Kidney Stones Are Prevalent in Individuals with Pseudoxanthoma Elasticum, a Genetic Ectopic Mineralization Disorder
Douglas Ralph, Rina Allawh, Ian F. Terry, Sharon F. Terry, Jouni Uitto,3, Qiao-Li Li,3,∗
1Department of Dermatology and Cutaneous Biology, The Sidney Kimmel Medical College, and The PXE International Center of Excellence in Research and Clinical Care, Thomas Jefferson University, Philadelphia, PA 19107, USA; 2PXE International, Inc.,Washington, DC 20008, USA; 3Jefferson Institute of Molecular Medicine, Thomas Jefferson University, Philadelphia, PA 19107,USA.
Abstract
Keywords: ectopic mineralization, kidney stones, pseudoxanthoma elasticum
Introduction
Pseudoxanthoma elasticum (PXE; OMIM #264800) is a rare autosomal recessive disorder characterized by ectopic calcium hydroxyapatite deposition in soft connective tissues, particularly the elastic structures.1-2The prevalence of PXE is estimated to be in the range of 1:50,000 to 1:70,000 with slight female preponderance. The primary clinical manifestations reside in the skin,eyes,and arterial blood vessels. The skin manifestations are primarily of cosmetic concerns but they signify later development of eye and cardiovascular complications causing significant morbidity and occasional mortality.3
PXE is caused by mutations in the ABCC6 gene,which encodes an efflux transporter protein ABCC6 expressed primarily in the liver.2Over 400 distinct mutations in ABCC6 have been reported in patients with classic PXE(https://www.ncbi.nlm.nih.gov/clinvar/?term=abcc6[gene]). The types of mutations include missense and nonsense mutations,mutations at the canonical splice sites causing aberrant splicing, as well as deletions and insertions. In some individuals the entire or part of the ABCC6 gene is deleted. Streamlined ABCC6 mutation detection strategies are readily available for individuals with a family history of PXE.
While PXE clinically affects skin,eyes,and vasculature,there are increasing reports of visceral involvements,including kidneys in patients with PXE.4-7Intrarenal calcifications may lie in the renal parenchyma(nephrocalcinosis) or collecting system (nephrolithiasis). Most kidney stones are made of calcium oxalate crystals.Nephrolithiasis is a major public health challenge since it can lead to colic nephritis,frequent hospitalizations and recurrent surgeries,infections, renal failure, and in some cases chronic kidney disease, hemodialysis, and kidney transplantation.8The sporadic reports of kidney stones in PXE raises the question whether the association of PXE with kidney stones is a coincidence, as nephrolithiasis is not rare in the general population,or if it in fact constitutes a rare manifestation of the PXE spectrum. Some sporadic reports in the literature suggest a possible association of kidney stones and PXE,that is, kidneys stones are part of the phenotype of PXE whereas other reports claimed rare association.9
Materials and methods
Patients and clinical evaluation of PXE
Informed consent was obtained from all patients. Clinical evaluations were performed at the Dermatology clinic at Thomas Jefferson University,Philadelphia,PA.Skin biopsies were fixed in 10%phosphate-buffered formalin,embedded in paraffin, sectioned (6μm), and stained with H&E,Verhoeff,and von Kossa stains using standard techniques.
Genetic analysis and bioinformatics predictions
Genomic DNA was isolated from saliva using an OrageneTMDNA collection kit (DNA Genotek Inc.,Ottawa, Canada). The entire coding region and intron/exon boundaries of ABCC6 were amplified using primers described previously and the products were analyzed via bidirectional Sanger sequencing.9Detection of the deletion of exons 23-29 in ABCC6 used primers described previously.10Recommendations of the Human Genome Variation Society (http://www.hgvs.org/mutnomen/) were followed for exon/intron numbering and mutation nomenclature.The+1 corresponds to the A nucleotide in the ATG translation initiation codon of the ABCC6 reference sequence(GeneBank accession no.AF076622).The consequence of splicing variant in the ABCC6 gene was analyzed by MutationTaster and Human Splicing Finder prediction programs.The consequence of small insertion variant in the ABCC6 gene was analyzed by MutationTaster and SIFT.The Combined Annotation Depletion score (CADD)integrating over 60 annotation features into one score,11-12was also used to assess the deleteriousness of sequence variants. The variant allele frequency in the general population was obtained from the Genome Aggregation Database(gnomAD)consisting of over 120,000 unrelated individuals sequenced in population genetic studies.
Survey of kidney stones in the U.S. general population
The National Health and Nutrition Examination Survey(NHANES) years 2007-2016 were used to determine the population prevalence of kidney stones in the United States. The study population consisting of participants aged 20 and older responded to the question, “Have you ever had a kidney stone?.” The self-reported responses were used to determine the population prevalence of kidney stone disease in the United States.
Survey of kidney stones in the U.S. PXE cohort
Of the approximately 4,600 patients enrolled into the PXE International registry, patients in the United States with available e-mail or home address were contacted to complete a questionnaire of 30 health-related questions including kidney stones. These individuals were prescreened as having PXE based on the PXE classification criteria developed in 2010.13There were two specific questions regarding kidney stones: “Have you ever had a kidney stone?” and “How old were you when you first experienced a kidney stone?.”Age, sex, and self-reported history of kidney stones were collected. This survey was approved by the Genetic Alliance Institutional Review Board (Approval No. PXE001).
Statistical analysis
Chi-squared test was used to determine the difference between percentages of kidney stones in different groups.Statistical significance was reached with P<0.05. All statistical analyses were conducted using Prism 8(Graph-Pad, San Diego, CA, USA).
Results
Clinical manifestations of patients with PXE
Two previously healthy female siblings of a Caucasian family were referred for evaluation of skin changes(Fig. 1A). Both parents were clinically normal. The older sister, 13 years of age, presented with a 2 year history of worsening textural changes around her neck.The younger sister, 11 years of age, presented with a 1 year history of similar changes on her neck.Over the past 6 months, the patients’ mother reported more prominent yellowish papules on the lateral neck and not elsewhere on the body; these lesions were asymptomatic and not bothersome to the patients. Physical examination revealed welldefined monomorphic yellowish papules of the skin on the bilateral neck in both patients, features characteristic of PXE (Fig. 1B). The diagnosis of PXE was confirmed by histopathologic examination of the lesional skin via hematoxylin and eosin and von Kossa stains which showed calcification in the mid-dermis. Verhoeff stain revealed disrupted, abnormal elastic structures surrounding mineral deposits(Fig.1C).The patients denied pain or irritation.They had been prescribed salicylic acid,yielding no improvement. Ophthalmologic and cardiac examinations were normal. No developmental and/or intellectual deficits were noted.The younger sister had a 3 year history of recurrent nephrolithiasis since a young age of 8 years.As kidney stones are rare in children,our studies expand the clinical spectrum in patients with ABCC6 mutations.
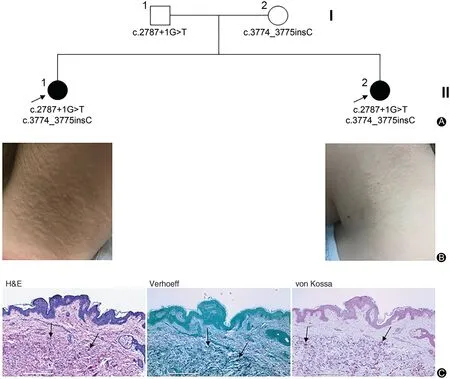
Figure 1. Nuclear pedigree and clinical features of the patients with PXE.A:Probands are indicated by arrows and the ABCC6 mutations are annotated under each family member.B:Physical examination revealed yellowish papules on the bilateral neck of the older(II-1)and younger(II-2)sister,characteristic of PXE.C:Histopathology of lesional skin in the older sister(II-1).H&E and von Kossa stains revealed mineral deposition in the mid-dermis (arrows). Verhoeff stain showed basophilic abnormal elastic structures in the dermis (arrows). Scale bar, 0.4mm. PXE:pseudoxanthoma elasticum.
Genetic testing
Mutation analysis in both patients identified two heterozygous ABCC6 variants, c.2787+1G>T in intron 21 and c.3774_3775insC in exon 27(Fig.2A).Both variants were previously reported in patients with PXE.14Father and mother were heterozygous carriers for c.2787+1G>T and c.3774_3775insC variant,respectively,consistent with the autosomal recessive inheritance mode of PXE. Bioinformatics analysis predicted their pathogenicity (Fig. 2B).Specifically, we used multiple publicly available and commonly used programs with different algorithms for prediction of pathogenicity. MutationTaster and Human Splicing Finder predict that the c.2787+1G>T variant most probably affects ABCC6 pre-mRNA splicing.MutationTasterandSIFT predictthatthec.3774_3775insC variant is a damaging mutation. Next, we determined the CADD score for each of the variants.CADD is a recently developed method for objective annotation of DNA sequence variants by combining more than 60 diverse annotations into a single measure (CADD score).11-12The CADD scores are over 20 for both variants. While the program does not declare a single universal cutoff value,a CADD score over 20 indicates that the variant is in the top 1% most deleterious to the human genome.In addition, both variants are exceedingly rare in over 120,000 healthy individuals in the Genome Aggregation Database(gnomAD)and no homozygous individuals were identified, suggesting that they are not genetic polymorphisms. Collectively, both variants are predicted to cause out-of-frame translation and premature stop codon, thus predicting synthesis of shortened, nonfunctional ABCC6 protein.
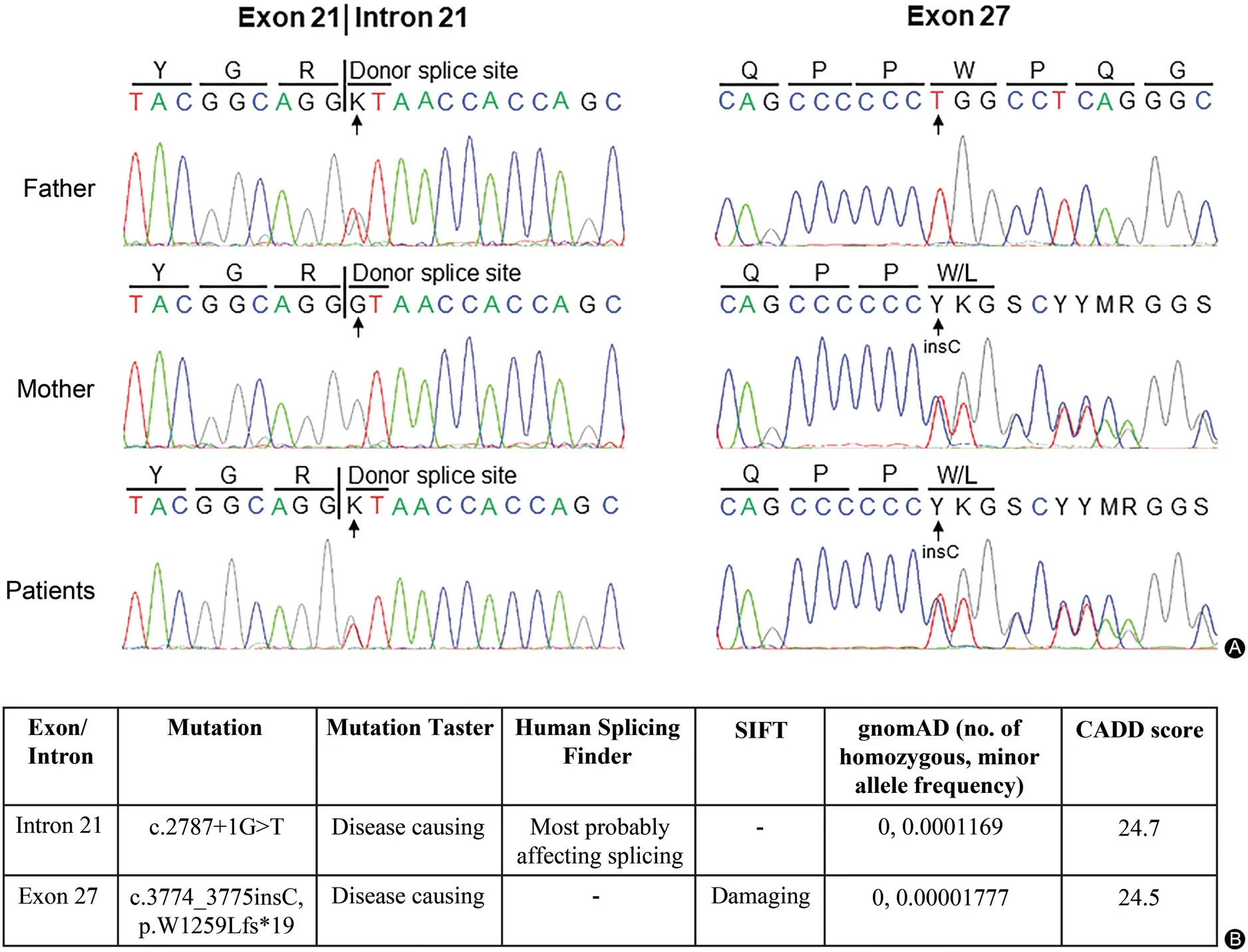
Figure 2. Mutation analysis of the ABCC6 gene in the family.A:Identification of a heterozygous c.2787+1G>T in the splicing donor site in intron 21 and a heterozygous c.3774_3775insC in exon 27 of the ABCC6 gene in the patients’DNA.The patients’parents are heterozygous carriers(arrows).B:Bioinformatics analysis of the ABCC6 mutations.Four independent programs,MutationTaster,Human Splicing Finder,SIFT,and CADD (the Combined Annotation Depletion score) were used to predict the consequences of the mutations. The allele frequency of c.2787+1G>T and c.3774_3775insC is exceedingly rare in over 120,000 healthy individuals in the Genome Aggregation Database (gnomAD), and thus, both variants are unlikely to be polymorphisms.
Frequency of kidney stones in the general population and patients with PXE
The NHANES, conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention, is a program of studies designed to assess the health and nutritional status of individuals in the United States.15Datasets in five independent and publicly available NHANES surveys from 2007 to 2016 were analyzed to evaluate the prevalence of kidney stones in the general population from 28,629 participants. All participants were 20 years old or older at the time of survey.One specific question related to kidney stones was “Have you ever had a kidney stone?.”Our analysis revealed that the prevalence of kidney stones from the last five cycles of the NHANES surveys remained relatively stable from 2007 to 2016 with an overall cumulative prevalence of 9.2%(2,635 of the 28,629 participants) (Fig. 3A).
PXE International,the premier patient advocacy organization supporting patients and families with PXE, conducted a survey of individuals diagnosed with PXE from August to December of 2019(Table 1).A total of 563 PXE patients answered the survey consisting of 30 health-related questions. The median age was comparable between the surveys, with NHANES at 49.0 years and PXE cohort at 51.5 years.To enable direct comparison of the prevalence of kidney stones in the general population,PXE International collected participants’ responses to “Have you ever had a kidney stone?,” the same question asked in the NHANES survey.Among 552 participants with 20 years of age and older from the PXE International survey, 129 (23.4%)responded that they have had a kidney stone, a greater prevalence than 9.2% from the NHANES surveys 2007-2016(P<0.01).The odds for kidney stone occurrence was 3.01 times greater among PXE participants as compared to odds in the NHANES controls. These results suggest that PXE patients have increased risk of developing kidney stones.Sex-matchedcomparisons alsorevealedsignificantly increased incidence of kidney stones in the PXE cohort(Fig. 3B). Males in both surveys had a higher prevalence comparedtofemales(Fig.3B).Whenthesurveyparticipants were divided by age groups there was also a significantly greater kidney stone prevalence in participants with PXE compared to age group-matched NHANES survey(Fig.3C).
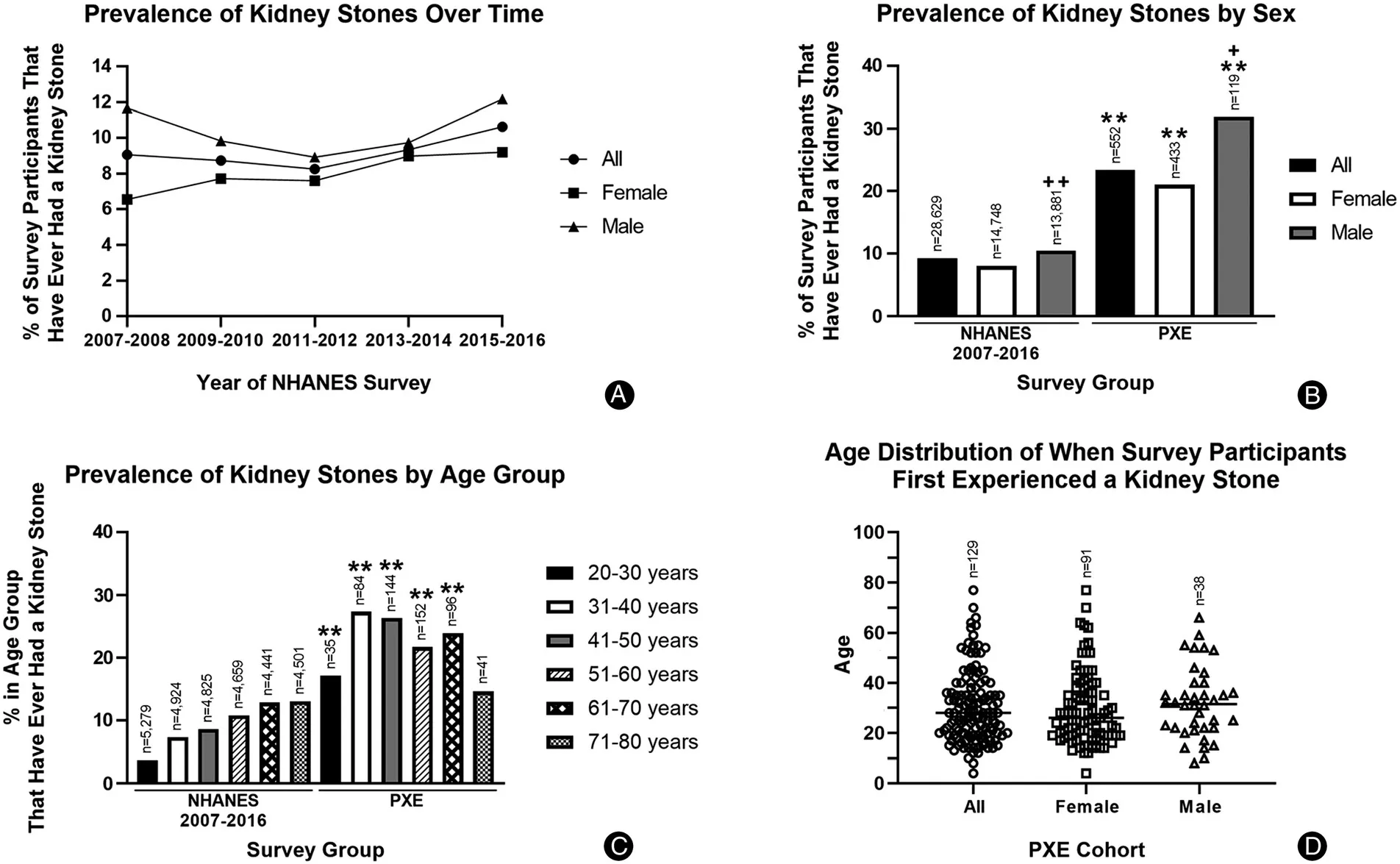
Figure 3. Comparison of kidney stone prevalence between the U.S. general population and the U.S. PXE cohort. A: The kidney stone prevalence in the U.S.general population, analyzed in five independent NHANES surveys from 2007 to 2016,remained relatively stable with overall cumulative prevalence of 9.2%(2,635 of the 28,629 total participants).B and C:Prevalence of kidney stones in the general population(NHANES 2007-2016)and our PXE cohort,across sex and age groups.Chi-squared test revealed greater incidence of kidney stones in the PXE cohort than the NHANES surveys.The n numbers represent the total number of survey respondents in different sex and age groups.D:Age distribution of PXE patients who first experienced a kidney stone.The n numbers represent the total number of survey respondents.∗∗P<0.01,compared to sex and age group-matched NHANES surveys; +P<0.05, ++P<0.01, compared to female participants within the NHANES(2007-2016)or PXE International surveys.Statistical difference was also found when each of the five independent datasets of NHANES survey years was compared to the PXE International survey data.NHANES:National Health and Nutrition Examination Survey;PXE:pseudoxanthoma elasticum.
To investigate the age onset of kidney stones in PXE patients, participants from PXE International also answered a question of “How old were you when you first experienced a kidney stone?”which was not asked in the NHANES surveys. Among 129 PXE patients who responded that they have had a kidney stone, 17.8%reported first experience of kidney stones before 18 years of age (Fig. 3D).
Discussion
We herein present the largest cohort of PXE in the U.S.with regards to the prevalence of kidney stones in patients with PXE.There is a significant increase in the prevalence of kidney stones in patients with PXE compared to the general population nationwide(P<0.01).Therefore,PXE patients have increased risk of developing kidney stones,a growing public health challenge,and an economic burden.While PXE patients have increased incidence of kidney stones,it is not clear whether PXE patients develop kidney stones at earlier age than the general population.
Patients affected by PXE are more prone to develop kidney stones than individuals in the general population in the NHANES survey in all age groups except 71-80. As vascular calcification is a major morbidity in PXE,sudden death has been reported in some patients involving accelerated coronary atherosclerosis with acute myocardial ischemia, mitral valve prolapse, restrictive cardiomyopathy,gastrointestinal hemorrhage,cerebral ischemia or hemorrhage, renal arterial intimal fibrosis, and nephrogenic hypertension.16-18While the majority of PXE patients have a normal life span, premature death in some individuals due to vascular complications might explain at least in part the lack of increased prevalence of kidney stones in the elderly individuals with PXE. In addition,the sample size of 41 PXE patients in the 71-80 age range is small, despite being part of the largest PXE cohort to date characterizing kidney stone prevalence in PXE.
The prevalence of kidney stones was also reported in other PXE cohorts. One publication reported 10.9%prevalence of kidney stones in 239 French PXE patients while another one reported 39.8%in a separate cohort of 113 French PXE patients.19-20Discrepancies betweenthese independent surveys may be explained in part by age demographics and geographic differences. Changes in dietary practices between regions is another contributing factor as dietary changes contribute to the risk of formation of calcium oxalate stones, the type of stone formed in the vast majority of kidney stone formers.21-22In addition, the self-reported data in all these surveys are subject to bias, a generally recognized limitation of selfreport surveys. While kidney stones are common in the general population, particularly in older individuals, they are relatively rare in children.23-24Both French cohorts included all age groups whereas our PXE International survey conducted in the U.S. excluded participants below age of 20, the same inclusion criteria set forth in the U.S.NHANES survey. Participants in our survey are age, sex,and location matched with that in the NHANES survey,allowing for a more direct comparison than the French cohorts. Further work is necessary to conduct a wellcontrolled prospective cohort study, from which relative risks can be calculated, to obtain the best estimates of kidney stones in PXE.
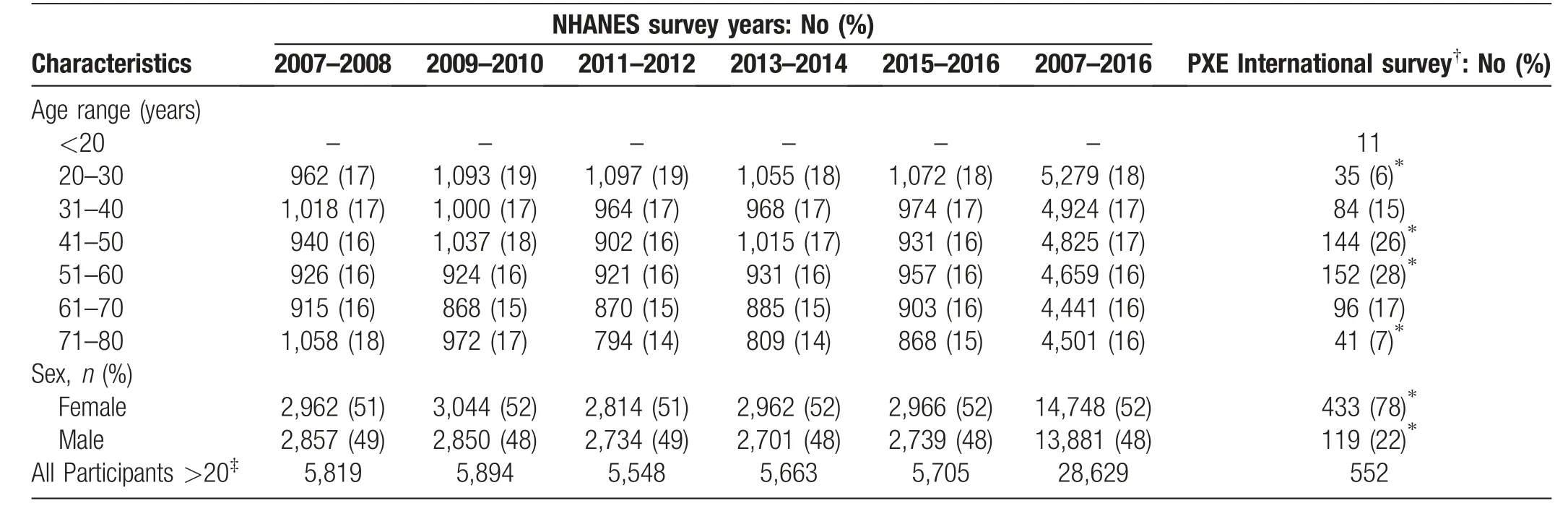
Table 1 Demographics of survey participants.
The internationally standardized scoring system for evaluation of PXE cases is the use of Phenodex Score.Phenodex Score assignment of patients with PXE to different phenotypic categories is currently based on clinical findings in five organ systems, that is, skin, eye,vascular, cardiac, and gastrointestinal symptoms.9,19,25Since kidney stones are an unrecognized but a prevalent feature of PXE, we propose that nephrolithiasis could be included in routine clinical evaluation and follow up visits beyond the skin, eyes, and cardiovascular system in patients with PXE as early detection and prevention is of paramount importance for clinical management of renal complications.In addition,surveys of kidney stones led us to propose,and supported by others,19that nephrolithiasis is a recurrent complication in PXE, which could be included in the Phenodex Score system for PXE.
Acknowledgments
This study was supported by the PXE International,NIH/NIAMS grants R01AR028450(JU)and R01AR072695(JU and QL).The authors thank our patients for their participation in our studies.Carol Kelly assisted in manuscript preparation.
- 国际皮肤性病学杂志的其它文章
- Adverse Skin Reactions to Personal Protective Equipment Among Health-Care Workers During COVID-19 Pandemic: A Multicenter Crosssectional Study in Indonesia
- Complete Draft Genome Sequence of Cutibacterium (Propionibacterium) acnes Type Strain ATCC6919
- The Immune Function of Keratinocytes in Anti-Pathogen Infection in the Skin
- A Case Report of Pemphigoid Nodularis as Masquerader of Neurotic Excoriations
- Co-occurrence of Vitiligo and Psoriasis in an 11-Year-Old Girl: A Case Report
- Erosive Adenomatosis of the Nipple Masquerading as Paget’s Disease:A Case Report

