虫害诱导植物信息化合物介导的植物间交流及机制
姚诚诚 杜立啸 李云河
摘要 :当遭受植食性昆虫取食时,植物会释放复杂和多样的挥发性有机化合物。虫害诱导的挥发物在调控植物与不同营养层昆虫群落相互关系中发挥重要作用。同时,邻近植物也能感知这种虫害诱导挥发物,对可能即将到来的虫害威胁做出防御准备。获得防御准备的植物在受到虫害损伤时,会立即启动相应的防御反应。为了应对植物的这种害虫防御策略,某些昆虫可通过调控植物挥发物信号来传递“假信息”,从而抑制相邻植物的防御反应。这种信息交流既可发生在同种植物之间,也可发生在不同科属的植物之间。近年,植物间的信息交流现象已得到广泛研究,但相关的生化和分子机制及生态学意义尚不清楚。本文主要探讨了虫害诱导信息化合物介导的植物间交流类型、机制和生态学意义,以及影响植物间交流的生物或非生物因子,分析了利用植物间信息交流开展害虫绿色防控的前景。
关键词 :虫害诱导的植物挥发物; 植物与植物交流; 植物与昆虫互作
中图分类号:
Q948.1, S433
文献标识码: A
DOI: 10.16688/j.zwbh.2020427
Plant-to-plant communications medicated by herbivore-induced plant volatiles and the mechanisms
YAO Chengcheng, DU Lixiao*, LI Yunhe*
(State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection,
Chinese Academy of Agricultural Sciences, Beijing 100193, China)
Abstract
Plants, when being attacked by herbivores, will constitutively emit a wide variety of volatile organic compounds, called herbivore-induced plant volatiles (HIPVs), which plays a key role in mediating the interactions between plants and diverse trophic levels of insects.In addition, neighboring plants can perceive these distress signals from herbivore-attacked plants and prepare well for incoming threats.Plants that have primed their defenses can immediately activate corresponding defensive responses once they were damaged by herbivorous insects.Conversely, some insects may manipulate their host plants to emit volatiles that disseminate the deceptive information to neighboring plants, which will suppress their defenses.The phenomenon of HIPVs-medicated plant-plant communication occurs among the plants of the same or different families and genera.Although the concept of this communication has been widely accepted, its biochemical and molecular mechanisms and ecological significances remain largely obscure.In this overview, we reviewed the types, mechanisms and significances of plant-plant communications mediated by HIPVs, and we focused on both ecological factors affecting plant-plant communications and the prospects for using volatiles to control pests based on plant-plant communications.
Key words
herbivore-induced plant volatiles; plant-plant communication; plant-insect interaction
當遭受植食性昆虫取食时,植物会产生一系列防御反应。一方面,植物会产生有毒化合物、抗营养酶类和抗消化酶类等物质直接毒杀或抑制植食性昆虫生长发育;另一方面,植物会释放挥发性化合物吸引捕食性或寄生性天敌来防御害虫[12]。此外,相邻植株也能感知这种虫害诱导植物挥发物(herbivore-induced plant volatiles, HIPVs),相邻植株接收HIPVs后做出的反应主要有两类:一类是直接激活自身的防御反应[35];另一类是不启动防御反应,而是“武装”自己,在受到植食性昆虫为害时,可启动更强或更快的防御反应[2, 58]。
虫害诱导植物挥发物介导的植物间交流现象最早发现于柳树中[9]。天幕毛虫Malacosoma californicum为害的柳树Salix sitchensis诱使其邻近的健康柳树植株对天幕毛虫的抗性增强。这一报道引起了研究者对植物间交流现象的广泛关注,相关报道日益增多[1014]。此外,也有研究发现,某些植食性昆虫会利用虫害诱导植物挥发物抑制相邻植物的防御反应,从而为种内其他昆虫创造更加有利的生存环境[1516]。本文通过探讨虫害诱导植物挥发物介导的植物间交流类型、机制和生态学意义,以及影响植物间交流的生物和非生物因子,分析利用植物间信息交流现象开展害虫绿色防控的前景,以期为该领域的研究者提供一定的启发和参考。
1 HIPVs介导的植物间交流
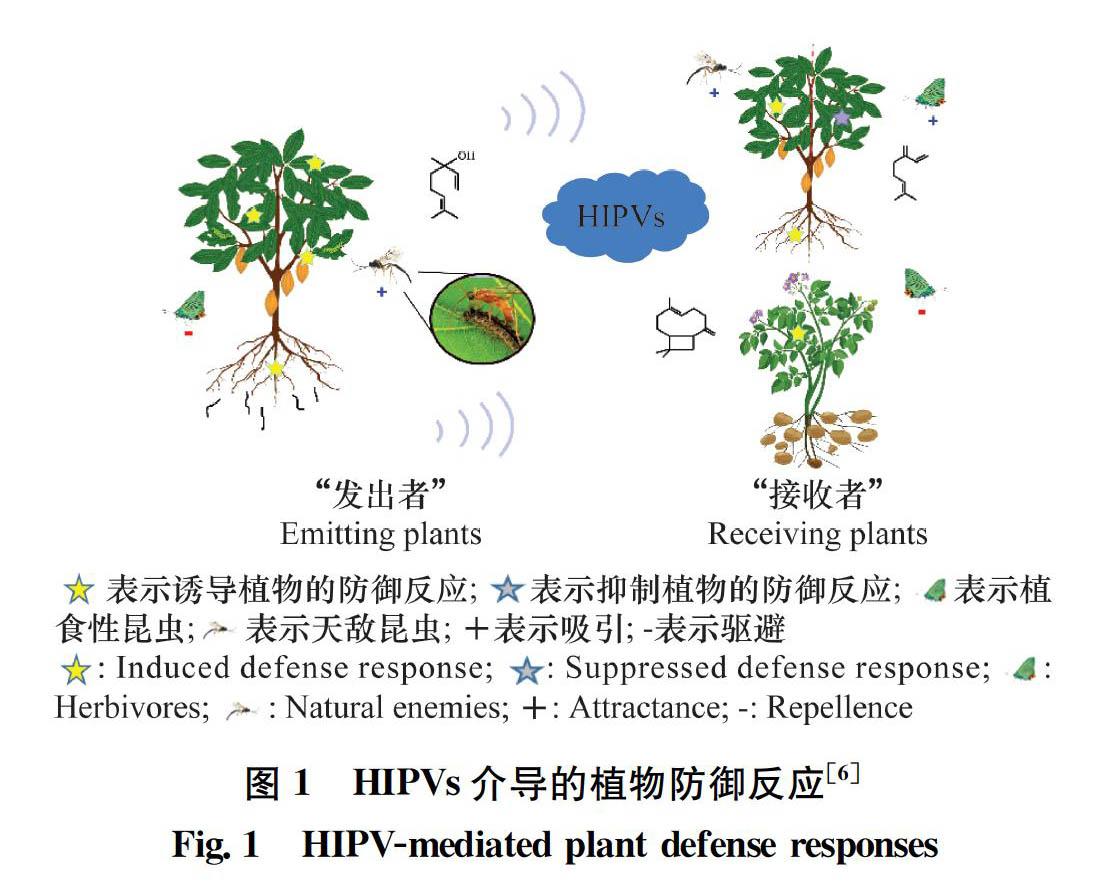
在自然界中,植物间交流的现象普遍存在[7, 1721]。当遭到植食性昆虫为害时,植物会释放HIPVs吸引害虫天敌或驱避成虫产卵,减轻危害;同时,邻近同种或异种植物能够感知这种HIPVs,对可能即将到来的虫害威胁进行预判,做出防御准备,提高对害虫的防御能力。然而,某些昆虫会利用植物的这种特性,调控植物向邻近植物传递“假信息”,抑制邻近植物的防御反应(图1)。下文对HIPVs调控邻近植物防御反应的两种类型分别进行阐述。
1.1 HIPVs诱导植物的防御反应
1.1.1 HIPVs诱导同种植物间的防御反应
目前,关于HIPVs诱导植物间防御反应的研究主要集中在同种植物之间[19]。研究发现,二斑叶螨Tetranychus urticae为害的棉花Gossypium hirsutum释放的植物挥发物会降低邻近棉花上二斑叶螨的落卵量[22];大菜粉蝶Pieris brassicae为害的甘蓝Brassica oleracea var.capitata释放的植物挥发物增强了邻近甘蓝对大菜粉蝶的抗性,同时还吸引菜粉蝶寄生蜂粉蝶盘绒茧蜂Cotesia glomerata[10];黏虫Mythimna separata为害的玉米Zea mays L.cv.Royal Dent释放的植物挥发物会导致邻近玉米上的黏虫生长发育受阻[11]。由于相同的遗传背景,同种植物在应对虫害时的防御反应一致,识别危险信号分子上也存在保守性,因此,在同种植物间利用HIPVs进行交流的效率更高[45, 23]。被植食性昆虫为害的植物释放HIPVs来调整邻近植物的状态,一方面干扰害虫的行为选择,来减轻对自身的为害;另一方面利用植物群体防御来提高物种自身的稳定性[45]。但是同种物种之间也存在着激烈的生存竞争,植物受到虫害后,释放HIPVs有自身防御的需要,但这种信号被邻近植物收到,是有意为之,还是邻近植物“窃听”的结果,目前尚无定论。
1.1.2 HIPVs诱导异种植物间的防御反应
与同种植物间的交流相比,HIPVs诱导异种植物防御反应的研究很少。研究显示,海灰翅夜蛾Spodoptera littoralis为害的棉花释放的植物挥发物可提高邻近紫苜蓿Medicago sativa的防御水平,导致海灰翅夜蛾在苜蓿上的产卵量下降[24]。这种跨越物种的交流现象,究竟是不同物种抵御相同害虫的需要还是自然进化过程中群体选择的需要,目前还存在争议[5]。
1.2 HIPVs抑制植物的防御反应
虽然多数研究表明,HIPVs可诱导邻近植物对植食性昆虫产生抗性,但最近的研究发现,昆虫也可通过调控植物挥发物,诱使邻近植物更适合昆虫的发育。譬如,烟粉虱Bemisia tabaci为害的番茄Lycopersicon esculentum cv.Moneymaker释放的植物挥发物不但没有诱导邻近番茄对烟粉虱的抗性,反而使其更适合烟粉虱取食[16]。小菜蛾Plutella xylostella为害的甘蓝释放的植物挥发物同样未能增强邻近甘蓝的抗虫性,反而增加了小菜蛾在邻近甘蓝上的落卵量[15]。
我们推测在长期的进化过程中,昆虫适应了寄主植物的防御反应,利用植物间交流的“漏洞”,通过调控受害植物的HIPVs向邻近植物传递“假”信号抑制邻近植物的防御反应,为其后代提供更有利的生存环境。有关HIPVs抑制邻近植物防御反应的现象还知之甚少,而HIPVs是否会抑制邻近异种植物防御反应目前也未见报道。
2 HIPVs调控植物防御反应的机制
研究者通过对“发出者(emitting plants)”释放的挥发物进行实时检测,以及对“接收者(receiving plants)”接收到化学信号后所做出的反应进行了研究,发现虫害诱导植物挥发物在植物间交流中发挥着媒介作用(表1),因此,这种虫害诱导挥发物常常被称为“植物语言(plant language)”[2530]。
2.1 HIPVs诱导邻近植物或同一植物不同组织防御反应的机制
HIPVs诱导邻近植物的防御反应包括直接防御和间接防御。直接防御是邻近植物接收到HIPVs信号后,通过诱导防御基因表达,调控植物激素和产生防御相关次生代谢物质等一系列过程,使植物对植食性昆虫产生直接的生理抗性[34, 3133];间接防御是被HIPVs诱导的邻近植物通过分泌花外蜜露(extrafloral nectars, EFNs)或释放挥发性化合物吸引捕食性或寄生性天敌昆虫,间接地降低植食性昆虫的危害[3438]。
2.1.1 HIPVs诱导邻近植物的直接防御机制
甘薯Ipomoea batatas遭到海灰翅夜蛾取食后迅速釋放出(E)-4,8-dimethyl-1,3,7-nonatriene (DMNT),DMNT诱导邻近甘薯中蛋白酶抑制剂(sporamin protease inhibitor, SPI)基因的表达上调,使叶片中胰蛋白酶抑制剂的含量提高,进而增强了邻近甘薯对海灰翅夜蛾的抗性[33]。欧洲桤木Alnus glutinosa受到蓝毛臀萤叶甲Agelastica alni为害后释放的DMNT、β-ocimene等会诱导邻近桤木蛋白酶抑制剂和酚类物质的活性提高[39];β-罗勒烯(β-ocimene)能够诱导邻近健康大白菜Brassica rapa var.glabra水杨酸和茉莉酸相关防御基因上调表达,提高芥子油苷的含量,从而抑制桃蚜Myzus persicae的生长发育,改变其取食行为[40]。这说明HIPVs作为植物间交流的信号分子可以激活植物防御反应,提高植物抗虫性。此外,邻近植物还可以直接利用HIPVs合成具有防御功能的化合物,抑制植食性昆虫生长发育。例如,番茄被斜纹夜蛾Spodoptera litura为害后释放出大量的(Z)-3-己烯醇[(Z)-3-hexenol],邻近番茄吸收(Z)-3-hexenol后会将其转化成(Z)-3-hexenylvicianoside,导致斜纹夜蛾生长发育受阻,成活率下降[41]。
研究表明,HIPVs诱导邻近植物防御反应主要受植物激素的调控,其中茉莉酸(jasmonic acid, JA)、茉莉酸异亮氨酸(jasmonoyl-isoleucine, JA-Ile)、水杨酸(salicylic acid, SA)和脱落酸(abscisic acid, ABA)等被证实在植物防御过程中发挥着重要调控作用[42]。例如,吲哚(indole)能够诱导邻近水稻Oryza sativa中JA途径相关防御基因的表达,以及12-oxophytodienoic acid (OPDA)和JA的累积量增加,增强水稻对草地贪夜蛾Spodoptera frugiperda幼虫的抗性[32];同时,吲哚还能够诱导邻近玉米中JA-Ile和ABA的累积量上升,进而增加绿叶挥发物和萜烯类化合物的释放量,增强玉米对海灰翅夜蛾幼虫的防御[12]。此外,无论是专食性害虫烟草天蛾Manduca sexta还是多食性害虫烟芽夜蛾Heliothis virescens为害本生烟Nicotiana benthamiana后植物释放的HIPVs均能够诱导邻近植物的防御准备;对邻近植物采用机械的方式损伤并涂抹任意一种害虫的口腔分泌物均会显著增加本生烟中JA含量,调控本生烟防御性挥发物的代谢过程,增加HIPVs的释放[43]。但也有研究表明,HIPVs诱导邻近植物的抗性反应独立于植物激素代谢通路。比如,海灰翅夜蛾幼虫为害的甘薯会诱导邻近甘薯叶片中胰蛋白酶抑制剂含量升高,增强对海灰翅夜蛾幼虫的抗性,但并未引起邻近叶片中JA的累积[33]。同时Sugimoto等[41]证实番茄吸收邻近虫害植株释放的(Z)-3-hexenol,将其转化成对斜纹夜蛾生长发育不利的(Z)-3-hexenylvicianoside,但植株中JA的含量并没有显著升高。
2.1.2 HIPVs诱导邻近植物的间接防御机制
HIPVs除了诱导邻近植物产生直接防御反应,也会诱导邻近植物释放挥发性有机化合物(volatile organic compounds,VOCs)吸引天敌而间接地防御植食性昆虫。番茄被海灰翅夜蛾为害后释放的植物挥发物诱导邻近番茄释放β-ocimene等物质吸引阿尔蚜茧蜂Aphidius ervi,降低海灰翅夜蛾的为害[14]。玉米受到斑禾草螟Chilo partellus (Swinhoe)为害后释放的植物挥发物会诱导邻近玉米释放DMNT吸引卵寄生蜂布氏赤眼蜂Trichogramma bournieri和幼虫寄生蜂大螟盘绒茧蜂Cotesia sesamiae [13]。海灰翅夜蛾为害的玉米释放的植物挥发物能够诱导邻近玉米释放芳香类和萜类化合物提高对缘腹绒茧蜂Cotesia marginiventris的引诱作用[44]。
此外,研究还发现,HIPVs会促进邻近植物分泌花外蜜露吸引天敌。例如,虫害诱导利马豆Phaseolus lunatus释放的(3Z)-hex-3-enyl acetate能够诱导邻近利马豆分泌花外蜜露吸引捕食性和寄生性天敌,降低甲虫和象甲等植食性昆虫的为害[35]。
2.1.3 HIPVs诱导同一植物邻近健康组织的防御机制
HIPVs能够诱导植物损伤部位的防御,同时还可以警告邻近组织做好应对虫害的准备。当遭到植食性昆虫取食时,已经接收到HIPVs的健康组织会立即启动防御反应降低害虫为害[3, 4547]。例如,被虫侵害的欧美杂交杨Populus deltoides × nigra叶片释放的萜烯化合物E-β-ocimene、DMNT、β-caryophyllene、germacrene D、α-farnesene均能够显著提高邻近叶片中萜烯化合物含量,增强杨树对舞毒蛾Lymantria dispar幼虫的抗性[48]。被虫侵害的玉米叶片释放的吲哚可以显著提高邻近叶片中萜烯类化合物含量[12]。对于植物自身而言,当某些部位不可避免要受到植食性昆虫为害时,它们就可能会释放HIPVs信号,克服长距离和维管束的限制,快速到达邻近组织警告威胁的存在,以便快速启动全身的防御系统;对于受害组织来说,释放HIPVs也是诱导受害组织启动防御反应抵御虫害的需要[45]。
2.2 HIPVs抑制邻近植物防御反应的机制
除了诱导邻近植物对植食性昆虫的防御,某些HIPVs还会充当植物防御抑制剂,抑制植物对害虫的防御反应[42]。比如,烟粉虱为害番茄释放的β-myrcene、ρ-cymene、β-caryophyllene能够显著提高邻近番茄中SA含量,诱导抗性基因PR-1a和PR-1b的上调表达,而抑制JA通路相关的抗性基因PⅠ-Ⅰ和PⅠ-Ⅱ的表达,进而使邻近番茄更容易受到烟粉虱的侵害[16]。田间试验也发现,田间释放绿叶挥发物(green leaf volatiles; GLVs)、(Z)-3-hexenal、(Z)-3-hexenol、(E)-2-hexenal和(Z)-3-hexenyl acetate,尽管可以提高玉米植株萜烯类化合物的释放,但并未降低植食性昆虫对玉米的为害,反而造成玉米受蟲害加重[49]。
3 影响HIPVs介导植物间交流的因子
HIPVs介导的植物间交流受到各种生物或非生物因素的影响,例如植物间的距离、空气流通程度、物种间亲缘关系、物种特异性等都会影响它们之间交流的效率。
3.1 距离
植物间交流的效率会随着植物间距离的增加而降低[51]。在同一浓度同一剂量下,野生烟草Nicotiana attenuata距离修剪的三齿蒿Artemisia tridentata 10 cm或15 cm之内时,受到害虫为害的程度会明显减低,然而随着距离增加,这种效应会逐渐降低[5556]。同种三齿蒿植株之间也能进行交流[57],这种交流能达到60 cm[58],利马豆间能达到50 cm[47],然而超过该阈值,植物间交流的效率会逐渐降低直至消失。研究表明植物接收HIPVs信号分子与细胞膜去极化和细胞质Ca2+浓度变化有关。而通过不同浓度的α-pinene 和β-caryophyllene处理番茄发现,高浓度化合物处理下番茄细胞膜去极化程度更高,这说明植物间交流存在浓度效应[52]。因此,随着植物间距离的增加,HIPVs由虫害植物传递到邻近植物的量必然会大大降低,它们交流的效率也会下降甚至消失。
3.2 空气流通
对于植物的地上部分来说,空气流通是植物间信号交流的必要因素[55, 5860]。机械损伤玉米叶片并涂抹海灰翅夜蛾口腔分泌物一段时间后,损伤邻近叶片并收集挥发物发现,萜烯类化合物含量显著增加;而当用聚四氟乙烯袋封闭虫害叶片,阻断虫害叶片和邻近叶片的空气流通时,同样时间段内在相同处理的邻近叶片中未发现萜烯类化合物含量的改变[12]。
3.3 亲缘关系
亲缘关系近的植物在信息化合物交流中可能会有额外的优势,这一优势被称为亲属选择假说(Kin-Selection Hypothesis)[61]。Karban等[23]认为来自亲属的挥发物信号可能更容易被同类所察觉。通过对两种不同品系玉米的研究发现,健康玉米暴露在同一品系玉米释放的经虫害诱导的挥发物中,对寄生蜂的吸引性更强[13]。这一现象在发草Deschampsia cespitosa[62]、拟南芥Arabidopsis thaliana[63]和三齿蒿[23, 59, 64]等植物中都有报道。
研究发现,某些植物只能和部分邻近植物进行交流,而对其他植物无反应,植物间的交流存在着物种特异性。譬如,海灰翅夜蛾为害的棉花植株释放出的挥发物增加了邻近棉花和苜蓿植物的抗虫性,降低了海灰翅夜蛾的产卵量;然而海灰翅夜蛾为害的苜蓿和埃及车轴草Trifolium alexandrinum植株释放的挥发物并没有增加邻近棉花的抗虫性[24]。玉米遭到海灰翅夜蛾为害后可以迅速地释放吲哚,显著增强邻近玉米的抗虫性,轻微地诱导邻近棉花的抗虫性,而没有诱导邻近豇豆Vigna unguiculata的抗虫性[12]。由此可见,植物能够特异性识别邻近植物释放的挥发物。因此,根据植物间交流的物种特异性,我们可以利用特定植物的挥发物防御害虫,实现绿色防控。
3.4 植食性昆虫
植食性昆虫也是驱动HIPVs调控植物间信息传递的重要因子。北美一枝黄花Solidago altissima被黄花叶甲虫Trirhabda virgate为害后释放的HIPVs能够激发邻近相同甚至不同基因型的植物提高对甲虫的防御水平;而采用未经甲虫诱导的挥发物处理植物,只有邻近相同基因型的植物会启动同等防御,不同基因型的植物没有表现同样的反应[17]。这一结果说明植食性昆虫会驱动植物进化出更开放的交流机制。
此外,植物个体的发育程度、植物暴露挥发物的时间和浓度、植食性昆虫为害的时间和强度以及气候变化等生物或非生物因素都影响着植物与植物交流的效率[15, 32, 6566]。
4 展望
在HIPVs介導的植物间交流中,HIPVs的释放和传递通常对“接收者”是有益的,但是对于“发出者”产生了一种耗能代价[6768]。提高邻近植物的抗性可能改变了“发出者”和“接收者”的竞争性平衡,这种平衡对“发出者”表现不利,但帮助“接收者”成功地降低了害虫的攻击[69]。植物之间互相竞争营养等资源,而植物间的交流为“接收者”提供了可靠的信息,但对“发出者”没有表现出明显的优势,为什么植物会浪费能量帮助自己的竞争对手去应对即将来临的威胁[70]?目前普遍认为,HIPVs的“发出者”可能并非是对“接收者”有意图的警告,更可能是“接收者”的窃听行为[19, 69, 7172]。因此,在未来的研究中,我们既要评估“发出者”和“接收者”的适应性,也要比较二者对资源的需求度,充分理解二者之间的关系,才能更好地利用HIPVs介导植物间的交流。
目前对植物间交流的探究多集中在实验室研究阶段,且多数研究使用人工合成的挥发物组分模拟HIPVs的释放,导致挥发物处理浓度远远高于HIPVs的真实释放量,由此得出的结果是否能够代表自然界中存在的真实情况还有待进一步的验证[45]。这也是目前限制HIPVs调控植物间信息传递研究继续深入的关键。因此,研究者应该更加关注自然界中植物间的交流,特别是自然界中HIPVs的浓度,这对未来利用HIPVs进行害虫的绿色防控至关重要。此外,邻近植物识别HIPVs的过程对于理解植物间交流至关重要,在植物体内是否存在类似昆虫嗅觉识别系统的神经调控网络,还不得而知[4];在接收到HIPVs后,植物如何利用这些化学信号来调控自身的防御系统,究竟是直接利用化合物进行调控还是这些化合物仅作为调控信号仍缺乏深入研究[45]。
近年来,对植物间交流的研究逐渐增多,利用植物间信息交流开展害虫绿色防控具有非常广阔的前景。比如,基于相邻植物间交流的理论,种植哨兵植物预先察觉即将到来的威胁,警告邻近植物做好防御准备[2, 73];或利用植物间信息交流中的生物活性挥发物组分,通过基因修饰(转基因植物)增加植物对昆虫的敏感性,快速释放关键活性挥发物警告邻近植物[74]。目前农业生产上采用的相邻植物间“推拉”调控策略效果显著,通过选择一种或多种驱避(推)害虫的植物以及吸引(拉)它们取食的植物,使被保护作物免受侵扰[2, 73, 75]。
除HIPVs介导植物间的交流外,病原菌诱导的植物挥发物(pathogen-induced plant volatiles; PIPVs)[7679]及机械损伤诱导的植物挥发物(wound-induced plant volatiles; WIPVs)[5658, 8083]也会介导植物间的交流,诱导邻近植物产生抗病性或抗虫性。因此,我们还可以采取一些机械方法在害虫为害之前预防并降低其为害,比如,对植株采取机械性修剪[59]、去顶[84]以及人为的干扰触动[85],一方面降低害虫对植物的为害,另一方面也诱导了其邻近植物的抗虫性。
此外,未受胁迫的植物释放的组成型挥发物也能够介导植物间的交流。健康洋葱Allium cepa释放的挥发物会改变邻近马铃薯Solanum tuberosum挥发物的释放模式,驱避桃蚜Myzus persicae[86]。我们推测,植物之间会相互竞争资源,“接收者”通过获取未受胁迫的“发出者”挥发物中的重要组分,从而提高自身的适应性。
参考文献
[1] POELMAN E H, DICKE M. Plant-mediated interactions among insects within a community ecological perspective [M]∥Annual plant reviews 47: insect-plant interactions. New York: Wiley, 2014: 309338.
[2] TURLINGS T C J, ERB M. Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential [J]. Annual Review of Entomology, 2018, 63(1): 433452.
[3] HEIL M, BUENO J C S. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature [J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(13): 54675472.
[4] HEIL M. Herbivore-induced plant volatiles: targets, perception and unanswered questions [J]. New Phytologist, 2014, 204(2): 297306.
[5] KARBAN R, YANG L H, ESWARDS K F. Volatile communication between plants that affects herbivory: a meta-analysis [J]. Ecology Letters, 2014, 17(1): 4452.
[6] DAS A, LEE S H, HYUN T K, et al. Plant volatiles as method of communication [J]. Plant Biotechnology Reports, 2013, 7(1): 926.
[7] DELORY B M, DELAPLACE P, FAUCONNIER M L, et al. Root-emitted volatile organic compounds: can they mediate belowground plant-plant interactions? [J]. Plant Soil, 2016, 402(1/2): 126.
[8] 王杰, 宋圓圆, 胡林, 等. 植物抗虫“防御警备”:概念、机理与应用[J]. 应用生态学报, 2018, 29(6): 20682078.
[9] RHOADES D F. Responses of alder and willow to attack by tent caterpillars and webworms: evidence for pheromonal sensitivity of willows [J]. American Chemical Society Symposium Series, 1983, 208: 5568.
[10]PENG J, VAN LOON J J A, ZHENG S, et al. Herbivore-induced volatiles of cabbage (Brassica oleracea) prime defence responses in neighbouring intact plants [J]. Plant Biology, 2011, 13(2): 276284.
[11]ALI M, SUGIMOTO K, RAMADAN A, et al. Memory of plant communications for priming anti-herbivore responses [J/OL]. Scientific Reports, 2013, 3: 1872. DOI: 10. 1038/srep01872.
[12]ERB M, VEYRAT N, ROBERT C A M, et al. Indole is an essential herbivore-induced volatile priming signal in maize [J/OL]. Nature Communications, 2015, 6: 6273. DOI: 10. 1038/ncomms7273.
[13]MUTYAMBAI D M, BRUCE T J A, BERG J V D, et al. An indirect defence trait mediated through egg-induced maize volatiles from neighbouring plants [J/OL]. PLoS ONE, 2016, 11(7): e0158744. DOI: 10. 1371/journal. pone. 0158744.
[14]COPPOLA M, CASCONE P, MADONNA V, et al. Plant-to-plant communication triggered by systemin primes anti-herbivore resistance in tomato [J/OL]. Scientific Reports, 2017, 7(1): 15522. DOI: 10. 1038/s41598-017-15481-8.
[15]LI Tao, BLANDE J D. Associational susceptibility in broccoli: mediated by plant volatiles, impeded by ozone [J]. Global Change Biology, 2015, 21(5): 19932004.
[16]ZHANG Pengjun, WEI Jianing, ZHAO Chan, et al. Airborne host-plant manipulation by whiteflies via an inducible blend of plant volatiles [J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(15): 73877396.
[17]KALSKE A, SHIOJIRI K, UESUGI A, et al. Insect herbivory selects for volatile-mediated plant-plant communication [J]. Current Biology, 2019, 29(18): 31283133.
[18]BALDWIN I T, HALITSCHKE R, PASCHOLD A, et al. Volatile signaling in plant-plant interactions: "talking trees" in the genomics era [J]. Science, 2006, 311(5762): 812815.
[19]KARBAN R. Plant behaviour and communication [J]. Ecology Letters, 2008, 11(7): 727739.
[20]MOREIRA X, ABDALA-ROBERTS L. Specificity and context-dependency of plant-plant communication in response to insect herbivory [J]. Current Opinion in Insect Science, 2019, 32: 1521.
[21]張苏芳, 张真, 王鸿斌, 等. 植物防御的新发现:植物植物相互交流[J]. 植物生态学报, 2013, 36(10): 11201124.
[22]BRUIN J, DICKE M, SABELIS M W. Plants are better protected against spider-mites after exposure to volatiles from infested conspecifics [J]. Experientia, 1992, 48(5): 525529.
[23]KARBAN A, SHIOJIRI K, ISHIZAKI S, et al. Kin recognition affects plant communication and defence [J/OL]. Proceedings of the Royal Society B: Biological Sciences, 2013, 280(1756): 20123062. DOI: 10.1098/rspb.2012.3062.
[24]ZAKIR A, SADEK M M, BENGTSSON M, et al. Herbivore-induced plant volatiles provide associational resistance against an ovipositing herbivore [J]. Journal of Ecology, 2013, 101(2): 410417.
[25]DICKE M, VAN LOON J J A. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context [J]. Entomologia Experimentalis et Applicata, 2000, 97(3): 237249.
[26]DICKE M, BALDWIN I T. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’ [J]. Trends Plant Science, 2010, 15(3): 167175.
[27]HOLOPAINEN J K, BLANDE J D. Molecular plant volatile communication [J]. Advances in Experimental Medicine and Biology, 2012, 739: 1731.
[28]MESCHER M C, DE MORAES C M. Role of plant sensory perception in plant-animal interactions [J]. Journal of Experimental Botany, 2015, 66(2): 425433.
[29]SIMPRAGA M, TAKABAYASHI J, HOLOPAINEN J K. Language of plants: Where is the word? [J]. Journal of Integrative Plant Biology, 2016, 58(4): 343349.
[30]孫仲享, 宋圆圆, 曾任森. 植物挥发物介导的种内与种间关系研究进展[J]. 华南农业大学学报, 2019, 40(5): 166174.
[31]NAGASHIMA A, HIGAKI T, KOEDUKA T, et al. Transcriptional regulators involved in responses to volatile organic compounds in plants [J]. Journal of Biological Chemistry, 2019, 294(7): 22562266.
[32]YE Meng, GLAUSER G, LOU Yonggen, et al. Molecular dissection of early defense signaling underlying volatile-mediated defense regulation and herbivore resistance in rice [J]. Plant Cell, 2019, 31(3): 687698.
[33]MEENTS A K, CHEN S P, REICHELT M, et al. Volatile DMNT systemically induces jasmonate-independent direct anti-herbivore defense in leaves of sweet potato (Ipomoea batatas) plants [J/OL]. Scientific Reports, 2019, 9(1): 17431. DOI: 10.1038/s41598019539460.
[34]KESSLER A, BALDWIN I T. Defensive function of herbivore-induced plant volatile emissions in nature [J]. Science, 2001, 291(5511): 21412144.
[35]KOST C, HEIL M. Herbivore-induced plant volatiles induce an indirect defence in neighbouring plants [J]. Journal of Ecology, 2006, 94(3): 619628.
[36]HEIL M. Indirect defence via tritrophic interactions [J]. New Phytologist, 2008, 178(1): 4161.
[37]HARE J D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects [J]. Annual Review of Entomology, 2011, 56(1): 161180.
[38]LI Tao, HOLOPAINEN J K, KOKKO H, et al. Herbivore-induced aspen volatiles temporally regulate two different indirect defences in neighbouring plants [J]. Functional Ecology, 2012, 26(5): 11761185.
[39]TSCHARNTKE T, THIESSEN S, DOLCH R, et al. Herbivory, induced resistance, and interplant signal transfer in Alnus glutinosa [J]. Biochemical Systematics & Ecology, 2001, 29(10): 10251047.
[40]KANG Zhiwei, LIU Fanghua, ZHANG Zhanfeng, et al. Volatile β-ocimene can regulate developmental performance of peach aphid Myzus persicae through activation of defense responses in Chinese cabbage Brassica pekinensis [J/OL]. Frontiers in Plant Science, 2018, 9: 708. DOI: 10. 3389/fpls. 2018. 00708.
[41]SUGIMOTO K, MATSUI K, IIJIMA Y, et al. Intake and transformation to a glycoside of (Z)-3-hexenol from infested neighbors reveals a mode of plant odor reception and defense [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(19): 71447149.
[42]ERB M. Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling [J]. Current Opinion in Plant Biology, 2018, 44: 117121.
[43]TIMILSENA B P, SEIDL-ADAMS I, TUMLINSON J H. Herbivore-specific plant volatiles prime neighboring plants for nonspecific defense responses [J]. Plant, Cell & Environment, 2019, 43(3): 787800.
[44]TON J, D’ALESSANDRO M, JOURDIE V, et al. Priming by airborne signals boosts direct and indirect resistance in maize [J]. Plant Journal, 2006, 49(1): 1626.
[45]GERSHENZON J. Plant volatiles carry both public and private messages [J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(13): 52575258.
[46]HEIL M, TON J. Long-distance signalling in plant defence [J]. Trends in Plant Science, 2008, 13(6): 264272.
[47]HEIL M, ADAME-ALVAREZ R M. Short signalling distances make plant communication a soliloquy [J]. Biology Letters, 2010, 6: 843845.
[48]FROST C J, APPEL H M, CARLSON J E, et al. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores [J]. Ecology Letters, 2007, 10(6): 490498.
[49]VON MEREY G, VEYRAT N, MAHUKU G, et al. Dispensing synthetic green leaf volatiles in maize fields increases the release of sesquiterpenes by the plants, but has little effect on the attraction of pest and beneficial insects [J]. Phytochemistry, 2011, 72(14/15): 18381847.
[50]ENGELBERTH J, ALBORN H T, SCHMELZ E A, et al. Airborne signals prime plants against insect herbivore attack [J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(6): 17811785.
[51]FROST C J, MESCHER M C, DERVINIS C, et al. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate [J]. New Phytologist, 2008, 180(3): 722734.
[52]ZEBELO S A, MATSUI K, OZAWA R, et al. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication [J]. Plant Science, 2012, 196: 93100.
[53]JING Tingting, DU Wenkai, GAO Ting, et al. Herbivore-induced DMNT catalyzed by CYP82D47 plays an important role in the induction of JA-dependent herbivore resistance of neighboring tea plants [J]. Plant, Cell & Environment, 2021,44(4):11781191.
[54]SU Qi, YANG Fengbo, ZHANG Qinghe, et al. Defence priming in tomato by the green leaf volatile (Z)-3-hexenol reduces whitefly transmission of a plant virus [J]. Plant, Cell & Environment, 2020, 43(11): 27972811.
[55]KARBAN R, BALDWIN I T, BAXTER K J, et al. Communication between plants: induced resistance in wild tobacco plants following clipping of neighboring sagebrush [J]. Oecologia, 2000, 125(1): 6671.
[56]KARBAN R, MARON J, FELTON G W, et al. Herbivore damage to sagebrush induces resistance in wild tobacco: evidence for eavesdropping between plants [J]. OIKOS, 2003, 100(2): 325332.
[57]KARBAN R, HUNTZINGER M, MCCALL A C. The specificity of eavesdropping on sagebrush by other plants [J]. Ecology, 2004, 85(7): 18461852.
[58]KARBAN R, SHIOJIRI K, MCCALL H A C. Damage-induced resistance in sagebrush: volatiles are key to intra- and interplant communication [J]. Ecology, 2006, 87(4): 922930.
[59]KARBAN R, SHIOJIRI K, ISHIZAKI S. An air transfer experiment confirms the role of volatile cues in communication between plants [J]. American Naturalist, 2010, 176(3): 381384.
[60]PEARSE I S, HUGHES K, SHIOJIRI K, et al. Interplant volatile signaling in willows: revisiting the original talking trees [J]. Oecologia, 2013, 172(3): 869875.
[61]KOBAYASHI Y, YAMAMURA N. Evolution of signal emission by uninfested plants to help nearby infested relatives [J]. Evolutionary Ecology, 2007, 21(3): 281294.
[62]SEMCHENKO M, SAAR S, LEPIK A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes [J]. New Phytologist, 2014, 204(3): 631637.
[63]BIEDRZYCKI M L, JILANY T A, DUDLEY SA, et al. Root exudates mediate kin recognition in plants [J]. Communicative & Integrative Biology, 2010, 3(1): 2835.
[64]KARBAN R, SHIOJIRI K. Self-recognition affects plant communication and defense [J]. Ecology Letters, 2009, 12(6): 502506.
[65]SHIOJIRI K, KARBAN R. Plant age, communication, and resistance to herbivores: young sagebrush plants are better emitters and receivers [J]. Oecologia, 2006, 149(2): 214220.
[66]GIRON-CALVA P S, MOLINA-TORRES J, HEIL M, et al. Volatile dose and exposure time impact perception in neighboring plants [J]. Journal of Chemical Ecology, 2012, 38(2): 226228.
[67]STRAUSS S Y, RUDGERS J A, LAU J A, et al. Direct and ecological costs of resistance to herbivory [J]. Trends in Ecology & Evolution, 2002, 17(6): 278285.
[68]HOBALLAH M E, KLLNER T G, DEGENHARDT J, et al. Costs of induced volatile production in maize [J]. OIKOS, 2004, 105(1): 168180.
[69]HEIL M, KARBAN R. Explaining evolution of plant communication by airborne signals [J]. Trends in Ecology & Evolution, 2010, 25(3): 137144.
[70]NINKOVIC V, MARKOVIC D, DAHLIN I. Decoding neighbour volatiles in preparation for future competition and implications for tritrophic interactions [J]. Perspectives in Plant Ecology Evolution and Systematics, 2016, 23: 1117.
[71]KARBAN R, MARON J. The fitness consequences of interspecific eavesdropping between plants [J]. Ecology, 2002, 83(5): 12091213.
[72]BALDWIN I T, KESSLER A, HALITSCHKE R. Volatile signaling in plant-plant-herbivore interactions: what is real? [J]. Current Opinion in Plant Biology, 2002, 5(4): 351354.
[73]PICKETT J A, KHAN Z R. Plant volatile-mediated signalling and its application in agriculture: successes and challenges [J]. New Phytologist, 2016, 212(4): 856870.
[74]ARIMURA G I, MUROI A, NISHIHARA M. Plant-plant-plant communications, mediated by (E)-β-ocimene emitted from transgenic tobacco plants, prime indirect defense responses of lima beans [J]. Journal of Plant Interactions, 2012, 7(3): 193196.
[75]PICKETT J A, WOODCOCK C M, MIDEGA C A, et al. Push-pull farming systems [J]. Current Opinion in Biotechnology, 2014, 26: 125132.
[76]SHULAEV V, SILVERMAN P, RASKIN I, et al. Airborne signalling by methyl salicylate in plant pathogen resistance [J]. Nature, 1997, 385(6618): 718721.
[77]KYUTARO K, KENJI M, RIKA O, et al. Volatile C6-aldehydes and allo-ocimene activate defense genes and induce resistance against Botrytis cinerea in Arabidopsis thaliana [J]. Plant & Cell Physiology, 2005, 46(7): 10931102.
[78]YI H S, HEIL M, ADAME-ALVAREZ R M, et al. Airborne induction and priming of plant defenses against a bacterial pathogen [J]. Plant Physiology, 2009, 151(4): 21522161.
[79]PIESIK D, LEMNCZYK G, SKOCZEK A, et al. Fusarium infection in maize: volatile induction of infected and neighboring uninfected plants has the potential to attract a pest cereal leaf beetle, Oulema melanopus [J]. Journal of Plant Physiology, 2011, 168(13): 15341542.
[80]FARMER E E, RYAN C A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves [J]. Proceedings of the National Academy of Sciences of the United States of America, 1990, 87(19): 77137716.
[81]DOLCH R, TSCHARNTKE T. Defoliation of alders (Alnus glutinosa) affects herbivory by leaf beetles on undamaged neighbours [J]. Oecologia, 2000, 125(4): 504511.
[82]KARBAN R. Communication between sagebrush and wild tobacco in the field [J]. Biochemical Systematics & Ecology, 2001, 29(10): 9951005.
[83]KIKUTA Y, UEDA H, NAKAYAMA K, et al. Specific regulation of pyrethrin biosynthesis in Chrysanthemum cinerariaefolium by a blend of volatiles emitted from artificially damaged conspecific plants [J]. Plant & Cell Physiology, 2011, 52(3): 588596.
[84]RENOU A, TRTA I, TOGOLA M. Manual topping decreases bollworm infestations in cotton cultivation in Mali [J]. Crop Protection, 2011, 30(10): 13701375.
[85]MARKOVIC D, COLZI I, TAITI C, et al. Airborne signals synchronize the defenses of neighboring plants in response to touch [J]. Journal of Experimental Botany, 2019, 70(2): 691700.
[86]NINKOVIC V, DAHLIN I, VUCETIC A, et al. Volatile exchange between undamaged plants-a new mechanism affecting insect orientation in intercropping [J/OL]. PLoS ONE, 2013, 8(7): e69431. DOI: 10. 1371/journal. pone. 0069431.
(責任编辑:杨明丽)

