MicroRNA-145 plays a role in mitochondrial dysfunction in alveolar epithelial cells in lipopolysaccharide-induced acute respiratory distress syndrome
Yi Han, Su-cheng Mu, Jian-li Wang, Wei Wei, Ming Zhu, Shi-lin Du, Min Min, Yun-jie Xu, Zhen-ju Song, Chao-yang Tong
Emergency Department, Zhongshan Hospital, Fudan University, Shanghai 200032, China
Corresponding Author: Chao-yang Tong, Email: tong.chaoyang@zs-hospital.sh.cn; Zhen-ju Song, Email: song.zhenju@zs-hospital.sh.cn; Yun-jie Xu, Email: xu.yunjie@zs-hospital.sh.cn
KEYWORDS: MicroRNA-145; Mitochondrial function; Lipopolysaccharide; Acute respiratory distress syndrome; Rats
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a common condition associated with critical illnesses,which causes substantial mortalities. Approximately 200,000 ARDS cases per year occur in the USA, and the mortality is as high as 36%-44%.[1,2]Sepsis is often the main cause of ARDS, which may lead to multiple organ failure.[3]Lung inflammation, hypoxemia, and non-cardiogenic pulmonary edema formation are characteristic features.[4,5]
Alveolar epithelium is one of the main sites of cell injuries in ARDS. Neutrophils contribute to lung inflammation and play important roles in the pathogenesis and progression of ARDS. The activated neutrophils damage epithelial cells,[6]which causes increased entry of fluid into the alveolar lumens,decreased clearance of fluid from the alveolar airspace,and decreased production of surfactant.[7]
MicroRNAs (miRNAs) are small noncoding ribonucleic acid (RNA) molecules and are recognized as endogenous physiological regulators of gene expression.[8]Given that an individual miRNA could potentially alter complicated cellular processes including cell growth,apoptosis, inflammatory-immune responses, and cellcell interaction,[9,10]it is not surprising that their wrong settings may be involved in the pathogenesis of ARDS.
Many miRNAs are expressed in the lung.MicroRNA-17 (miR-17), microRNA-92a (miR-92a),and microRNA-127 (miR-127) regulate the lung development.[11,12]Although miRNAs play a dominating role in several physiological functions, they are also involved in the pathogenesis of many diseases. The abnormal expression of miRNAs is associated with cardiac disorders,[13]vascular diseases,[14]cancers,[15]and pulmonary diseases like ARDS.[16]
As an important miRNA, microRNA-145 (miR-145) has been studied in various cancers.[15,17]It could potentially alter complex cellular processes, such as cell growth, cell cycle, apoptosis, and invasion.[18]A previous study reported that the expression of miR-145 was significantly reduced in myocardial ischemia/reperfusion (I/R) injury in the rats,[19]indicating that the abnormal expression of miR-145 was involved in myocardial I/R injury. Hypoxia could promote umbilical cord mesenchymal stem cell (UCMSC) differentiation into alveolar epithelial cells, and this effect was mainly mediated by miR-145.[20]Lipopolysaccharide (LPS)-induced liver inflammation was probably mediated by miR-145 through interleukin-1 receptor-associated kinase 1 (IRAK1) and nuclear factor-kappa B (NF-κB)pathways.[21]Furthermore, miR-145 suppression reversed the LPS-induced inf lammatory injury on ATDC5 cells.[22]
However, the role of miR-145 in ARDS has not been investigated. In the present study, we aimed to identify miRNA-145 involved in ARDS by using an animal model of ARDS. In addition, we tried to focus on the relationship between miR-145 and mitochondrial function, which plays a critical role in regulating the cell injury of ARDS.
METHODS
Animals
The study was proved by the Ethics Department of Zhongshan Hospital, Fudan University. A total of 24 male Sprague-Dawley rats, aged 6-8 weeks, purchased from the Animal Center of Fudan University, and bred under pathogen free conditions, were housed separately in a temperature-controlled room with a 12-hour light/12-hour dark cycle. Animals were allowed free access to food and water.
Animal treatment
Rats were randomly assigned into two groups. They were anesthetized with an intraperitoneal injection of avertin (25 mg/kg) and f ixed at a 60° angle on a table in a supine position. The oropharynx was lifted with forceps,allowing for the direct visualization of the trachea. LPS at a dose of 0.5 mg/kg (Sigma, USA) was injected into the trachea using an 18G catheter attached to a 1 mL syringe as previously described.[23]Control animals received an equal volume of phosphate-buffered saline (PBS). Rats were sacrificed at 6, 24, and 72 hours after LPS/PBS instillation after the intraperitoneal injection of avertin(25 mg/kg).
RNA isolation and analysis
RNA was isolated from the median and caudal lobe of the right lung using the Qiagen RNeasy Mini Kit following the manufacturer’s instructions. Quantitative polymerase chain reaction (qPCR) was performed for expressions of miRNAs using the primers for miR-145 as follows: 5′-GUCCAGUUUUCCCAGGAAUCCCU-3′.
Determination of the lung water content
The right main bronchus was ligated, and the cranial and accessory lobes of the right lung were excised. After wet weights were measured, the cranial and accessory lobes of the right lung were placed in an oven at 60 °C for 72 hours to allow determination of the wet-to-dry (W/D) weight ratio.
Bronchoalveolar lavage (BAL)
The BAL was performed in the left lung. Totally 2 mL PBS (4 °C) was slowly infused. The fluid was slowly withdrawn and reinfused for another two times. The recovered f luid was collected for further analysis.
Cytokines in bronchoalveolar lavage f luid (BALF)
Tumor necrosis factor-α (TNF-α) and interleukin-6(IL-6) levels in BALF were measured using rat TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA)kits (R&D Systems) according to the manufacturer’s recommendations.
Hematoxylin and eosin (H&E) staining
Lung tissues were fixed and processed for H&E staining. Briefly, lung tissues were fixed by 10% PBSbuffered formalin through trachea catheterization at a transpulmonary pressure of 15 cmH2O (1 cmH2O=0.098 kPa), and then overnight at 4 °C with agitation. After paraffin processing, the tissues were cut into semi-thin 4-5 μm thick and stained with H&E for histological analysis.
Transmission electron microscope
The preparation of lung tissues for transmission electron microscopy was made following the procedure described previously.[24]Lung samples were obtained and f ixed with 2.5% glutaraldehyde in PBS buffer. Then,lung samples were post-fixed with 1% OsO4in PBS buffer for 1 hour, followed by dehydration. Tissues were embedded in 50% propylene oxide/50% resin. Sectioning was performed on an ultramicrotome (60 nm thickness).Samples were stained with lead citrate, and examined with an electron microscope (Hitachi H-600, Japan).
Target gene prediction of miRNA
The target genes of prognostic miR-145 were predicted using TargetScan and miRDB analysis tools.The overlapping genes were analyzed. Gene Ontology(GO) and Kyoto Encyclopedia of Genes and Genomes(KEGG) pathway enrichment analyses were performed for the target genes. TheP-value <0.05 and gene count≥3 were set as the cut-off criteria.
Statistical analysis
The data were expressed as mean±standard deviation(SD). The expression levels of miRNAs in ARDS and control rats were analyzed by Wilcoxon signed-rank test. TheP-value <0.05 was considered statistically signif icant. The statistical analysis was performed using SPSS software and Prism 6.0.
RESULTS
MiR-145 down-regulated in LPS-induced ARDS rat lung
With H&E staining, there were few inflammatory cells or lymphocyte infiltrations, and the structure of alveolar was almost intact in the control group;at 6 hours after LPS injection, the lymphocytes in the alveolar were significantly increased, especially increased around bronchi and lung vessels, and there was protein-like fluid filled in the alveola; at 24 hours after LPS injection, there were more lymphocyte inf iltrations,the alveolar structures were disrupted and filled with inflammatory cells, and alveolar interval was much thicker than that in the control group; at 72 hours after LPS injection, the lymphocyte infiltrations still existed,but decreased compared with those at 24 hours (Figures 1A[a-d]). According to the lung injury scoring system,[25]scores significantly increased in LPS instillation lungs,especially at 24 hours and 72 hours after LPS injection(Figure 1B). At 6 hours after LPS injection, the W/D ratio of LPS lungs was about 7.5 compared with 3.0 in the control group; at 24 hours after LPS injection, the W/D ratio of LPS lungs was about 7.0, much higher than that in the control group (P<0.05); at 72 hours after LPS injection, the W/D ratio of LPS lungs was about 4.5,higher than that in the control group (P<0.05) (Figure 1C). The cytokines in BALF were measured at 6 hours(n=4), 24 hours (n=4), and 72 hours (n=4) after LPS instillation. The results showed that TNF-α peaked at 6 hours after LPS and gradually decreased; IL-6 peaked at 24 hours after LPS and gradually decreased to baseline at 72 hours (Figures 1D, E). The miR-145 messenger RNA(mRNA) expression was measured at 6, 24, and 72 hours after LPS instillation using qPCR. The results showed that with LPS instillation, the miR-145 expression was signif icantly decreased (Figure 1F).
Mitochondrial dysfunction found in the
epithelial cells of LPS-induced ARDS rat lung
The ultrastructure of alveolar epithelial cells was detected using a transmission electron microscope.There was a clear nuclear and lamellar bodies in the control lung epithelial cells, and the electron density of mitochondria was homogeneous. At 6 hours after LPS,the villi of epithelial cells turned incomplete, while there were still intact lamellar bodies and mitochondria.At 24 hours after LPS injection, the structures of the lamellar bodies and mitochondrial cristae were in disorder, and the electron density of mitochondria was gradually increased. At 72 hours after LPS injection,there was obvious structure disturbance of mitochondria and lamellar bodies, with the absence of mitochondrial cristae, which was the obvious evidence of mitochondrial injury (Figures 2A-D).
Predicted target genes of miR-145 related to mitochondrial dysfunction
TargetScan and miRDB databases were used to search miR-145 so as to predict the target genes. A total of 428 overlapping genes were identified (Figure 3A).The enrichment analysis was performed to elucidate the biological function of target genes. The KEGG pathways were significantly enriched in mitogenactivated protein kinase (MAPK) signaling pathway and RAS signaling pathway. In addition, the GO biological process was mainly enriched in gene binding, signal transduction, and transcription regulation (Figures 3B,C). Moreover, using the Rat Genome Database at the National Center for Biotechnology Information (NCBI),seven mitochondria-associated genes regulated by miR-145 were identif ied, includingSlc1a2,Cftr,Ogt,Acs14,Camk2d,Slc8a3, andSlc25a25. All seven target genes were verified using qPCR in ARDS lungs. The results showed thatOgt,Camk2d,Slc8a3, andSlc25a25were slightly up-regulated at 24 hours after LPS injection,while signif icantly down-regulated at 72 hours after LPS injection (P<0.05) (Figure 3D).

Figure 2. TEM structure of alveolar epithelial cell (×20,000). Red star:nuclear of alveolar epithelial cell; blue arrow: lamellar bodies; green arrow:mitochondria; black arrow: villus of alveolar epithelial cell; A: control lung(72 hours after PBS); B: 6 hours after LPS instillation; C: 24 hours after LPS instillation; D: 72 hours after LPS instillation; TEM: transmission electron microscope; PBS: phosphate-buffered saline; LPS: lipopolysaccharide.
DISCUSSION
ARDS is one of the most critical diseases in intensive care units (ICUs), which seriously affects the prognosis and life quality of critically ill patients. It is characterized by the acute onset of respiratory failure associated with diffuse interstitial pulmonary edema in the absence of left ventricular failure. It has been proved that the degeneration of surfactant is one of the most important causes of ARDS.Reduced secretion of surfactant is associated with worse outcome. A potential role for intact mitochondria in surfactant production and secretion is supported by studies reporting that intramitochondrial delivery of glutathione in rats significantly preserved surfactant producing and secreting functions of type II cells.[26]
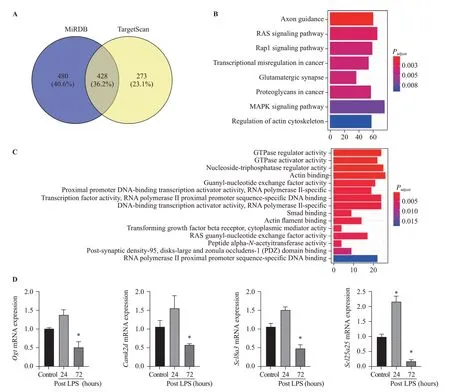
Figure 3. Bioinformatics data of microRNA-145 target genes and pathways. Compared with control, *P<0.05; A: target genes of microRNA-145 predicted using TargetScan and miRDB tools; B: KEGG pathway analysis (Y-axis representing the enriched KEGG terms, X-axis representing the amount of the microRNA-145-related mRNAs enriched in KEGG terms); C: GO biological process (Y-axis representing the enriched GO terms,X-axis representing the amount of the microRNA-145-related mRNAs enriched in GO terms); D: Ogt, Camk2d, Scl8a3, and Scl25a25 mRNAs were slightly up-regulated at 24 hours post LPS instillation, while signif icantly down-regulated at 72 hours post LPS instillation; MAPK: mitogenactivated protein kinase; KEGG: Kyoto Encyclopedia of Genes and Genomes; GO: Gene Ontology; LPS: lipopolysaccharide.
LPS could induce inflammatory responses in various diseases, including ARDS.[21-23]The intratracheal instillation of LPS is proved to be an excellentin vivomodel of lung injury, and it is widely used for investigating ARDS. In our study, the results revealed that LPS up-regulated IL-6 and TNF-α expression,promptly stimulated cytokines responses, and signif icantly damaged the epithelial barrier. Inf lammatory cells infiltrated in the alveolar air space and the paravascular space, and the separation of alveola was much thickened after LPS instillation, which would finally form the hyaloid membrane in ARDS. With these pathological manifestations, we scored the inf lammatory and structure disruption levels based on the previous study,[25]and we concluded that intratracheal instillation of LPS was a convenient and sufficient way to set up an ARDS model in rats.
The miRNAs are small non-coding RNAs that play a crucial role in many disease processes, including malignancy and inflammatory processes. Abnormal expression of miRNAs, such as miR-126-5p, miR-1246,miR-34a, miR-27a, and miR-223, has been found in lung injury.[23,27-30]MiR-145 is an important molecular marker,which has been proven to mediate cell proliferation, cell cycle, apoptosis, and invasion.[15]Researchers also found that miR-145 played a role in cervical epithelial cell barrier.[18]These studies demonstrated that miR-145 was associated with epithelial cell injury in cancer. However,whether miR-145 was involved in regulating LPS-induced ARDS remains unknown.
In our study, we found that the expression of miR-145 decreased in ARDS lungs, which was corresponding to the level of mitochondrial damage observed by transmission electron microscope (TEM) in lung epithelial cells. We found that the electron density of mitochondria began to increase at 24 hours after LPS injection, and there was obvious structure disturbance of mitochondria and lamellar bodies, as well as with the absence of mitochondrial cristae. As described in previous studies, miR-145 played an important role in regulating the mitochondrial apoptotic pathway in tumor cells, partly through its ability to target various anti-apoptotic molecules.[31]Moreover, the abnormal expression of miR-145 was associated with vascular smooth muscle cells’ response to hydrogen peroxideinduced oxidative stress, indicating that miR-145 may participate in the regulation of the oxidative stress-triggered apoptosis and the regulation of the mitochondrial apoptotic pathway. Furthermore,programmed cell death 4 (PDCD4) was identified as a novel target of miR-145 in cardiomyocyte, and the overexpression of PDCD4 could remarkably restore the miR-145-inhibited cardiomyocyte apoptosis and mitochondrial dysfunction after hypoxia injury.[32]However, little is known about whether miR-145 is associated with lung epithelial cell apoptosis or how it interferes with the mitochondrial apoptotic pathway.
Previous studies have reported that inherited mitochondrial polymorphisms, genes, and pathways were associated with epithelial ovarian cancer risk, including TERF and PPARGC1a.[33]Cystic fibrosis transmembrane conductance regulator (CFTR) silencing results in lipid homeostasis disruption and mitochondrial dysfunction in intestinal epithelial cells, and it regulates neuronal apoptosis following cerebral I/R via mitochondrial oxidative stressdependent pathway.[34,35]Ogtis catalytically activein vivoand supports mitochondrial structure, health, and survival.[36]In our study, we found thatOgt,Camk2d,Slc8a3, andSlc25a25were significantly down-regulated at 72 hours after LPS injection, which verif ied the results thatOgt,Camk2d,Slc8a3, andSlc25a25were target genes of miR-145. Further studies are needed to confirm how miR-145 regulates its target genes, and to confirm the pathways we speculated from the bioinformatics data.
CONCLUSIONS
The current study provided evidence related to the role of miR-145 in mitochondrial function in LPS-induced ARDS. The miR-145 was down-regulated in LPS-induced lung injury, which might affect its downstream genes targeting mitochondrial functions such asOgt,Camk2d,Slc8a3, andSlc25a25. Bioinformatics data indicated that the regulation of miR-145 may be through MAPK and RAS signaling pathways. These results provide evidence that miR-145 may play a role in inf lammatory-related epithelial barrier disruption, and further studies are needed to elucidate the specif ic mechanisms.
Funding:None.
Ethical approval:All applicable international, national, and institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants.
Conflicts of interests:The authors declare that they have no competing interests.
Contributors:YH and SCM contributed equally to this work. CYT conceived the project, conducted the study, and gave administrative support to this study. SCM and JLW analyzed and interpreted the data. WW, MZ, and SLD contributed to data acquisition. MM helped with data analysis. ZJS and YJX revised the manuscript.CYT, ZJS, and YJX were co-corresponding authors. All authors read and approved the f inal manuscript.
 World journal of emergency medicine2021年1期
World journal of emergency medicine2021年1期
- World journal of emergency medicine的其它文章
- Trends and challenges of emergency and acute care in Chinese mainland: 2005-2017
- Factors associated with refractory pain in emergency patients admitted to emergency general surgery
- Identifying critically ill patients at risk of death from coronavirus disease
- Clinical correlates of hypotension in patients with acute organophosphorus poisoning
- Effects of viral infection and microbial diversity on patients with sepsis: A retrospective study based on metagenomic next-generation sequencing
- Effects of metabolic syndrome on onset age and long-term outcomes in patients with acute coronary syndrome
