Measurement of viscoelastic properties of injured mouse brain after controlled cortical impact
Yu Chen,Suhao Qiu,Cheng Wang,Xiaowei Li,Yaohui Tang,Yuan Feng✉
1Institute for Medical Imaging Technology,School of Biomedical Engineering,Shanghai Jiao Tong University,Shanghai 200030,China
2School of Biomedical Engineering and Med-X Research Institute,Shanghai Jiao Tong University,Shanghai 200030,China
3Bio-ID Center,School of Biomedical Engineering,Shanghai Jiao Tong University,Shanghai 200240,China
Abstract Mechanical properties of brain tissue can provide vital information for understanding the mechanism of traumatic brain injury(TBI).As mouse models were commonly adopted for TBI studies,a method to produce injury to the brain and characterize the injured tissue is desired.In this paper,a complete work flow of TBI induction,sample preparation,and biomechanical characterization is presented for measurement of the injured brain tissue.A controlled cortical impact device was used to induce injury to the brain.By setting the angle,speed,and position of the impact,the level of brain injuries could be controlled.Viscoelastic properties of both injured and non-injured brain tissues were measured using a ramp-hold indentation test.Regions of interests(ROIs)were tested and compared to contralateral corresponding counterparts.Methods introduced in this paper could be easily extended to produce and test a variety of other injured soft biological tissues.
Keywords Controlled cortical impact,Traumatic brain injury,Viscoelastic properties,Mouse model
INTRODUCTION
Traumatic brain injury(TBI)is one of the major causes of morbidity and mortality worldwide,with more than 50 million patients each year and causing 400 billion dollars of global economy loss annually(Maas et al.2017).TBI is induced by a mechanical process.Many studies have shown that mechanical properties of the brain are closely related to its development and diseases(Goriely et al.2015).Biomechanical testing of the brain tissue could provide key physical parameters for the modeling and prediction of TBI(Ganpule et al.2017;Lu et al.2019;Madhukar and Ostoja-Starzewski 2019;Zhao et al.2017;Zhou et al.2019).Comparisons of mechanical properties between the healthy and injured brain tissues can provide important clues to understand the mechanism of TBI(Feng et al.2017a;Qiu et al.2020).Therefore,understanding the biomechanical properties of the brain tissue is important to decipher the myths of TBI.
Animal models are indispensable for TBI research since injury-level experiments are impractical for human subjects.Despite the fact that the animal models cannot completely represent human brain injury,animal models of TBI still offer the best alternative to examine the biomechanical,cellular,and molecular mechanism of TBI-associated neuropathological progression(Ma et al.2019).Animal models such as pig(Pasquesi and Margulies 2018),mouse(Chen et al.2014),and monkey(King et al.2010)have been used for TBI studies.Among them,mouse model is relatively easy to implement and provides various genetic manipulation options for translational studies.To induce brain injury,typical techniques include blast wave,weight-drop,lateral fluid percussion,and control cortical impact(CCI).The blast wave technique is usually carried out in closed skull,inducing severe brain injuries.It is relatively difficult to carry out and hard to quantify.The other three methods are generally carried out under the condition of open skull and the injury is relatively easy to quantify.The weight-drop method is straightforward to conduct,but can only mimic a vertical impact on brain.The lateral fluid percussion method is usually used to induce diffuse axonal injury(DAI),but the speed of water is hard to control.The CCI method uses electric or pneumatic devices to induce injuries via a striking tip.It provides the best control of impact velocity,depth,and direction and thus the best control of injury levels.However,CCI is only suitable for small animals.Therefore,we adopted an in vivo mouse model to investigate the injured brain tissue using CCI.
To acquire the biomechanical properties of the brain tissue,many different methods have been adopted to characterize the brain tissue(Goriely et al.2015).Tension tests(Rashid et al.2012),shear tests(Destrade et al.2015;Rashid et al.2013),and indentation tests(Budday et al.2015;Feng et al.2017b;van Dommelen et al.2010;MacManus et al.2017a)were the mostly used.Tension tests such as uniaxial or biaxial tests could provide uniform deformation,facilitating measurements backed by analytical models.Shear tests can also provide uniform shear deformation and a direct measurement of the shear modulus.However,both tension and shear tests require the sample to be properly fixed at the boundaries.This poses a great challenge for brain tissue.In addition,it is especially challenging to conduct tension or shear tests on a specific region of brain.Indentation does not need special fixations for samples.Therefore,it provides a great convenience for testing brain tissue.In addition,indentation tests have also shown to be effective in testing specific regions in various scales(Hu et al.2020;Qiu et al.2020).
In this method study,viscoelastic properties of the brain tissue after CCI were measured using indentation.Both the CCI and indentation devices were custom-built.As a demonstration of method,we induced mild injuries to the mouse brain.Two regions of interests(ROIs),the cortex and hippocampus,were tested in both the ipsilateral and contralateral hemisphere.The proposed method of viscoelastic characterization was not limited to measure brain tissue,but could also be used to measure any soft biological tissues.
EXPERIMENTAL SECTION
CCI device
A custom-built CCI device was built for inducing injury to the mouse brain.It has a cylindrical tip to produce impact on the mouse brain(Fig.1).The impact motion was generated using a voice coil(SMAC Corporation,Carlsbad,California,USA).The impact speed and acceleration were controlled using an industrial workstation(LCC-10 controller).The workstation was connected to the voice coil as a control console.The impact angle was adjusted with an angle dial gauge.To fix the mouse head firmly,a stereotaxic head frame was placed under the voice coil,with its center aligned along the impact tip.A custom-written program(based on SMAC LAC-X Editor V1.0)was used to control the impact speed.
Indentation device
A custom-built indentation device was used to characterize the viscoelastic properties of the brain tissue.The indentation motion was produced with an electric motor(SMAC Corporation,Carlsbad,California,USA).A cylindrical indenter with a flat tip and a diameter of 2 mm was rigidly connected to the motor for indentation(Fig.2).A laser reflection plate was used for displacement measurement.The motion of the indenter was recorded with a laser sensor(Model HG-C1050 30 mm,Panasonic,Japan).A load cell(GSO-10,Transducer Technique,USA)connected to the indenter tip was used to measure the reaction force during the indentation.Signals from the sensors were collected with a data acquisition board(PCI-1706U,Advantech Co.,Ltd.,China)at a sampling rate of 1 kHz.A graphical user interface(GUI)based on LABVIEW(National Instruments,TX,USA)was designed for data acquisition and indentation control(Fig.2).
Measurement protocol
Female mice(balb/c,20 g,SPF grade,certificate No.SCXK 2018-0006)were used for demonstrating the protocol.For the open-skull impact,each mouse was anesthetized with chloride hydrate(10% concentration).After shaving hairs on the top of mouse head,a 5-mm-diameter trephine(Roboz Surgical Instrument Co.,Gaithersburg,MD,USA)was used for craniotomy.Taking the left hemisphere as an example,the ROIs were chosen at the left rear of the anterior fontanel,about 3 mm to the left of the midline of skull.A circular shaped bone flap with a diameter of 3 mm was removed to expose the ROIs after the craniotomy(Fig.3).The skin wrapping the skull was peeled off after the mouse was anesthetized(Fig.3).The impact position was taken at the middle of bregma and lambda with a 1.5 mm lateral distance on the left hemisphere.The ROIs were delineated on the skull before impact.
A cylindrical impact head with a diameter of 4 mm was fixed to the voice coil motor to produce the controlled impact.To avoid severe brain injury and skull rupture,the impact velocity was set to be less than 2 m/s and the controlled depth of the impact was about 1 mm for close-skull CCI.After CCI,the skin incision was sutured with a curved needle and the animals were housed in warm and clean environment supplied with food and water to reduce infection and mortality.
The brain was removed from skull before indentation test.Along the midline of skull,the skin wrapping the skull was cut to expose the skull.A small scissor was used to cut the soft tissue along the medullary foramen until it touched the skull.Then small slit was cut at the end of midline of the skull and the skull was peeled off carefully with forceps to expose the mouse's brain.Once the dissection was done,the whole brain could be removed from the skull.
Coronal slices of the brain were sectioned with a coronal slicing matrix(RWD Life Science,Shenzhen,China).Each slice was about 3 mm thick.The slice was stored in phosphorous-buffered saline(PBS)shortly before the indentation test.The pH value of the PBS solution(MA0015,Meilun Biotechnology,Dalian,China)was between 7.2 and 7.4.In this method study,four ROIs were chosen for comparative analysis as a demonstration:left cortex(LC),left hippo(LH),right cortex(RC),and right hippo(RH)(Fig.3).
The indentation test includes five steps:(1)Measurement of the slice thickness.The distances with and without the sample were recorded by the laser sensor.The difference of the two measurements was taken as the thickness of the slice.(2)ROI selection.The selected ROI for measurement was placed directly below the indenter.(3)Contact.The indenter was lowered to be in contact with the sample surface.(4)Indentation.Each sample was indented within 8% of its sample thickness to minimize the boundary effect(Budday et al.2015).Relaxation was followed for measurement of viscoelastic properties.The displacement and reactive force were recorded for 180 s since the start of the indentation.(5)All the recorded data were saved.
A flow chart of the protocol is summarized in Fig.4.The CCI and indentation control,data acquisition,and result visualization were all carried out using a single PC(Fig.5).A custom-written GUI was used to control all instrument,and the data communication was done via RS-232 ports.
Estimation of viscoelastic parameters
The displacement of indenter during ramp-hold test protocol is a piecewise function:
where V is the indentation velocity and tRis the indentation ramp time.Therefore,the corresponding reaction force based on 2-term Prony series is(Qiu et al.2018)given as follows:
where R is the radius of the indenter,C0,Ciand τiare components of Prony series,X represents the compensation factor(Dimitriadis et al.2002),and h and H are the displacement of indenter and the thickness of the sample,respectively.
She gave him food, and when he had finished his meal she told him how his brothers had come to the town where she lived with her sisters, how they had each chosen a bride, and, taking herself with them, had started for home
The viscoelastic parameters were estimated by a custom-written MATLAB(Mathworks,Natick,MA,USA)code with an objective function:
where w1and w2were taken as 0.5.The n and m are the number of data points in each section.The corresponding instantaneous shear modulus G0and longtime shear modulus G∞are given as follows:
RESULTS
Typical experimental and fitted force vs.time curves were shown in Fig.5.As an illustration,the estimated viscoelastic model parameters for the four ROIs 1 h post injury are summarized(Table 1).A comparison of G0and G∞values is shown in Fig.6.We observed higher mean G0values of LC and LH(1.31 kPa,1.35 kPa),compared to that of RC and RH(1.02 kPa,1.05 kPa).Student t-tests showed significant differences(p<0.05)between LC/RC and LH/RH pairs in terms of G0.However,the mean differences of the G0values between the two pairs were almost the same(0.29 kPa,0.30 kPa).The G∞mean values of LC and LH(0.12 kPa,0.16 kPa)were slightly higher than that of RC and RH(0.11 kPa,0.13 kPa).
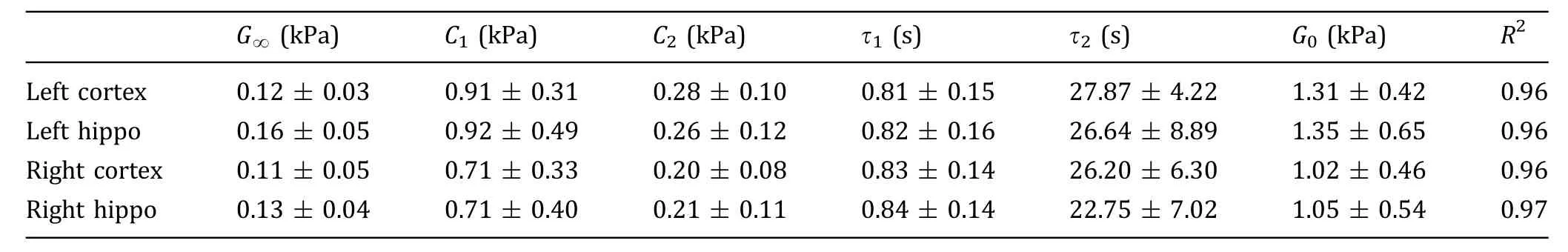
Table 1 Measured viscoelastic properties of the brain tissue 1 h post injury with 95% confidence intervals
DISCUSSION
In this method,the CCI was introduced for producing injuries to the brain.The indentation test was used for measuring the viscoelastic properties of the brain tissue.By controlling the cortical impact magnitude and position,brain injuries with different levels and positions could be produced for a variety of TBI studies.
The impact depth and velocity are crucial parameters,although there is a wide range of impact velocities used in CCI studies,from 5-6 m/s(Lu and Mao 2019;Mao et al.2013)to around 1 m/s.It is shown that for producing mild TBI in an open-skull condition,the impact depth and velocity should be less than 1 mm and 4 m/s,respectively(Ma et al.2019).The impact velocities used here(1 mm depth and 2 m/s velocity)were similar to that of reported CCI studies using mouse model(Qiu et al.2020;Toshkezi et al.2018;Xu et al.2019),producing mostly mild,no more than the moderate level of injury.Compared with the mice that did not receive CCI,the mice after CCI had a longer recovery time.In addition,the skull of the injured mice was intact and there was no obvious bleeding in the injured area.These experimental observations also confirmed the introduction of mild TBI.
With respect to the impact location,most animal studies focused on the area between lambda and bregma(Chen et al.2014;Pleasant et al.2011;Xu et al.2014).This is because impacting this location could produce injury to hippocampus,cortex,and subcortical white matter simultaneously.The impact tip with a diameter of 4 mm was suitable for the brain of 8-weekold mouse.The 2-mm-diameter indenter tip could provide the best testing resolution for the small-sized mouse brain.In previous studies,we have also noticed that the impact angle significantly influenced instantaneous shear modulus after TBI(Qiu et al.2020).Although most of the brain injury studies based on CCI adopted open-skull impact,85%-89% of the TBI cases happened in closed skull(Myburgh et al.2008).In this method study,we demonstrated the measurement protocol for inducing and measuring a mild injury.It should be noticed that the methods could be easily extended to testing brain tissues with different injury levels with the corresponding impact velocity,depth,and angle.
Studies have shown that dynamic measurement could provide more information.However,dynamic testing poses additional challenges of measurements(MacManus et al.2017a).Compared with the indirect measurement of slice thickness(Wang et al.2020),a direct measurement using an independent laser sensor could be more accurate.However,one of the limitations is dehydration of the sample for viscoelastic measurement.For example,for a ramp-hold test,relaxation section could last as long as 3 min.A practical solution for preventing the dehydration is spraying PBS solution to the sample to keep it moisturized.
CONCLUSIONS
We introduced a method that could provide wellcontrolled velocity and angle for inducing impact injuries to specific brain regions using CCI.The indentation test procedure facilitated convenient sample preparation for brain tissue.By inducing a mild TBI to mouse brain,we demonstrated the method to measure the viscoelastic properties of both the injured and noninjured ROIs in the brain.Brain tissues with different injury levels could be produced by changing the impact speed and depth.In addition,it is easy to apply the indentation method to test other soft biological tissues such as liver,breast,and tumor.
AcknowledgmentFunding support from National Natural Science Foundation of China(31870941)and Shanghai Science and Technology Committee(1944190700)are acknowledged.Technical assistance and helpful discussions from Jianhui Zhang and Dongjing Lu are acknowledged.
Compliance with Ethical Standards
Conflict of interestYu Chen,Suhao Qiu,Chen Wang,Xiaowei Li,Yaohui Tang,and Yuan Feng declare that they have no conflict of interest.
Human and animal rights and informed consentAll of the animal procedures and protocols were approved by the Institutional Animal Care and Use Committee of the Shanghai Jiao Tong University and all institutional and national guidelines for the care and use of laboratory animals were followed.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License,which permits use,sharing,adaptation,distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s)and the source,provide a link to the Creative Commons licence,and indicate if changes were made.The images or other third party material in this article are included in the article's Creative Commons licence,unless indicated otherwise in a credit line to the material.If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use,you will need to obtain permission directly from the copyright holder.To view a copy of this licence,visit http://creativecommons.org/licenses/by/4.0/.
- Biophysics Reports的其它文章
- The emerging role of tubulin posttranslational modifications in cilia and ciliopathies
- ‘‘At last in’’the physiological roles of the tubular ER network
- Long-term high-resolution in vivo imaging of cerebral cortical structures following ischemic stroke
- Prediction of RNA secondary structure with pseudoknots using coupled deep neural networks
- Epigenetic regulation of covalently closed circular DNA minichromosome in hepatitis B virus infection

