respectively.In another study,TPGS1000 was also served as drug stabilizer to prepare UA nanoemulsion by emulsion solvent evaporation method.This nanoemulsion improved the equilibrium solubility of UA in deionized water up to 23.99-fold,and increased the oral bioavailability of UA up to 2.68-fold [99].Even though the preliminary studies showed the stability of carrier-free nano-drugs in the absence of stabilizers is not as good as that of traditional carrier-based nano-drugs,they still contribute a lot to the development of nano-drugs.
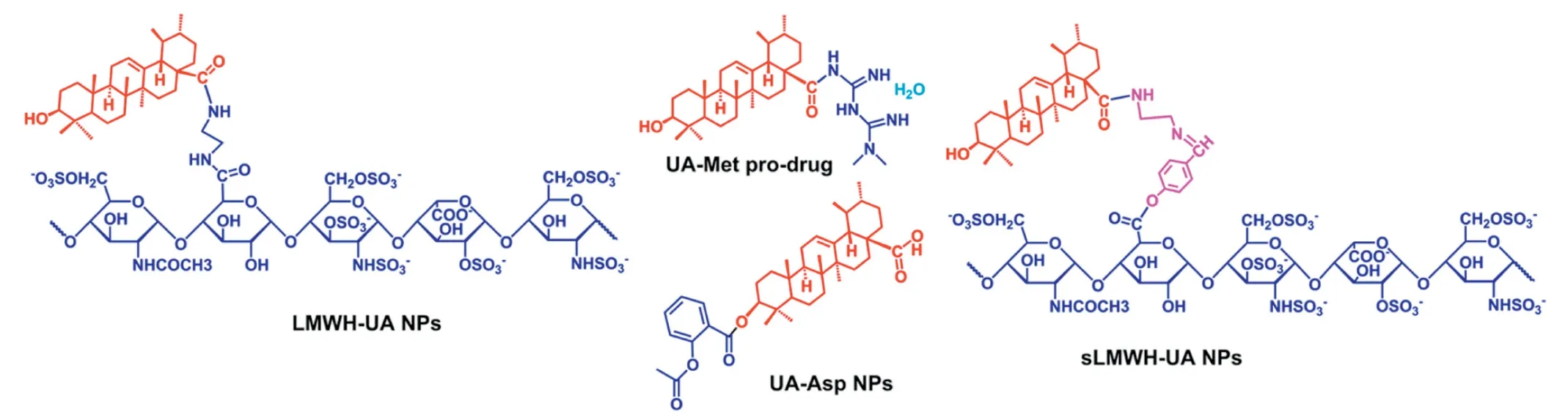
Fig.5-Structure of conjugates of UA with clinical drugs by the covalent link[92-94,101].
4.2.Carrier-free nano-drug by co-assembly of chemotherapeutic agent and photosensitizer
Phototherapy,which contains photothermal therapy and photodynamic therapy,is characterized by high tumor ablation efficiency.It has been well-reported that co-delivery of chemotherapeutic agents and photosensitizers could enhance the therapeutic effect of cancer.A novel carrierfree nano-drug delivery system (ICG@UA/PTX NPs) for codelivery of a photosensitizer (Indocyanine green,IGG) and chemotherapy drugs(UA and PTX)was developed by a solvent exchanging method [89].The carrier-free small molecule nano-drug via self-assembly demonstrated excellent ability of tumor targeting and tumor growth suppressing with no sign of recurrence under NIR laser irradiation [89].In a recently alternative study,we developed a nano-drug(UA-LAICG NPs)by co-assembly of UA,active targeting ligand LA and ICG.The UA-LA-ICG NPs exhibited anti-proliferative activity on asialoglycoprotein receptor(ASGPR)-overexpressing HepG2 cells.It was also shown that UA-LA-ICG NPs+NIR irradiation treatment significantly inhibited the development of tumors in H22 tumor-bearing mice[90].
4.3.Carrier-free nanosize drug by co-assembly of chemotherapeutic agents
Many researchers found that the carrier-free delivery systems based on combination of UA with other chemotherapeutic agents were able to display synergistic anticancer effects.A composite carrier-free system has been developed by the selfassembly of UA,aptamer and doxorubicin(Apt/UD NPs)[91].In vitro/vivostudies,the Apt/UD NPs showed superior inhibitory and synergistic anticancer effects.Our group developed the prodrug of UA and aspirin (Asp) into a nano-drug via the solvent exchange method [94,100].Of note,this nano-drug could release over 80% of UA after 48 h at pH 5.5,while only release of UA about 40% at pH 7.4 [94].Similarly,the UA-Metprodrug synthesized by our group exhibited a dose-dependent anti-proliferation effect on various cancer cells and displayed synergistic inhibition of cancer cell metastasis and invasion[101].
Cheng et al.developed an amphiphilic molecule (LMWHUA),in order to achieve chemo-and anti-angiogenic combined therapy.The degradation rate of LMWH-UA in plasma and tumor homogenate was 6.64% ± 0.87% and 39.35% ± 5.47%,respectively,which confirmed that the micelles existed excellent stability in plasma and controlled release manner at tumor tissues [92].Based on the above experiments,Xiong et al.constructed a novel prodrug of UA (sLMWH-UA) by linking with LMWH and UA using the Schiff base (-CH=N-)bond.The diameter size of sLMWH-UA was 223.1 ± 0.9 nm,and the critical aggregation concentration was 38.30 μg/ml,indicating its excellent stability in the systemic circulation.The addition of Schiff base bond promoted the micelles to hydrolyze and then release drugs under acidic conditions[93].Overall,these studies demonstrated that conjugating UA with other chemotherapeutic agents(summarized in Fig.5)may be a promising strategy for cancer therapy.
5.Nanosystem studies with ursolic acid in clinical trials
Clinical trials are essential for validating the efficacy and safety concerns of formulations.Extensive studies have confirmed the significant anticancer effect of UA nanosystems in pre-clinical studies,but the clinical studies of UA nanosystems are comparatively limited.In 2011,the UA liposomes (UA NLs) were firstly evaluated in human pharmacokinetic study by Tianjin Medicinal University Cancer Institute.The clinical data reported that UA NLs achieved a meanCmaxof 3404.6±748.8 ng/ml,and AUC0-∞value of 9918.4±1215.2 ng·h/ml [102].Wang et al.evaluated the toxicity and single-dose pharmacokinetics of UA NLs.It was shown that the maximum tolerated dose (MTD)of UA NLs was 98 mg/m2and the dose-limiting toxicity was hepatotoxicity and diarrhea[103].In another pharmacokinetic study,Zhu et al.evaluated the single-dose and multiple-dose pharmacokinetic study of UA NLs,in which twenty-four healthy volunteers received a single-dose of UA NLs (37,74,98 mg/m2) while eight cancer patients received a multipledose of UA NLs (every day 74 mg/m2of UA NLs for 14 d).The study revealed that the UA NLs exhibited a relatively linear pharmacokinetic behavior at dose levels between 37 and 98 mg/m2[104].In a recent clinical study of UA NLs,twenty-one patients with solid tumors were intravenously administered with doses of 56,74 and 98 mg/m2UA for 14 d consecutivly over a 21 d treatment cycle.Indeed,60% of subjects achieved satisfactory therapeutic effect after two treatment cycles [105].The results of these clinical phases I trials all confirmed that UA NLs could show high tolerance and low toxicity in healthy volunteers.Nevertheless,more extensive studies and investigations should be carried out in patients with different solid tumors.
6.Advantage and challenge of ursolic acid nanosystem
Up to now,UA nanosystems have made significant contribution to cancer therapy.We summarized thein vivoantitumor studies of UA nanoformulations to demonstrate that the applications of nanotechnology are able to improve antitumor efficacy of UA(Table 3).Additionally,we presented the unique advantages and challenges of UA nanosystems for cancer therapy below.
6.1.Enhancing the bioavailability of UA
Bioavailability is an essential index of pharmacokinetic property that mainly depending on drug solubility,stability,metabolism,and degradation [106].It has been widely accepted that the poor water solubility greatly limited the bioavailability of chemotherapeutic agents.Using TPGS1000 acted as a drug stabilizer could effectively improve the solubility of drugs and subsequently enhance their oral bioavailability [88].For nano-delivery system,the rigidity of nanoparticle displays a significant influence on its stability,drug release and accumulation time.Specifically,modifying nanoparticle with water-soluble polyethylene glycol (PEG)can significantly ameliorate its rigidity [45].In addition,the physicochemical properties of the nanoparticles including morphology,size,surface charge and surface hydrophilicity all have great effects on their circulating half-life and metabolism[107,108].A short description of the nano-delivery system of UA that significantly improved its pharmacokinetics and bioavailability is provided in Table 4.
6.2.Targeted drug delivery
Targeted drug delivery systems are able to selectively deliver drug into the specific sites,thereby enhancing its therapeutic efficacy and reducing toxic effect [109,110].Targeted drug delivery system can be divided into active and passive targeting systems.The nanoparticles with a size of 10-200 nm are able to effectively permeate into tumor tissues with the help of passive targeting [111].Our group evaluated the passive tumor-targetability of nanoparticles by NIR fluorescence imaging technology.After 24 h,30%fluorescence intensity of ICG in nanoparticles at the tumor sites was observed,while there was no fluorescence intensity tumor accumulation of free ICG group(Fig.6)[89].However,the size,zeta potential,solubility or dispersion of nanoparticles may also exhibit an impact on its targetability [112].Additionally,the main factor of passive tumor-targetability of nanoparticles is the heterogeneity of tumor and its stroma,such as a hypoxic gradient [113].Meanwhile,the increased interstitial fluid pressure is found to hamper the penetration of nanoparticles into neoplastic tissues [114].Most of the active targeting systems were prepared by surface modification with targeting-ligands (such as protein,sugar,amino acids,enzymes).Modified by multifarious ligands,enhancement of cellular uptake and interaction with tumor surface receptors of nanoparticles have been documented [108].To obtain effective targeting,the nanoparticles should be stable and also can avoid rapid recognition by RES.Accordingly,it is crucial to optimize the density of targeting-ligands on the surface of nanosystems.In conclusion,the nanosystems with effective passive or active tumor targetability have achieved substantial advantage over the free molecules[115].
6.3.Controlled drug release
Controlled-release drug delivery systems can release drugs with a certain speed at a predetermined time [116,117].Delivery systems with different drug release manners such as extended-release,sustained-release,delayedrelease,and targeted-release,are collectively referred to as controlled-release delivery systems[118].The plasma drug concentration of nanoparticles will be very low initially and then progressively increased,which led to a broad and flat AUC [119].By contrast,free drugs will commonly achieve their plasmaCmaxduring the intravenous infusion period and followed by a rapid drug concentration decrease,and therefore theirAUCare likely to be a peak shape with a tail [119].Accordingly,nanoparticles are of more potential to maintain drug concentration within the therapeutic window than free drugs [120].To precisely control the release of drug,stimuli-responsive nanomaterials have been developed and utilized.They can release drugs in response to specific triggers,including exogenous stimuli (variations in temperature,magnetic field,ultrasound intensity,light or electric pulses)and endogenous stimuli(variations in enzyme concentration or redox gradients) [121,122].Nevertheless,these newer delivery systems are still developed at the proofof-concept level.Taken together,the development of new nanoparticles-based strategy is critical for enhancing drug delivery into tumors.
6.4.Challenge and prospect of UA nanosystem
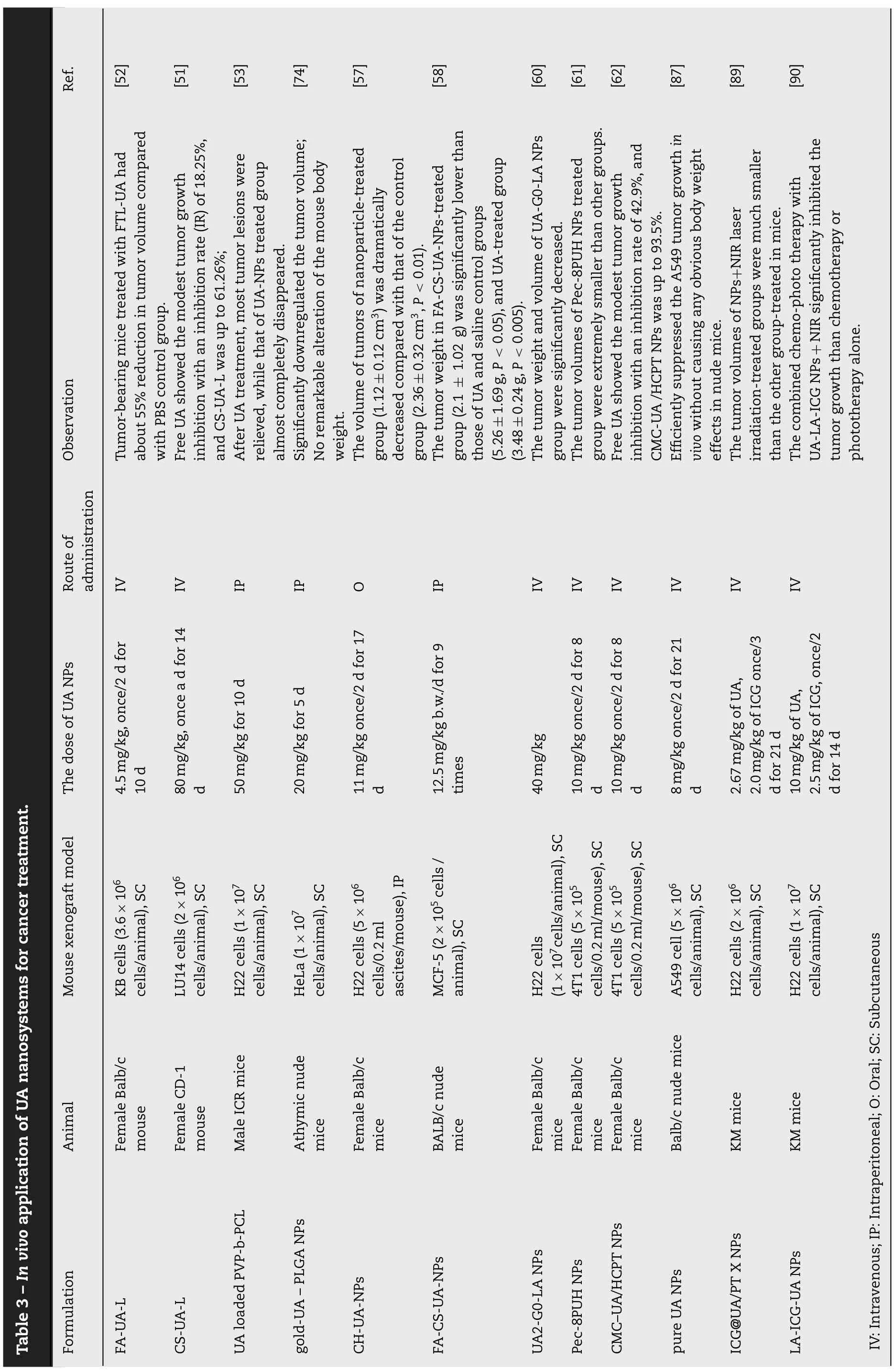
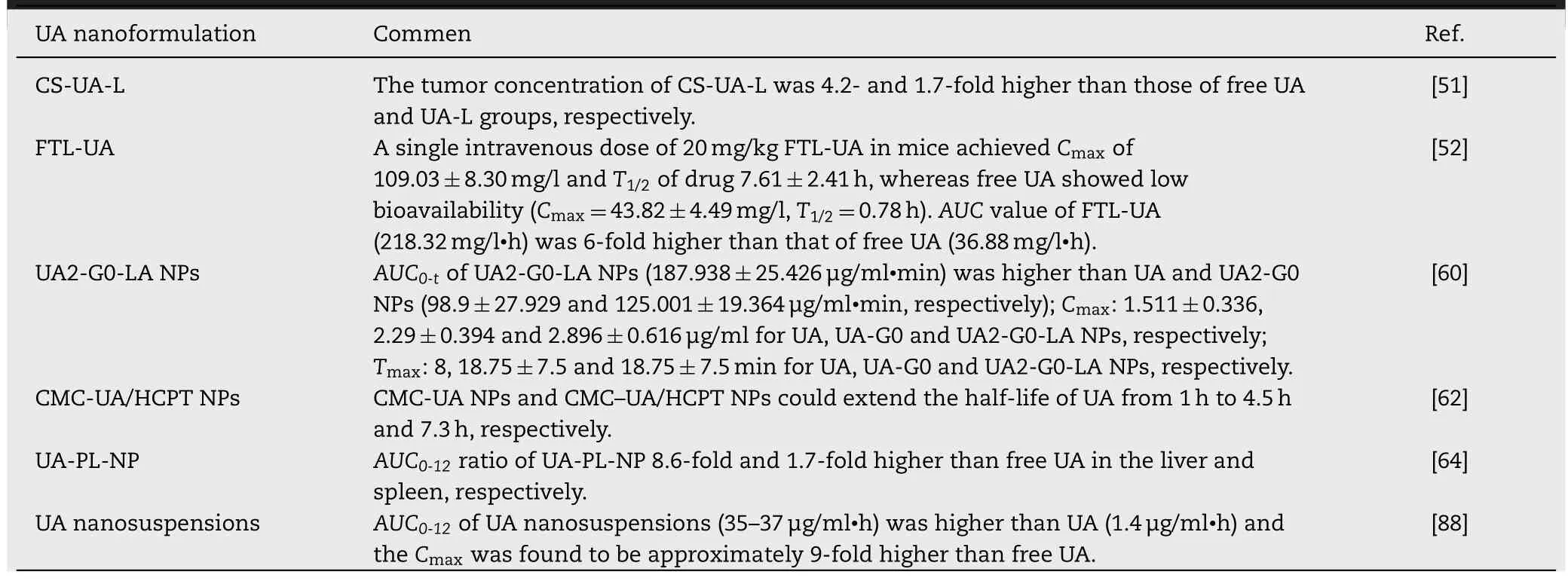
Table 4-Pharmacokinetics properties and bioavailability of various UA nanosystems.

Fig.6-In vivo targeting efficiency of ICG@UA/PTX NPs compared with that of free ICG.(A)Schematic of ICG-UA/PTX NPs for cancer imaging and chemo-phototherapy.(B)NIR fluorescence images of free ICG and NPs injected into tumor-bearing mice were recorded at 0.5,1,3,6,12 and 24 h,indicating that the NPs can efficaciously target tumor site.Reproduce with permission[89].Copyright 2017,American Chemical Society.
With significant advances achieved in nanotechnologies,constructing drug-loaded nanosystems is considered to be one of the most promising strategies for cancer therapy.The advantages and disadvantages of different UA nanosystems are sequentially discussed in Table 5.Despite numerous progress has been made in recent years,there are still some challenges in the clinical applications of UA nanosystems.(i)Some nanocarriers of UA possibly aggregate or agglomerate with other biomolecules or biological fluidsin vivo[123].(ii)The stability and storage aspects of UA nanosystems are still needed to be further improved.(iii)Complex preparation methods of UA nanosystems will lead to some problems such as potentially toxic to healthy tissues,indistinct metabolism,as well as biodegradation problems.(iv) Many physiological barriers will certainly limit the deep penetration of UA nanosystems into the tumor tissues and subsequently impact the therapeutic outcome.These challenges must be addressed in order to promote UA nanosystems to become the nextgeneration nanomedicine.
The following several aspects should be considered in promoting UA nanomedicines from preclinical levels to the clinical settings:(i)The implementation of US Food and DrugAdministration (FDA)-approved materials as nanocarriers of UA can consequently expedite its clinical transformation.(ii) To address the issues of short blood circulation halflife and nonspecific protein adsorption,the surface of UA nanoparticles can be modified by various materials,such as PEG or polysorbate 80,dysopsonins proteins (e.g.,clusterin and albumin),and self-markers (e.g.,CD47 peptides) and membrane of erythrocytes,leukocytes,cancer cells or thrombocytes [36,119].(iii) Additionally,the physicochemical characterization of UA nanoparticles should be carried out under similar clinical conditions.(iv) Moreover,the rational design of nanosystems is important to develop UA nanosystems with high loading capacity,which will further affect its dosage and therapeutic efficacy in clinical treatment[124].Therefore,the development of nanosystems with active targeting-ligands or stimuli-responsive properties is valuable for improving the targetability and therapeutic efficacy of conventional UA nanosystems.More comprehensive preclinical and clinical trials should be conducted to further validate its therapeutic efficacy.It is also noteworthy that the combination of UA with other therapeutic agents could result in synergistic effects and offer a better therapeutic index.On the other hand,the novel composite nanosystems with two or more anticancer agents will help to reduce adverse effects by reducing the single dosage of chemotherapeutic agent.In addition to these considerations,manufacturing UA nanosystems at an industrial level should consider the cost-benefit and follow GLP and GMP (good laboratory and manufacturing practice).
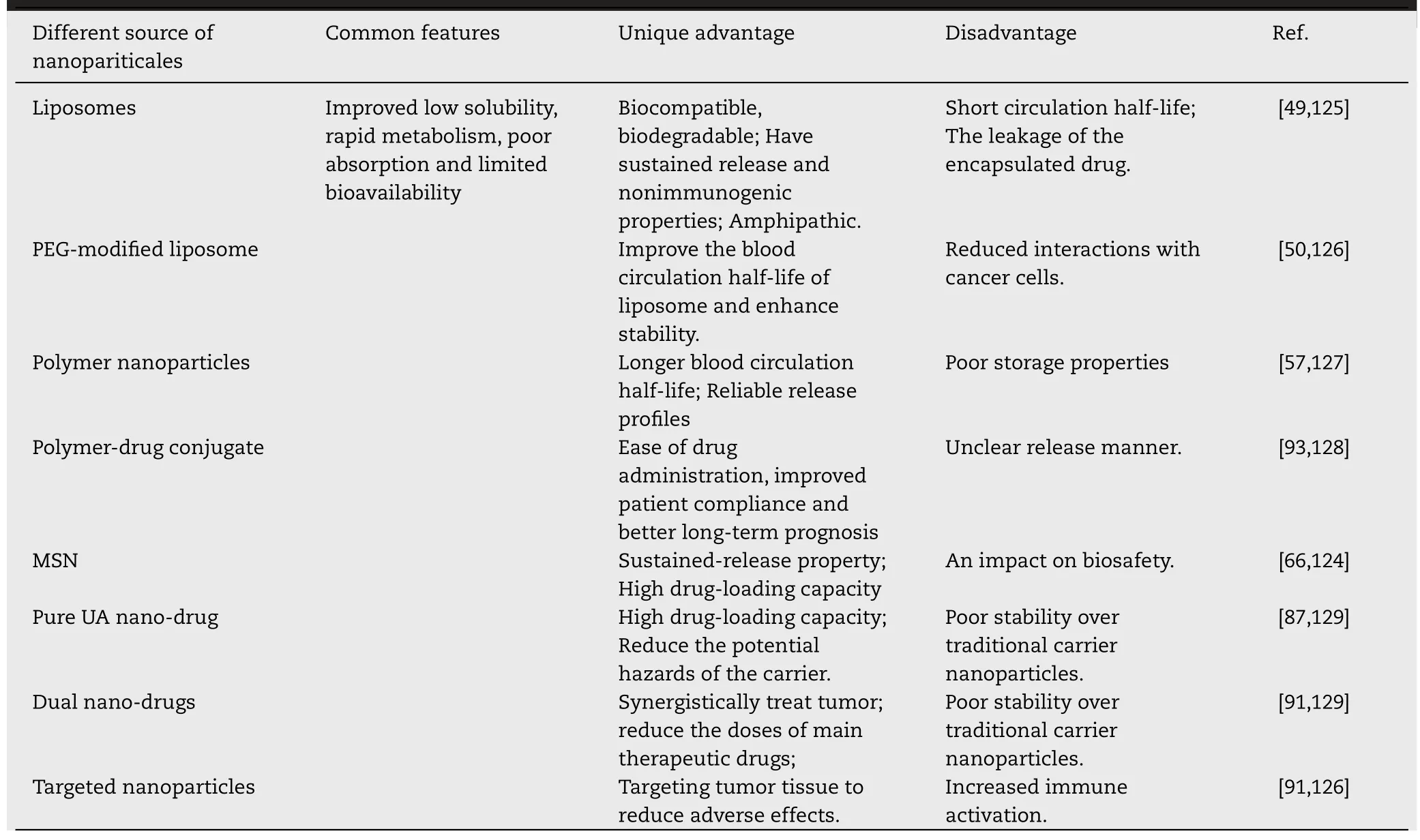
Table 5- Summary of the differences,advantages,and disadvantages of different type nanosystems of UA in cancer therapy.
7.Conclusion
In summary,this review mainly describes the recent advance of UA nanosystems for cancer treatment,with an emphasis on different nanocarrier delivery systems and carrierfree delivery systems.Large amounts ofin vitroandin vivoexperiments have provided evidence that these UA nanosystems displayed improved efficacy,targetability and reduced systemic toxicity.At present,most of the UA nanosystems have been developed and optimized at the laboratory scale and comprehensive preclinical tests to confirm their potential effect and safety.The phase I clinical trials confirm that UA liposomes are systemically safe,and can effectively improve the bioavailability of UA.Additionally,the potential toxicity of some UA nanosystems in humans remain unclear,hence future studies should focus on complementary toxicity assays of UA nanosystems.It is worth noting that the combination of UA nanosystems with phototherapy or other chemotherapeutic agents resulted in a promising therapeutic effect in cancer therapy.Therefore,a composite system of UA with other anticancer agents can be a promising approach to enhance anticancer efficacy and reduce systemic toxicity.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This project was supported by the National Natural Science Foundation of China(81972832,81472767),Scientific Research Training Program for Undergraduate of Fuzhou University(25055,25068).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.ajps.2020.03.001.
REFERENCES
[1]Roy PS,Saikia BJ.Cancer and cure:a critical analysis.Indian J Cancer 2016;53(3):441-2.
[2]Bray F,Ferlay J,Soerjomataram I,Siegel RL,Torre LA,Jemal A.Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries.CA Cancer J Clin 2018;68(6):394-424.
[3]Miller KD,Siegel RL,Lin CC,Mariotto AB,Kramer JL,Rowland JH,et al.Cancer treatment and survivorship statistics,2016.CA Cancer J Clin 2016;66(4):271-89.
[4]Miller KD,Nogueira L,Mariotto AB,Rowland JH,Yabroff KR,Alfano CM,et al.Cancer treatment and survivorship statistics,2019.CA Cancer J Clin 2019;69(5):363-85.
[5]Reyes-Habito CM,Roh EK.Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer:part I.Conventional chemotherapeutic drugs.J Am Acad Dermatol 2014;71(2)203 e1-12.
[6]Lee YT,Tan YJ,Oon CE.Molecular targeted therapy:treating cancer with specificity.Eur J Pharmacol 2018;834:188-96.
[7]Emens LA.Breast cancer immunotherapy:facts and hopes.Clin Cancer Res 2018;24(3):511-20.
[8]Fairchild A,Tirumani SH,Rosenthal MH,Howard SA,Krajewski KM,Nishino M,et al.Hormonal therapy in oncology:a primer for the radiologist.AJR Am J Roentgenol 2015;204(6):W620-30.
[9]Li YJ,Lei YH,Yao N,Wang CR,Hu N,Ye WC,et al.Autophagy and multidrug resistance in cancer.Chin J Cancer 2017;36(1):52.
[10]Hu LY,Mi WL,Wu GC,Wang YQ,Mao-Ying QL.Prevention and treatment for chemotherapy-induced peripheral neuropathy:therapies based on CIPN mechanisms.Curr Neuropharmacol 2019;17(2):184-96.
[11]Zong S,Wang X,Yang Y,Wu W,Li H,Ma Y,et al.The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice.Eur J Pharm Biopharm 2015;93:127-35.
[12]Markov AV,Zenkova MA,Logashenko EB.Modulation of tumour-related signaling pathways by natural pentacyclic triterpenoids and their semisynthetic derivatives.Curr Med Chem 2017;24(13):1277-320.
[13]Zhang J,Xu HY,Wu YJ,Zhang X,Zhang LQ,Li YM.Neutrophil elastase inhibitory effects of pentacyclic triterpenoids from eriobotrya japonica(loquat leaves).J Ethnopharmacol 2019;242:111713.
[14]Alqahtani A,Hamid K,Kam A,Wong KH,Abdelhak Z,Razmovski-Naumovski V,et al.The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications.Curr Med Chem 2013;20(7):908-31.
[15]Janicsák G,Veres K,Kakasy AZ,Máthé I.Study of the oleanolic and ursolic acid contents of some species of the lamiaceae.Biochem System Ecol 2006;34(5):392-6.
[16]Silva MG,Vieira IG,Mendes FN,Albuquerque IL,dos Santos RN,Silva FO,et al.Variation of ursolic acid content in eight Ocimum species from northeastern Brazil.Molecules 2008;13(10):2482-7.
[17]Kashyap D,Tuli HS,Sharma AK.Ursolic acid(UA):a metabolite with promising therapeutic potential.Life Sci 2016;146:201-13.
[18]Jang SM,Yee ST,Choi J,Choi MS,Do GM,Jeon SM,et al.Ursolic acid enhances the cellular immune system and pancreatic beta-cell function in streptozotocin-induced diabetic mice fed a high-fat diet.Int Immunopharmacol 2009;9(1):113-9.
[19]Kim MH,Kim JN,Han SN,Kim HK.Ursolic acid isolated from guava leaves inhibits inflammatory mediators and reactive oxygen species in LPS-stimulated macrophages.Immunopharmacol Immunotoxicol 2015;37(3):228-35.
[20]Wojciak-Kosior M,Paduch R,Matysik-Wozniak A,Niedziela P,Donica H.The effect of ursolic and oleanolic acids on human skin fibroblast cells.Folia Histochem Cytobiol 2011;49(4):664-9.
[21]Broniatowski M,Flasinski M,Hac-Wydro K.Antagonistic effects of alpha-tocopherol and ursolic acid on model bacterial membranes.Biochim Biophys Acta 2015;1848(10 Pt A):2154-62.
[22]Yuliang W,Zejian W,Hanlin S,Ming Y,Kexuan T.The hypolipidemic effect of artesunate and ursolic acid in rats.Pak J Pharm Sci 2015;28(3):871-4.
[23]Shao JW,Dai YC,Xue JP,Wang JC,Lin FP,Guo YH.In vitroandin vivoanticancer activity evaluation of ursolic acid derivatives.Eur J Med Chem 2011;46(7):2652-61.
[24]Yin R,Li T,Tian JX,Xi P,Liu RH.Ursolic acid,a potential anticancer compound for breast cancer therapy.Crit Rev Food Sci Nutr 2018;58(4):568-74.
[25]Wang J,Jiang Z,Xiang L,Li Y,Ou M,Yang X,et al.Synergism of ursolic acid derivative US597 with 2-deoxy-D-glucose to preferentially induce tumor cell death by dual-targeting of apoptosis and glycolysis.Sci Rep 2014;4:5006.
[26]Yang X,Li Y,Jiang W,Ou M,Chen Y,Xu Y,et al.Synthesis and biological evaluation of novel ursolic acid derivatives as potential anticancer prodrugs.Chem Biol Drug Des 2015;86(6):1397-404.
[27]Chen Q,Luo S,Zhang Y,Chen Z.Development of a liquid chromatography-mass spectrometry method for the determination of ursolic acid in rat plasma and tissue:application to the pharmacokinetic and tissue distribution study.Anal Bioanal Chem 2011;399(8):2877-84.
[28]Liao Q,Yang W,Jia Y,Chen X,Gao Q,Bi K.LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional chinese medicinal preparation Lu-Ying extract.Yakugaku Zasshi 2005;125(6):509-15.
[29]Zhang H,Li X,Ding J,Xu H,Dai X,Hou Z,et al.Delivery of ursolic acid(UA)in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2(COX-2).Int J Pharm 2013;441(1-2):261-8.
[30]Yu MK,Park J,Jon S.Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy.Theranostics 2012;2(1):3-44.
[31]Rajeshkumar S,Bharath LV.Mechanism of plant-mediated synthesis of silver nanoparticles-A review on biomolecules involved,characterisation and antibacterial activity.Chem Biol Interact 2017;273:219-27.
[32]Kuai Q,Wang Y,Gao F,Qi Y,Wang R,Wang Y,et al.Peptide self-assembly nanoparticles loaded with panobinostat to activate latent human immunodeficiency virus.J Biomed Nanotechnol 2019;15(5):979-92.
[33]Jadhav NR,Nadaf SJ,Lohar DA,Ghagare PS,Powar TA.Phytochemicals formulated as nanoparticles:inventions,recent patents and future prospects.Recent Pat Drug Deliv Formul.2017;11(3):173-86.
[34]Tiwari SB,Amiji MM.A review of nanocarrier-based CNS delivery systems.Curr Drug Deliv 2006;3(2):219-32.
[35]Chari RV.Targeted delivery of chemotherapeutics:tumor-activated prodrug therapy.Adv Drug Deliv Rev 1998;31(1-2):89-104.
[36]Norouzi M,Amerian M,Amerian M,Atyabi F.Clinical applications of nanomedicine in cancer therapy.Drug Discov Today 2020;25(1):107-25.
[37]Valdes K,Morales J,Rodriguez L,Gunther G.Potential use of nanocarriers with pentacyclic triterpenes in cancer treatments.Nanomedicine Lond 2016;11(23):3139-56.
[38]Luo J,Hu YL,Wang H.Ursolic acid inhibits breast cancer growth by inhibiting proliferation,inducing autophagy and apoptosis,and suppressing inflammatory responses via the PI3K/AKT and NF-kappaB signaling pathwaysin vitro.Exp Ther Med 2017;14(4):3623-31.
[39]Liu L,Zhang J,Li M,Zhang X,Zhang J,Li Z,et al.Inhibition of HepG2 cell proliferation by ursolic acid and polysaccharides via the downregulation of cyclooxygenase-2.Mol Med Rep 2014;9(6):2505-11.
[40]Guo JL,Han T,Bao L,Li XM,Ma JQ,Tang LP.Ursolic acid promotes the apoptosis of cervical cancer cells by regulating endoplasmic reticulum stress.J Obstet Gynaecol Res 2019;45(4):877-81.
[41]Liu K,Guo L,Miao L,Bao W,Yang J,Li X,et al.Ursolic acid inhibits epithelial-mesenchymal transition by suppressing the expression of astrocyte-elevated gene-1 in human nonsmall cell lung cancer A549 cells.Anticancer Drugs 2013;24(5):494-503.
[42]Harmand PO,Duval R,Delage C,Simon A.Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells.Int J Cancer 2005;114(1):1-11.
[43]Weng H,Tan ZJ,Hu YP,Shu YJ,Bao RF,Jiang L,et al.Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells.Cancer Cell Int 2014;14(1):96.
[44]Gai WT,Yu DP,Wang XS,Wang PT.Anti-cancer effect of ursolic acid activates apoptosis through ROCK/PTEN mediated mitochondrial translocation of cofilin-1 in prostate cancer.Oncol Lett 2016;12(4):2880-5.
[45]Dougherty U,Sehdev A,Cerda S,Mustafi R,Little N,Yuan W,et al.Epidermal growth factor receptor controls flat dysplastic aberrant crypt foci development and colon cancer progression in the rat azoxymethane model.Clin Cancer Res 2008;14(8):2253-62.
[46]Liu P,Du R,Yu X.Ursolic acid exhibits potent anticancer effects in human metastatic melanoma cancer cells(SK-MEL-24)via apoptosis induction,inhibition of cell migration and invasion,cell cycle arrest,and inhibition of mitogen-activated protein kinase(MAPK)/ERK signaling pathway.Med Sci Monit 2019;25:1283-90.
[47]Meng Y,Lin ZM,Ge N,Zhang DL,Huang J,Kong F.Ursolic acid induces apoptosis of prostate cancer cells via the PI3K/Akt/mTOR pathway.Am J Chin Med 2015;43(7):1471-86.
[48]Kim K,Shin EA,Jung JH,Park JE,Kim DS,Shim BS,et al.Ursolic acid induces apoptosis in colorectal cancer cells partially via upregulation of microrna-4500 and inhibition of JAK2/STAT3 phosphorylation.Int J Mol Sci 2018;20(1):E114.
[49]de Araujo Lopes SC,Vinicius Melo Novais M,Salviano Teixeira C,Honorato-Sampaio K,Tadeu Pereira M,Ferreira LA,et al.Preparation,physicochemical characterization,and cell viability evaluation of long-circulating and pH-sensitive liposomes containing ursolic acid.Biomed Res Int 2013;2013:467147.
[50]Zhao T,Liu Y,Gao Z,Gao D,Li N,Bian Y,et al.Self-assembly and cytotoxicity study of PEG-modified ursolic acid liposomes.Mater Sci Eng C Mater Biol Appl 2015;53:196-203.
[51]Wang M,Zhao T,Liu Y,Wang Q,Xing S,Li L,et al.Ursolic acid liposomes with chitosan modification:promising antitumor drug delivery and efficacy.Mater Sci Eng C Mater Biol Appl 2017;71:1231-40.
[52]Yang G,Yang T,Zhang W,Lu M,Ma X,Xiang G.In vitroandin vivoantitumor effects of folate-targeted ursolic acid stealth liposome.J Agric Food Chem 2014;62(10):2207-15.
[53]Zhang H,Zheng D,Ding J,Xu H,Li X,Sun W.Efficient delivery of ursolic acid by poly(N-vinylpyrrolidone)-block-poly(epsilon-caprolactone)nanoparticles for inhibiting the growth of hepatocellular carcinomain vitroandin vivo.Int J Nanomed 2015;10:1909-20.
[54]Antonio E,Antunes ODRJ,de Araujo IS,Khalil NM,Mainardes RM.Poly(lactic acid)nanoparticles loaded with ursolic acid:characterization andin vitroevaluation of radical scavenging activity and cytotoxicity.Mater Sci Eng C Mater Biol Appl 2017;71:156-66.
[55]Baishya R,Nayak DK,Kumar D,Sinha S,Gupta A,Ganguly S,et al.Ursolic acid loaded PLGA nanoparticles:in vitroandin vivoevaluation to explore tumor targeting ability on B16F10 melanoma cell lines.Pharm Res 2016;33(11):2691-703.
[56]Oprean C,Zambori C,Borcan F,Soica C,Zupko I,Minorics R,et al.Anti-proliferative and antibacterialin vitroevaluation of the polyurethane nanostructures incorporating pentacyclic triterpenes.Pharm Biol 2016;54(11):2714-22.
[57]Jin H,Pi J,Yang F,Wu C,Cheng X,Bai H,et al.Ursolic acid-loaded chitosan nanoparticles induce potent anti-angiogenesis in tumor.Appl Microbiol Biotechnol 2016;100(15):6643-52.
[58]Jin H,Pi J,Yang F,Jiang J,Wang X,Bai H,et al.Folate-Chitosan nanoparticles loaded with ursolic acid confer anti-breast cancer activitiesin vitroandin vivo.Sci Rep 2016;6:30782.
[59]Gao Y,Li Z,Xie X,Wang C,You J,Mo F,et al.Dendrimeric anticancer prodrugs for targeted delivery of ursolic acid to folate receptor-expressing cancer cells:synthesis and biological evaluation.Eur J Pharm Sci 2015;70:55-63.
[60]Shen Z,Li B,Liu Y,Zheng G,Guo Y,Zhao R,et al.A self-assembly nanodrug delivery system based on amphiphilic low generations of PAMAM dendrimers-ursolic acid conjugate modified by lactobionic acid for HCC targeting therapy.Nanomedicine 2018;14(2):227-36.
[61]Liu Y,Liu K,Li X,Xiao S,Zheng D,Zhu P,et al.A novel self-assembled nanoparticle platform based on pectin-eight-arm polyethylene glycol-drug conjugates for co-delivery of anticancer drugs.Mater Sci Eng C Mater Biol Appl 2018;86:28-41.
[62]Liu YX,Liu KF,Li CX,Wang LY,Liu J,He J,et al.Self-assembled nanoparticles based on a carboxymethylcellulose-ursolic acid conjugate for anticancer combination therapy.RSC Adv 2017;7(58):36256-68.
[63]Nahak P,Karmakar G,Chettri P,Roy B,Guha P,Besra SE,et al.Influence of lipid core material on physicochemical characteristics of an ursolic acid-loaded nanostructured lipid carrier:an attempt to enhance anticancer activity.Langmuir 2016;32(38):9816-25.
[64]Zhou XJ,Hu XM,Yi YM,Wan J.Preparation and body distribution of freeze-dried powder of ursolic acid phospholipid nanoparticles.Drug Dev Ind Pharm 2009;35(3):305-10.
[65]Biswas S.,Mukherjee P.K.,Harwansh R.K.,Bannerjee S.,Bhattacharjee P.Enhanced bioavailability and hepatoprotectivity of optimized ursolic acid-phospholipid complex.Drug Dev Ind Pharm 2019:1-13.
[66]Li T,Chen X,Liu Y,Fan L,Lin L,Xu Y,et al.pH-Sensitive mesoporous silica nanoparticles anticancer prodrugs for sustained release of ursolic acid and the enhanced anti-cancer efficacy for hepatocellular carcinoma cancer.Eur J Pharm Sci 2017;96:456-63.
[67]Jiang K,Chi T,Li T,Zheng G,Fan L,Liu Y,et al.A smart pH-responsive nano-carrier as a drug delivery system for the targeted delivery of ursolic acid:suppresses cancer growth and metastasis by modulating P53/MMP-9/PTEN/CD44 mediated multiple signaling pathways.Nanoscale 2017;9(27):9428-39.
[68]Zhao R,Li T,Zheng G,Jiang K,Fan L,Shao J.Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex.Biomaterials 2017;143:1-16.
[69]Zhong Y,Wang J,Wang Y,Wu B.Preparation and evaluation of liposome-encapsulated codrug LMX.Int J Pharm 2012;438(1-2):240-8.
[70]Zamboni WC.Liposomal,nanoparticle,and conjugated formulations of anticancer agents.Clin Cancer Res 2005;11(23):8230-4.
[71]Sapra P,Tyagi P,Allen TM.Ligand-targeted liposomes for cancer treatment.Curr Drug Deliv 2005;2(4):369-81.
[72]Malhotra M,Tomaro-Duchesneau C,Prakash S.Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases.Biomaterials 2013;34(4):1270-80.
[73]Bhavna MdS,Ali M,Baboota S,Sahni JK,Bhatnagar A,et al.Preparation,characterization,in vivobiodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting.Drug Dev Ind Pharm 2014;40(2):278-87.
[74]Wang S,Meng X,Dong Y.Ursolic acid nanoparticles inhibit cervical cancer growthin vitroandin vivovia apoptosis induction.Int J Oncol 2017;50(4):1330-40.
[75]Ekladious I,Colson YL,Grinstaff MW.Polymer-drug conjugate therapeutics:advances,insights and prospects.Nat Rev Drug Discov 2019;18(4):273-94.
[76]Tapeinos C,Battaglini M,Ciofani G.Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases.J Control Release 2017;264:306-32.
[77]Garces A,Amaral MH,Sousa Lobo JM,Silva AC.Formulations based on solid lipid nanoparticles(SLN)and nanostructured lipid carriers(NLC)for cutaneous use:a review.Eur J Pharm Sci 2018;112:159-67.
[78]Beloqui A,Solinis MA,Rodriguez-Gascon A,Almeida AJ,Preat V.Nanostructured lipid carriers:promising drug delivery systems for future clinics.Nanomedicine 2016;12(1):143-61.
[79]Shang F,Sun J,Wu S,Liu H,Guan J,Kan Q.Direct synthesis of acid-base bifunctionalized hexagonal mesoporous silica and its catalytic activity in cascade reactions.J Colloid Interface Sci 2011;355(1):190-7.
[80]Liu T,Li L,Teng X,Huang X,Liu H,Chen D,et al.Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice.Biomaterials 2011;32(6):1657-68.
[81]He Q,Gao Y,Zhang L,Zhang Z,Gao F,Ji X,et al.A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance.Biomaterials 2011;32(30):7711-20.
[82]Li L,Tang F,Liu H,Liu T,Hao N,Chen D,et al.In vivodelivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy.ACS Nano 2010;4(11):6874-82.
[83]Lu J,Liong M,Zink JI,Tamanoi F.Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs.Small 2007;3(8):1341-6.
[84]Liu G,Gao J,Ai H,Chen X.Applications and potential toxicity of magnetic iron oxide nanoparticles.Small 2013;9(9-10):1533-45.
[85]Jia L,Zhao Y,Liang XJ.Fast evolving nanotechnology and relevant programs and entities in China.Nano Today 2011;6(1):6-11.
[86]Song J,Wang Y,Song Y,Chan H,Bi C,Yang X,et al.Development and characterisation of ursolic acid nanocrystals without stabiliser having improved dissolution rate andin vitroanticancer activity.AAPS PharmSciTech 2014;15(1):11-19.
[87]Fan L,Zhang B,Xu A,Shen Z,Guo Y,Zhao R,et al.Carrier-free,pure nanodrug formed by the self-assembly of an anticancer drug for cancer immune therapy.Mol Pharm 2018;15(6):2466-78.
[88]Ge ZQ,Du XY,Huang XN,Qiao B.Enhanced oral bioavailability of ursolic acid nanoparticles via antisolvent precipitation with TPGS1000 as a stabilizer.J Drug Deliv Sci Technol 2015;29:210-17.
[89]Guo Y,Jiang K,Shen Z,Zheng G,Fan L,Zhao R,et al.A small molecule nanodrug by self-assembly of dual anticancer drugs and photosensitizer for synergistic near-Infrared cancer theranostics.ACS Appl Mater Interfaces 2017;9(50):43508-19.
[90]Zhao R,Zheng G,Fan L,Shen Z,Jiang K,Guo Y,et al.Carrier-free nanodrug by co-assembly of chemotherapeutic agent and photosensitizer for cancer imaging and chemo-photo combination therapy.Acta Biomater 2018;70:197-210.
[91]Jiang K,Han L,Guo Y,Zheng G,Fan L,Shen Z,et al.A carrier-free dual-drug nanodelivery system functionalized with aptamer specific targeting HER2-overexpressing cancer cells.J Mater Chem B 2017;5(46):9121-9.
[92]Cheng W,Dahmani FZ,Zhang J,Xiong H,Wu Y,Yin L,et al.Anti-angiogenic activity and antitumor efficacy of amphiphilic twin drug from ursolic acid and low molecular weight heparin.Nanotechnology 2017;28(7):075102.
[93]Xiong H,Wu Y,Jiang Z,Zhou J,Yang M,Yao J.pH-activatable polymeric nanodrugs enhanced tumor chemo/antiangiogenic combination therapy through improving targeting drug release.J Colloid Interface Sci 2019;536:135-48.
[94]Li C,Lin J,Wu P,Zhao R,Zou J,Zhou M,et al.Small molecule nanodrug assembled of dual-anticancer drug conjugate for synergetic cancer metastasis therapy.Bioconjug Chem 2018;29(10):3495-502.
[95]Müller RH,Shegokar R,Gohla S,Keck CM.Nanocrystals:production,cellular drug delivery,current and future products.Intracellular delivery.Springer;2011.p.411-32.
[96]Junghanns J-UA,Müller RH.Nanocrystal technology,drug delivery and clinical applications.Int J Nanomed 2008;3(3):295.
[97]Pi J,Liu Z,Wang H,Gu X,Wang S,Zhang B,et al.Ursolic acid nanocrystals for dissolution rate and bioavailability enhancement:influence of different particle size.Curr Drug Deliv 2016;13(8):1358-66.
[98]Wang Y,Song J,Chow SF,Chow AH,Zheng Y.Particle size tailoring of ursolic acid nanosuspensions for improved anticancer activity by controlled antisolvent precipitation.Int J Pharm 2015;494(1):479-89.
[99]Qiu L,Zhao X,Zu Y,Zhang Y,Liu Y,Wu W,et al.Ursolic acid nanoparticles for oral delivery prepared by emulsion solvent evaporation method:characterization,in vitroevaluation of radical scavenging activity and bioavailability.Artif Cells Nanomed Biotechnol 2019;47(1):610-21.
[100]Tang Q,Liu Y,Li T,Yang X,Zheng G,Chen H,et al.A novel co-drug of aspirin and ursolic acid interrupts adhesion,invasion and migration of cancer cells to vascular endothelium via regulating EMT and EGFR-mediated signaling pathways:multiple targets for cancer metastasis prevention and treatment.Oncotarget 2016;7(45):73114-29.
[101]Zheng G,Shen Z,Xu A,Jiang K,Wu P,Yang X,et al.Synergistic chemopreventive and therapeutic effects of co-drug UA-Met:implication in tumor metastasis.J Agric Food Chem 2017;65(50):10973-83.
[102]Xia Y,Wei G,Si D,Liu C.Quantitation of ursolic acid in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its pharmacokinetic study.J Chromatogr B Analyt Technol Biomed Life Sci 2011;879(2):219-24.
[103]Wang XH,Zhou SY,Qian ZZ,Zhang HL,Qiu LH,Song Z,et al.Evaluation of toxicity and single-dose pharmacokinetics of intravenous ursolic acid liposomes in healthy adult volunteers and patients with advanced solid tumors.Expert Opin Drug Metab Toxicol 2013;9(2):117-25.
[104]Zhu Z,Qian Z,Yan Z,Zhao C,Wang H,Ying G.A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors.Int J Nanomed 2013;8:129-36.
[105]Qian Z,Wang X,Song Z,Zhang H,Zhou S,Zhao J,et al.A phase I trial to evaluate the multiple-dose safety and antitumor activity of ursolic acid liposomes in subjects with advanced solid tumors.Biomed Res Int 2015;2015:809714.
[106]Yallapu MM,Jaggi M,Chauhan SC.Curcumin nanomedicine:a road to cancer therapeutics.Curr Pharm Des 2013;19(11):1994-2010.
[107]Kalyane D,Raval N,Maheshwari R,Tambe V,Kalia K,Tekade RK.Employment of enhanced permeability and retention effect(EPR):nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer.Mater Sci Eng C Mater Biol Appl 2019;98:1252-76.
[108]Farokhzad OC,Langer R.Impact of nanotechnology on drug delivery.ACS Nano 2009;3(1):16-20.
[109]Guan J,Jiang Z,Wang M,Liu Y,Liu J,Yang Y,et al.Short peptide-mediated brain-targeted drug delivery with enhanced immunocompatibility.Mol Pharm 2019;16(2):907-13.
[110]Srinivasarao M,Galliford CV,Low PS.Principles in the design of ligand-targeted cancer therapeutics and imaging agents.Nat Rev Drug Discov 2015;14(3):203-19.
[111]Torchilin V.Tumor delivery of macromolecular drugs based on the EPR effect.Adv Drug Deliv Rev 2011;63(3):131-5.
[112]Yingchoncharoen P,Kalinowski DS,Richardson DR.Lipid-based drug delivery systems in cancer therapy:what is available and what is yet to come.Pharmacol Rev 2016;68(3):701-87.
[113]Wicki A,Witzigmann D,Balasubramanian V,Huwyler J.Nanomedicine in cancer therapy:challenges,opportunities,and clinical applications.J Control Release 2015;200:138-57.
[114]Jain RK,Stylianopoulos T.Delivering nanomedicine to solid tumors.Nat Rev Clin Oncol 2010;7(11):653-64.
[115]Dissanayake S,Denny WA,Gamage S,Sarojini V.Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides.J Control Release 2017;250:62-76.
[116]Zhang Y,Wu M,Dai W,Li Y,Wang X,Tan D,et al.Gold nanoclusters for controlled insulin release and glucose regulation in diabetes.Nanoscale 2019;11(13):6471-9.
[117]Yasser M,Teaima M,El-Nabarawi M,El-Monem RA.Cubosomal based oral tablet for controlled drug delivery of telmisartan:formulation,in vitroevaluation andin vivocomparative pharmacokinetic study in rabbits.Drug Dev Ind Pharm 2019:1-14.
[118]Bhusal P,Harrison J,Sharma M,Jones DS,Hill AG,Svirskis D.Controlled release drug delivery systems to improve post-operative pharmacotherapy.Drug Deliv Transl Res 2016;6(5):441-51.
[119]Shi J,Kantoff PW,Wooster R,Farokhzad OC.Cancer nanomedicine:progress,challenges and opportunities.Nat Rev Cancer 2017;17(1):20-37.
[120]Ediriwickrema A,Saltzman WM.Nanotherapy for cancer:targeting and multifunctionality in the future of cancer therapies.ACS Biomater Sci Eng 2015;1(2):64-78.
[121]Mura S,Nicolas J,Couvreur P.Stimuli-responsive nanocarriers for drug delivery.Nat Mater 2013;12(11):991-1003.
[122]Lu Y,Sun W,Gu Z.Stimuli-responsive nanomaterials for therapeutic protein delivery.J Control Release 2014;194:1-19.
[123]Bertrand N,Leroux JC.The journey of a drug-carrier in the body:an anatomo-physiological perspective.J Control Release 2012;161(2):152-63.
[124]Shen S,Wu Y,Liu Y,Wu D.High drug-loading nanomedicines:progress,current status,and prospects.Int J Nanomed 2017;12:4085-109.
[125]Wong A.Quantitative modeling of the high-throughput production andin vivokinetics of(drug-encapsulating)liposomes.PLoS ONE 2010;5(4):e10280.
[126]Wolfram J,Ferrari M.Clinical cancer nanomedicine.Nano Today 2019;25:85-98.
[127]Sarcan ET,Silindir-Gunay M,Ozer AY.Theranostic polymeric nanoparticles for NIR imaging and photodynamic therapy.Int J Pharm 2018;551(1-2):329-38.
[128]Greco F,Vicent MJ.Combination therapy:opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines.Adv Drug Deliv Rev 2009;61(13):1203-13.
[129]Yang MY,Zhao RR,Fang YF,Jiang JL,Yuan XT,Shao JW.Carrier-free nanodrug:a novel strategy of cancer diagnosis and synergistic therapy.Int J Pharm 2019;570:118663.
 Asian Journal of Pharmacentical Sciences2020年6期
Asian Journal of Pharmacentical Sciences2020年6期
- Asian Journal of Pharmacentical Sciences的其它文章
- Recent trends on wound management:New therapeutic choices based on polymeric carriers
- BioPerine Encapsulated Nanoformulation for Overcoming Drug-Resistant Breast Cancers
- NIR-triggered thermo-responsive biodegradable hydrogel with combination of photothermal and thermodynamic therapy for hypoxic tumor
- Dynamic micelles with detachable PEGylation at tumoral extracellular pH for enhanced chemotherapy
- Regorafenib-loaded poly(lactide-co-glycolide)microspheres designed to improve transarterial chemoembolization therapy for hepatocellular carcinoma
- Oral uptake and persistence of the FnAb-8 protein characterized by in situ radio-labeling and PET/CT imaging
