Reevaluation of the Holotype of Odorrana schmackeri Boettger,1892(Amphibia:Anura:Ranidae) and Characterization of One Cryptic Species in O.schmackeri sensu lato through Integrative Approaches
Huijun SHEN,Yanjun ZHU,Zhen LI,Zhuo CHEN and Xiaohong CHEN
College of Life Sciences,Henan Normal University,Xinxiang 453007,Henan,China
Abstract The Odorrana schmackeri sensu lato is a group of cascade frogs that are widely distributed in southern China.However,their taxonomy has long been unresolved and several cryptic species have been reported in the past decade.Previous analyses revealed the existence of three sympatric species (i.e.,O.schmackeri,O.hejiangensis and O.sp2) at the type locality of O.schmackeri (Gaojiayan Town,Yichang City,Hubei Province,China).However,the lack of an original description for the holotype specimen (SMF6241) of O.schmackeri poses a confusing and controversial problem in species nomination within this group.Here,we reevaluate the taxonomic affinities of SMF6241 and examine the distinctness of O.sp2 based on specimens from the type locality.We use integrative approaches that combine morphology with 10 microsatellite loci and bioacoustics.Canonical discriminant analysis of morphological characteristics revealed three distinct groupings (i.e.,O.schmackeri,O.hejiangensis and O.sp2) and attributed SMF6241 to O.schmackeri.Genetic clustering analysis of nuclear DNA also identified three corresponding clusters.In addition,analysis of interspecific variation in morphometrics and acoustics showed divergence between O.sp2 and its sympatric congeners.All lines of evidence from morphological,molecular and bioacoustic approaches suggest the species status of O.sp2,namely Odorrana ichangensis Chen,sp.nov. Overall,this study resolves the taxonomic assignment of the holotype of O.schmackeri and provides an example for species delimitation using integrative approaches.
Keywords integrative taxonomy,new species,Odorrana ichangensis Chen,sp.nov.,Odorrana schmackeri sensu lato,reevaluation
1.Introduction
The two fundamental tasks of taxonomy are species delimitation and classification (Sauer and Hausdorf,2011;Boury-Esnaultet al.,2013).The odorous frogs belong to the genusOdorranaand they inhabit montane streams throughout subtropical or tropical regions of East and Southeast Asia,ranging across southern China,Japan,northeastern India and Southeast Asian countries (Feiet al.,2012; Frost,2020).This genus represents a highly diverse species group containing 58 recognized species,and more than 20 new species have been described in the past decade (Phamet al.,2016; Frost,2020).However,the phylogenetic relationships and species validity within this genus have long been controversial due to limited and incomplete original description,insufficient type material and lost holotype specimens (Hallowell,1861; Boettger,1892;Matsui,1994; Bainet al.,2003; Kurabayashiet al.,2010; Chenet al.,2013; Rujirawanet al.,2018; Chenet al.,2020).
Odorrana schmackeri(Boettger,1892) was erected based on only one male specimen SMF6241 (=1054,2a) from Gaojiayan Town(=Kao-cha-hien) of Yichang (Ichang) City,Hubei Province in central China (Boettger,1892).However,the lack of original descriptions,diagnostic characters,pictures and etymological meaning of the type specimen limited our knowledge of this species for many years (Supplementary Figure S1).Liu and Hu(1961) provided a relatively comprehensive description ofO.schmackeribased on 9 specimens from the area near the type locality (i.e.,Wushan and Xiushan County in Chongqing City),and it was accepted universally.Due to similar morphology and color patterns,theO.schmackerisensu lato was formerly regarded as a single,widespread species in southern China over a long time (Feiet al.,1990; Feiet al.,2012).However,the results of comparative morphological and molecular studies revealed substantial species-level morphological and genetic variation among geographic populations,suggesting thatO.schmackeriwas paraphyletic with several discrete species,forming “theO.schmackerispecies complex” (Chenet al.,2013; Zhu,2016;Heet al.,2017; Liet al.,2017).Four other distinctive species,i.e.,O.hejiangensis,O.huanggangensis,O.tianmuiiandO.kweichowensis,have been described in this complex(Deng and Yu,1992; Chenet al.,2010a; Chenet al.,2010b; Liet al.,2018).
Using 1021 samples from 85 sites across 15 provinces (or municipalities) of China,a mtDNA-based phylogeographic study (Zhu,2016) demonstrated thattheO.schmackerisensu lato was paraphyletic and included six independent clades (Clade A-F):O.schmackeri(Clade A),O.kweichowensis(Clade B; named as “O.sp1” by Zhu),O.hejiangensis(Clade C),O.huanggangensis(Clade E),O.tianmuii(Clade F) and one cryptic species (Clade D; i.e.,O.sp2).The mtDNA topology suggested the monophyly of all samples ofO.sp2 and a sister-group relationship toO.hejiangensiswith strong support.The sister-group relationship betweenO.schmackeriandO.kweichowensisand betweenO.huanggangensisandO.tianmuiiwerealso well supported.The fine geographical distribution ranges of the species in this complex were also revealed by Zhu (2016) and several other studies (Heet al.,2017; Liet al.,2017; Wanget al.,2019),and the results were different from previous studies (Feiet al.,2009; Feiet al.,2012).However,due to the lack of morphological,acoustic and nuclear evidence,the validity of the cryptic speciesO.sp2 remains unresolved.In addition,field surveys indicated that three species of this complex (i.e.,O.schmackeri,O.hejiangensisandO.sp2)were sympatrically distributed at the type locality (Gaojiayan Town,Hubei Province) ofO.schmackeri(Figure 1).Furthermore,the affinity of the holotype (SMF6241) ofO.schmackerito which of the three sympatric species is still unknown.
Here,we used morphological data and performed canonical discriminant analysis to address the taxonomic attribution of the holotype (SMF6241) ofO.schmackeri.We also used integrative data of morphological characteristics,ten nuclear loci and male advertisement calls to test the taxonomic validity of the cryptic speciesO.sp2.Furthermore,we provided the taxonomic descriptions of the new species.
2.Materials and methods
2.1.SamplingSpecimens and tissues used in this study were collected from field surveys,and the majority of them were kept in the Amphibian and Reptile Laboratory of Henan Normal University.A total of 190 adult specimens were collected from eight localities (2-144 samples per site; Supplementary Table S1).Specifically,specimens ofO.sp2 (51♂,31♀) were from the eight sites,while specimens ofO.schmackeri(31♂,23♀) andO.hejiangensis(33♂,21♀) were all from Gaojiayan Town,Hubei Province (i.e.,the type locality).All the samples were measured for morphological analysis,and 92 of them were used for molecular analysis.
2.2.Morphological measurementsAll measurements were made with a digital caliper to the nearest 0.1 mm precision from preserved adult specimens.The following 24 morphometric measurements were taken:snout-vent length (SVL; length from tip of snout to vent); head length (HL; distance between angle of jaws and snout-tip); head width (HW; width of head at level of maxilla articulation); snout length (SL; length from the front of eye to the tip of snout); nostril-snout tip distance (NSD; distance from anterior margin of nostril to snout tip); nostril-eye length(NEL; distance between anterior corner of eye and nostril center) ; internarial distance (IND; distance between central points of nostrils); interorbital distance (IOD; shortest distance between medial edges of eyeballs in dorsal view); IFE (distance between anterior corner of eyes); IAE (distance between posterior corner of eyes); eye diameter (ED; eye diameter from anterior to posterior corner); maximum width of upper eyelid(UEW; maximum distance between medial edge of eyeball and lateral edge of upper eyelid); tympanum diameter (TD;maximum diameter of tympanum); tympanum-eye distance(TED; distance from anterior edge of tympanum to posterior corner of the eye); length of lower arm and hand (LAHL);lower arm diameter (LAD; maximum diameter of lower arm);hand length (HAL; length from elbow to the tip of third finger);length of hind leg (HLL; length from vent to tip of the longest toe); tibia length (TL; length from outer edge of flexed knee to heel); tibia width (TW; maximum width of tibia); foot and tibiotarsus length (FTL; length from tibiotarsal joint to end of fourth toe); foot length (FL; length from the base of inner metatarsal tubercle to the tip of fourth toe); outer metatarsal tubercle length (OMTL; maximum length of outer metatarsal tubercle); inner metatarsal tubercle width (IMTW; maximum width of inner metatarsal tubercle) (see Feiet al.,2009).We measured the above 24 morphological variables of collected specimens ofO.schmackeri,O.hejiangensisandO.sp2.We also obtained 18 variables (SVL,HL,HW,SL,NSD,NEL,IOD,ED,UEW,TD,TED,LAHL,HAL,TL,TW,FTL,FL and OMTL)of the holotype specimen (SMF6241) preserved at the Muséum national d’ Histoire naturelle,Paris.For the holotype of the new species (HNNU1807I139),another two traits (FPW,finger disk width at the widest part of the pad of finger III and DPW,width of distal phalanx of finger III) were also measured in addition to the above 24.For comparisons,morphological data forO.schmackeriandO.hejiangensiswere based on specimens in this study and the original descriptions from Liu and Hu(1961) and Deng and Yu (1992).The morphological data ofO.kweichowensis,O.tianmuiiandO.huanggangensiswere taken from Liet al.(2018) and Chenet al.(2010a,b).
2.3.Microsatellite loci genotyping and genetic structureNinety-two individuals from Gaojiayan Town were used in the molecular analysis (48O.schmackeri,21O.hejiangensisand 23O.sp2).Total genomic DNA was extracted using a standard phenol/chloroform procedure (Sambrook and Russell,2001).A panel of ten microsatellite markers (Table 1) was used (Gaoet al.,2013; Qiaoet al.,2015).Genotypes for the microsatellite markers were conducted following Qiaoet al.(2015).Micro-Checker v.2.2.3 (Van Oosterhoutet al.,2004) was used to remove genotyping errors as the presence of null alleles for each microsatellite locus.Gene pop on web 4.0 was used to test Hardy-Weinberg equilibrium and linkage disequilibrium between each pair of loci (Raymond and Rousset,1995).Observed (HO) heterozygosity,expected (HE) heterozygosity,the polymorphism information content (PIC),and number of alleles per microsatellite locus were calculated using Cervus 2.0(Kalinowskiet al.,2007).
We used the Bayesian-based genetic clustering program STRUCTURE 2.3 to infer the number of clusters and estimate admixture among these sympatric lineages based on microsatellite data (Pritchardet al.,2000).A correlated allele frequency model allowing for admixture of individuals was assumed.Independent runs were performed by setting the number of clusters (K) from 1 to 10.Each run comprised 200 000 burn-in iterations,followed by 1 200 000 generations of the Monte Carlo Markov Chain (MCMC) to estimate LnP (D).The successive changes of LnP (D) values and the delta K value were used to assess the optimal K value (Evannoet al.,2005; Earl and Vonholdt,2012).
2.4.Acoustic analysisAs a key role in reproductive isolation(Hoskin and Higgie,2010; Wilkinset al.,2013),bioacoustic analysis is currently recommended as a powerful tool for anuran species delimitation (Lacerdaet al.,2011; Alström and Ranft,2013; Köhleret al.,2017).We recorded advertisement calls of twoO.schmackeriand fiveO.sp2 separately on July 21 and 26,2018 at Gaojiayan Town,Hubei Province.Calls of one individual ofO.hejiangensiswere recorded on July 8,2018 in Wanyuan City,Sichuan Province.The local air temperature and humidity were measured with a digital thermo-hygrometer to the nearest 0.1°C.
Male calls were recorded with a Marantz PMD661MKIII digital recorder connected to a Sennheiser ME 66 directional microphone capsule and a Sennheiser K6 powering module in wave format files with a sampling rate of 44.1 kHz and 16-bit resolution.The microphone was placed approximately 0.5-1 m away from the calling frogs (Köhleret al.,2017).
Calls were analyzed using the sound analysis software Raven Lite 2.0 (Cornell Laboratory of Ornithology,Ithaca,NY,USA) and Adobe Audition 3.0 (Adobe Systems,San Jose,CA,USA).Spectral and temporal variables were measured in a single call.Spectral measurements were taken through fast Fourier transform set at a length of 512 points.Sound figures were obtained using PRAAT software (Boersma,2001).
2.5.Statistical analysisA canonical discriminant analysis(CDA) was performed to discriminate species and predict group membership of SMF6241,as in other cases (Parsons and Jones,2000; Polaszeket al.,2010; Xionget al.,2015).We first carried out the CDA based on 24 morphological variables of measured individuals in males and females,respectively.Then,we performed the CDA again on the 18 variables from SMF6241 and all male individuals used above.In the CDA,canonical functions can be derived to compute the squared Mahalanobis distance between individuals and group centroids (Cerqueira and Lemos,2000).The classification of SMF6241 was then allowed to be determined by the smallest squared Mahalanobis distance.The statistical significance of discrimination was determined by Wilk’s test.Figures were plotted using the ggplot2 package in R version 3.2.2 (Wickham,2009).
To examine the morphological intraspecific variance and screen for useful variables,we ran one-way analysis of variance(ANOVA) and pair-wise multiple comparisons by Dunn’s test.Size differences between males and females were tested by the analysis of covariance (ANCOVA) with SVL as covariate.Datawere log-transformed for these statistical analyses.However,mean values (± standard deviation) and ranges of variations are presented as non-transformed data.Inter-species differences in acoustic parameters were evaluated using a Kruskal-Wallis ANOVA on Ranks adjusted by Bonferroni-Holm.All statistical analyses were performed using SPSS software (Version 25.0,SPSS Inc,Chicago,IL,USA).
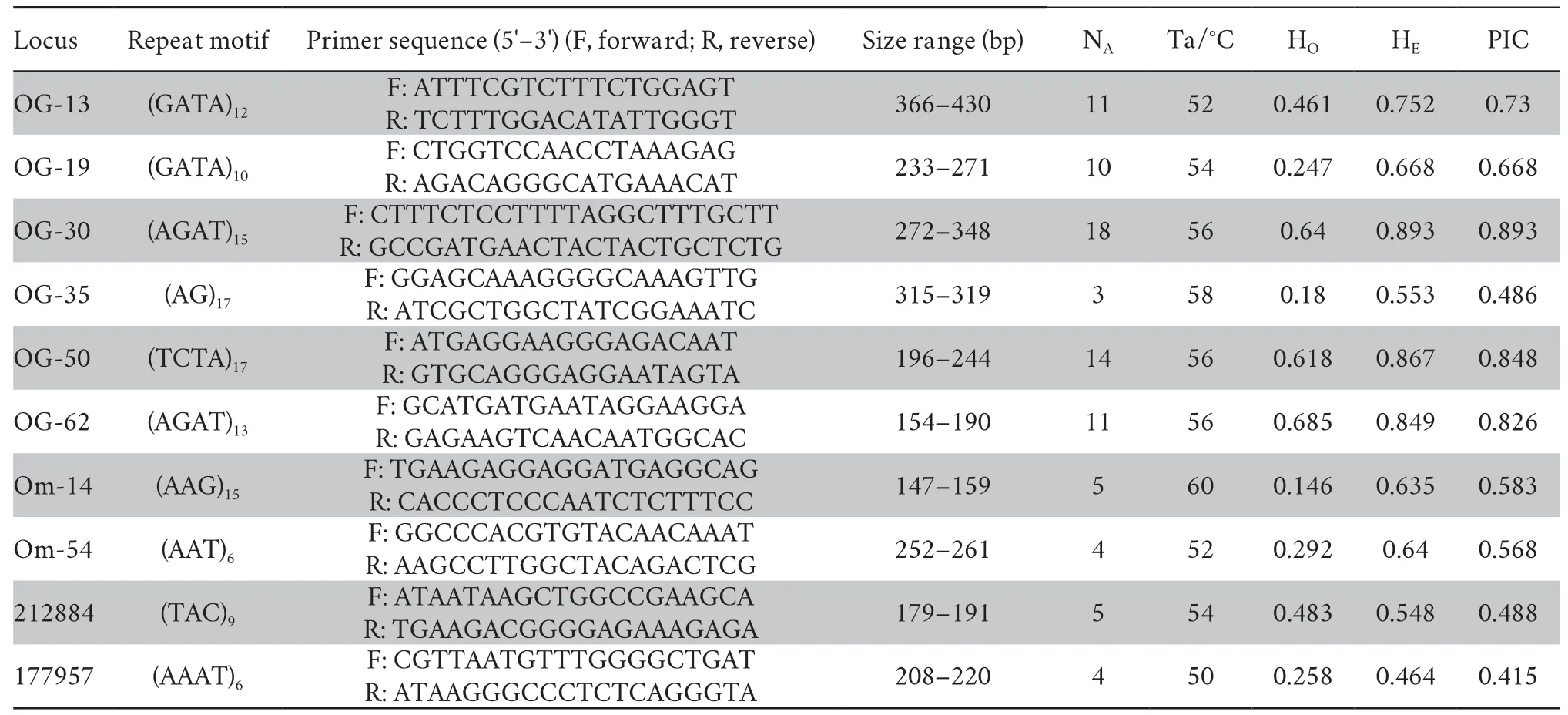
Table 1 Characterization of 10 microsatellite markers for O.schmackeri,O.hejiangensis and O. sp2.
3.Results
3.1.MorphologyThe canonical discriminant function based on 24 morphometric characters significantly (allP<0.05; Supplementary Table S2) differentiated all individuals(witha priorispecies determination) into a specific group (i.e.,100% accurate classification rate in raw data),corresponding toO.schmackeri,O.hejiangensisorO.sp2 for both males and females (Figures 2A,B).The leave-one-out cross-validation tests were further performed to evaluate the accuracy,and the classification accuracy was 91.7% in males and 77.6% in females,respectively.The CAN1 (explaining 87.9% and 77.1% of the whole variance in males and females,respectively) suggested the differentiation ofO.hejiangensisfromO.schmackeriandO.sp2,mainly contributed by TL and TD in males,and SL and SVL in females,respectively (Supplementary Table S2).The CAN2(explaining 12.1% and 22.9% of the whole variance in males and females,respectively) differentiatedO.schmackeriandO.sp2,largely based on HW and FL in males,and NSD and FTL in females (Supplementary Table S2).
The CDA,utilizing 18 morphometric variables from SMF6241(male) and the above male samples,was also conducted to reevaluate the taxonomic attribution of SMF6241.The discriminant functions yielded significant Wilks’λ(P<0.001 for two axes; Supplementary Table S3),and the accurate classification rate was 98.8% in raw data and 95.2% in leaveone-out cross-validation,respectively.The plot of SMF6241 was grouped withO.schmackeriand greatly varied from the other two groups (Figure 2C).The squared Mahalanobis distances from SMF6241 toO.schmackeri,toO.sp2,andtoO.hejiangensiswere 10.209,30.809 and 97.481,respectively.
Furthermore,we used univariate assessments to explore the morphological differences betweenO.sp2,O.schmackeriandO.hejiangensis(Table 2).The morphological descriptive statistics for each measurement are shown in Supplementary Table S4.For males,O.sp2 was extremely different fromO.hejiangensisforall the 24 characters with nearly allP< 0.05 in the ANOVA,whereas only nine traits (SVL,HW,SL,NEL,IFE,TD,HLL,TL and TW) betweenO.sp2 andO.schmackeriweresignificantly different.For females,however,less morphological variance among species was present than in males,and it varied among species pairs.TheO.sp2 can be distinguished fromO.hejiangensisin 10 characters (SVL,HW,IAE,TED,LAHL,HAL,HLL,TL,FTL and FL) withP< 0.05,while it diverged fromO.schmackeriin 8 traits (IOD,IFE,IAE,HAL,HLL,FTL,FL and OMTL).Between sexes,the difference was significant in all the three species with females being larger than males in both body size and body shape (ANOVA,P< 0.001; Figure 1;Supplementary Table S5).However,O.sp2 exhibited distinct sexual dimorphism both in magnitude and direction.AlthoughO.sp2 had the largest ratio (1.9) of female SVL to male SVL thanO.schmackeri(1.8) andO.hejiangensis(1.4),it showed the fewest size-corrected traits which were significantly different between sexes than the other two species.Only seven traits (i.e.,IOD,LAHL,HAL,HLL,TL,FTL and FL) in females were found to be significantly larger than in males inO.sp2 (ANOVA,P< 0.05; Supplementary Table S5),whereas more traits were significantly different between sexes inO.schmackeri(i.e.,HW,TD,TED,LAHL,HAL,HLL,TL,FTL,FL and OMTL) andO.hejiangensis(i.e.,HW,IOD,ED,TED,LAD,LAHL,HAL,HLL,TL,FTL,FL,TW and IMTW).In addition,O.sp2 displayed reversals of sexual dimorphism in head shape and lower arm diameter,but the differences were not statistically significant(Supplementary Table S5).
3.2.Genetic structure with microsatellite DNAAccording toΔK,the best-supported number of clusters in STRUCTURE was three (ΔK=3; Figure 3).As shown in Figure 3,the entire population was divided into three genetic clusters.Nearly all the individuals ofO.schmackeri(N=48),O.hejiangensis(N=21) andO.sp2 (N=23) were assigned to one of the three homogenous gene pools(q> 0.95; 1=pure cluster).Only one individual ofO.sp2 showed a slightly lowerqvalue of 0.94.
3.3.VocalizationCall parameter statistics are shown in Table 3.We recorded and analyzed the spontaneous vocalizations from five males ofO.sp2,two males ofO.schmackeriand one male ofO.hejiangensis,respectively.Male advertisement calls of these species were short and discontinuously separated by long silent intervals and were reminiscent of a bird’s call“qiu,qiu”.The oscillograms and spectrograms of calls showed species-specific patterns (Figure 4).Male calls ofO.sp2 andO.schmackeriwere composed of 1-2 short single-pulsed notes with 4frequency bands.In contrast,O.hejiangensisproduced both short calls (Figure 4) and long calls (not shown here).The short calls ofO.hejiangensisincluded3-4 pulses with energy in 2 frequency bands,and the long calls were composed of pulse series.
In acoustic features,O.sp2 was distinct from its congeners(Table 3).The average note duration ofO.sp2 (mean 0.053 ±0.018 s,rang 0.017-0.100 s,N=77) was prominent shorter than both ofO.schmackeri (mean 0.078 ± 0.145 s,rang 0.053-0.111s,N=21) andO.hejiangensis(mean 0.121 ± 0.016 s for short calls,rang 0.102-0.145 s,N=12; Kruskal-Wallis test,bothP< 0.001).The second harmonic was the dominant frequency (DF) in all the three species.The DF ofO.sp2 (3.679 ± 0.460 kHz,N=77) was significantly lower thanO.schmackeri(3.965 ± 0.272 kHz,N=21; Kruskal-Wallis test,P=0.009) but much higher than that ofO.hejiangensis(2.807 ± 0.045 kHz,N=12; Kruskal-Wallis test,P< 0.001).Significant differences in fundamental frequency (FF)were found only betweenO.sp2 andO.schmackeri(P=0.001).In addition,the average maximum frequency (MAXF) ofO.sp2 (7.806 ± 0.360 kHz,ranging 7.231-8.913 kHz,N=30) was lower than that ofO.schmackeri(8.474 ± 0.509 kHz,ranging 7.786-9.336 kHz,N=16; Kruskal-Wallis test,P=0.003),but much higher than that ofO.hejiangensis(Kruskal-Wallis test,P< 0.001).
In brief,integrative evidence of morphology,molecules(mitochondrial and nuclear makers) and acoustics suggested the species status ofO.sp2,and we proposed and describedO.sp2 asOdorrana ichangensisChen,sp.nov.
3.4.Species Accounts
Odorrana ichangensis Chen,sp.nov.(Figures 1A,5-7; Table 4)
HolotypeHNNU1807I139,an adult male (Figures 1A; 5A,B,E),collected by H.J.SHEN,M.Y.XU and C.KE on 24 July 2018 from Gaojiayan Town (N30°38′45.6″,E110°57′7.2″; 323 m elevation),Changyang Co.,Yichang City,Hubei Prov.,China.
AllotypeHNNU1807I155,an adult female (Figures 1A; 5C,D,E),collected simultaneously and syntopically with the holotype(HNNU1807I139).

Figure 2 Plots of discriminant scores of O.schmackeri,O.hejiangensis,O. sp2 and SMF6241 based on morphometric characters:(A)males,(B) females and (C) males incorporating the holotype of O.schmackeri (SMF6241).Different species are denoted as different colors and shapes.
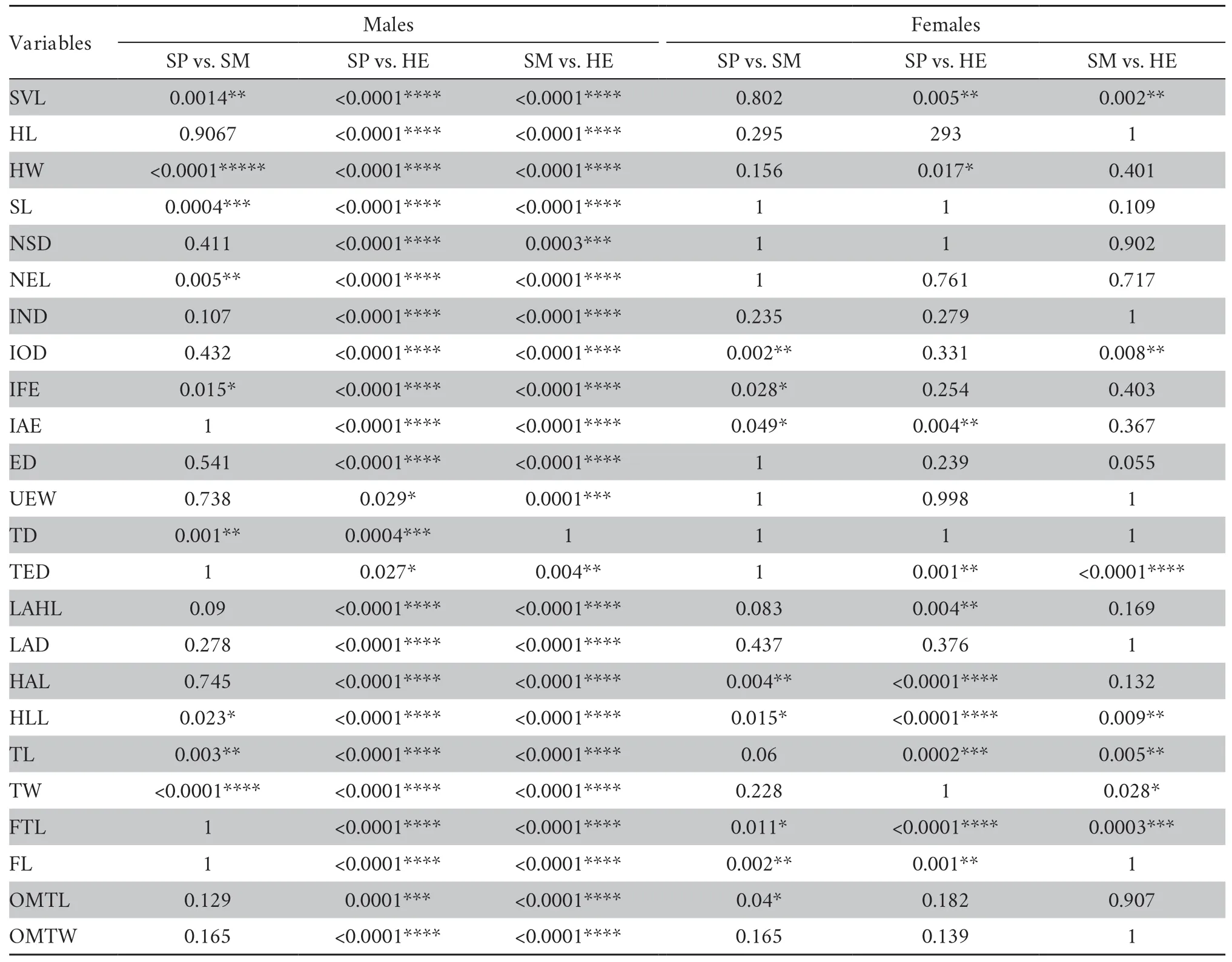
Table 2 Results of one-way ANOVA with corrected P values for differences in morphometric variables among O.schmackeri,O.hejiangensis and O. sp2,separated by sex.

Figure 3 Bayesian clustering analysis based on ten microsatellite loci using STRUCTURE.Each individual is represented by a vertical bar and grouped by population and species (A).Different species are denoted as different colors (Purple:O.hejiangensis;Green:O.schmackeri; Red:O. sp2.).The optimal K value is three (B).
ParatypesFifty male and thirty female adult specimens were collected by X.H.CHEN,L.QIAO,Y.Q.LU,Y.J.ZHU,Q.Q.FENG,L.M.CHANG,Y.K.WAN,H.J.SHEN,M.Y.XU and C.KE from 2009 to 2018 from eight localities in China (Supplementary Table S6).All the type specimens were deposited in the Herpetological Research Laboratory,College of Life Science,Henan Normal University.
DiagnosisOdorrana ichangensissp.nov.differs from its congeners by the following combination characters:(1) snoutvent length (SVL) of adult females approximately twice as long as males,82.4 ± 4.5 (72.4-93.0 mm,N=30) in females,42.9 ± 2.4(38.2-47.1 mm,N=50) in males; (2) head length greater than width in males,smaller than width in females; (3) tympanum large,greater than one-half diameter of the eye; (4) eyes big and prominent,width of upper eyelid (UEW) approximately 3/4 of eye diameter (ED); (5) dorsolateral fold absent; (6) legs long,tibiotarsal articulation reaching to nostril when leg stretched forward; (7) dorsum emerald green to yellow green in life,scattered irregular dark brownish spots; (8) lips with black spots; (9) dorsum of limbs with black-brown transverse bars;(10) disks on all digits with lateroventral grooves; (11) relative finger lengths:III>IV>II>I; (12) feet fully webbed; (13) males with paired subgular vocal sacs located at corners of throat;(14) pinkish lineae musculinae on dorsal side in males; (15) graywhite pads on dorsal surface of first finger in males; (16) white spinules on ventral surface of throat,chest,abdomen,limbs and upper and lower lips in males and females; (17) females having creamy yellow eggs without pigmentation,mean diameter of 2.3 mm (Figure 5F).
Description of holotypeBody miniaturized (SVL 38.5 mm);head length equal to width; snout obtusely pointed in profile,projecting beyond lower jaw; nostril lateral,distance to snout tip equal to eye; canthus rostralis distinct; loreal region concave and oblique; internasal distance larger than interorbital distance;tympanum-eye distance small (TED/TD=0.3); tympanum distinct,rounded,75% eye diameter,not depressed relative to skin of temporal region; tympanic rim elevated relative to tympanum; vomerine teeth well developed,on two oblique ridges,separated by a distance barely equal to length of one;tongue narrowly cordiform,deeply notched posteriorly.

Figure 4 Oscillograms (left) and spectrograms (right) of the advertisement calls from O.schmackeri (A,B; air temperature=26.2 °C;air humidity=99.9%),O.hejiangensis (C,D; air temperature=25.9 °C; air humidity=94.2%) and O. sp2 (E,F; air temperature=26.2°C; air humidity=96.4%).

Table 3 Acoustic parameters and P values in Kruskal-Wallis ANOVA on ranks adjusted by Bonferroni-Holm for repeated comparison of call traits among O.schmackeri,O.hejiangensis and O. sp2.Values are presented as mean ± standard deviation (SD) followed by range.

Table 4 Morphometric measurements (in mm) of the type series of O.ichangensis sp.nov.from eight populations across its distribution:mean± SD (range) and ratio of each measurement to the SVL (%).
Hand moderately long,hand length (HAL) 0.3 times of SVL;tips of fingers moderately dilated,becoming pointed,cordiform disks with lateroventral grooves on all fingers; width of finger III disc slightly larger than width of distal phalanx (FPW/DPW=1.1),approximately 55% the diameter of tympanum;fingers bearing narrow lateral fringes; relative finger length:III>IV>II>I; subarticular tubercles ovoid,markedly elevated and prominent:1,1,2,2; weak supernumerary tubercles below base of finger II,III and IV; inner metacarpal tubercle oval,elongated,larger than length of finger I; outer two metacarpal tubercle small,feebly prominent; fingers without web (Figures 5A,B).
Hind limbs comparatively robust and long,tibia length 63%of SVL; tibiotarsal articulation of the adpressed limb reaching to nostril; foot slightly shorter than tibia (FL/TL=0.9); relative toe lengths:IV>V≥III>II>I; tip of toe V extending to the distal edge of distal subarticular tubercle on toe IV; tarsal fold absent; tips of toes tapering dilated into narrow and long triangular disks;lateroventral grooves present; feet entirely webbed to disks; web becoming narrower and reaching the base of disk except toe IV webbed to distal subarticular tubercle; subarticular tubercles prominent,ovoid; inner metatarsal tubercle elongated; outer metatarsal tubercle absent; inner tarsal fold absent (Figures 5A,B).
Skin on dorsum slightly shagreened; dense granules on the entire dorsum and upper sides of limbs; small round tubercles on lores,temporal region,and posterior to tympanum; larger flat tubercles on flanks of trunk from back of eye to base of forelimb; no dorsolateral fold; two rictal glands; small white spinules on ventral surface of throat,chest,abdomen,limbs and upper and lower lips.
Color of holotype in lifeDorsum emerald green; small and irregular dark brownish spots on dorsal surface of head,back,limbs and flank; small and black spots on the margin of snout tip and lower jaw; dorsal surface of limbs emerald green with narrow,diffuse dark brown crossbars (4-5 strips on thigh),interspersed with small dark brown spots; tympanum brownish,center and margin yellow; pinkish lineae musculinae on dorsal side; venter ivory to yellowish-brown with diffuse brown markings on chest; brownish pigmentation on gular pouch.
Color of holotype in preservativeDorsum black-brown; side of head,supratympanic fold,upper and lower lip yellowishgray with small dark brown spots; flanks whitish-gray with fewer light brown spots and creamy-white flat tubercles; rictal glands creamy-white; nuptial pad whitish-gray; tympanum brown,margin dark brown; dorsal limbs with black-brown spots and banding; venter yellowish-gray with diffuse dark brown markings on gular pouch and chest.
Sexual dimorphismMales small (SVL,mean 42.9 ± 2.4 mm,range 38.2-47.1 mm),on average half of females (SVL,mean 82.4 ± 4.5 mm,range 72.4-93.0 mm),but obviously larger than females in the relative size of ED and TD (Table 4); nuptial pads whitish-gray develop on the inner aspect of the first fingers,paired subgular vocal sacs located at corners of throat,pinkish lineae musculinae on dorsal side in males.
Variation in the type seriesVariation in morphometric measurements of the type series from eight populations across its distribution range are listed in Table 4.Basically,the type series agrees with the holotype coloration,but also shows differences in color pattern and spots (Figures 6-7).The venter surface is grey white with light brown mottling on the lower jaw,throat,chest and upper abdomen in male HNNU1007I063(Figure 6F).The color of the dorsum surface is gray-brown in female HNNU1408II140 (Figure 7G).Large dark brown spots are present on the dorsum of the posterior body in female HNNU1507I304 (Figure 7D),and the lateral side of flanks formed networks in female HNNU1408II133 (Figure 7F).
EtymologyThe specific epithet “ichangensis” refers to the type locality of the new species as well asO.schmackeri,Yichang City,Hubei Province,China.The “ichang” is an old spelling and a transliteration for “Yichang”.The suggested common English name is the “Yichang Odorous Frog” for this species.
Distribution and ecologyOdorrana ichangensissp.nov.inhabits in mountain streams in western China,distributed in Qionglai Mountain,north of Dalou Mountain,Wushan and Daba Mountains,from the western of Sichuan Basin along its southern edge up to the northeastern edge,reaching the eastern edge of Wuling Mountain.
All specimens were collected at night on rocks and stream banks along slow flowing canyon streams in broadleaved forests at 246-1025 m elevations (Figure 8).Breeding season was approximately from late July to Mid-August.The amplexus and spawn behaviors were found on July 27,2018 at Changyang Co.,Hubei Prov.(N30°36′23.97″,E111°2′.58.8″,260-336 m elevations; Figure 1A),July 29,2015 at Xuyong Co.,Sichuan Prov.(662-747 m elevations),and August 16,2014 at Emei Mountain,Sichuan Province of China (611-672 m elevations).Adult female spawn 1300-1784 eggs once a time with a mean diameter of 2.29 mm.Mature eggs are creamy yellow,without pigment (Figure 5F).
ComparisonsOdorrana ichangensissp.nov.closely resemblesO.schmackeriinmorphology,and both were collected from Gaojiayan Town,Hubei Province.simultaneously and syntopically.However,O.ichangensissp.nov.differs fromO.schmackeriby having the dorsum green with small,irregular,dark brown spots (dorsum green with distinct,round,large light brown spots inO.schmackeri),having males with greater relative head length,head width and internarial distance than females,having supernumerary tubercles and lateral fringes on toe V (absent inO.schmackeri),absence of stripes on canthus rostralis (O.schmackeriwith narrow brown stripes on canthus rostralis,from tip of snout along margin of upper eyelid to posterior corner of upper eyelid),the absence of skin fold(present inO.schmackeri),and females with pigmentless eggs(eggs beige with black poles inO.schmackeri).

Figure 5 (A,B) Dorsolateral and ventral views of the holotype(HNNU1807I139,male).(C,D) Dorsolateral and ventral views of the allotype (HNNU1807I155,female).(E) Dorsolateral view of the amplexus of the holotype (upper) and allotype(lower).(F) Eggs of female O.ichangensis sp.nov.Photographs by H.J.SHEN.
Odorrana ichangensissp.nov.differs fromO.hejiangensisby having a greater ratio of female SVL to male SVL (1.9 inO.ichangensissp.nov.,1.4 inO.hejiangensis,males SVL 47.3-57.8 mm,mean 52.7 mm,females 67.9-81.5 mm,mean 75.9 mm),having males with greater relative head length,head width and internarial distance than females,having the dorsum green with narrow crossbars on limbs (anterior half of dorsum green,posterior half of dorsum brown with wide crossbars on limbs inO.hejiangensis),having small,irregular,dark brown spots on dorsum (distinct,round,large brown spots on dorsum,blending into shadow at rear of back),the absence of white spinules on dorsum (O.hejiangensiswith white spinules on surface of upper eyelid,limbs,tympanic region and rear of back; Deng and Yu,1992).

Figure 6 Variation of males in morphology and color patterns of O.ichangensis sp.nov.in life.(A,B) Dorsolateral views of HNNU1507I067 and HNNU1507I061 from Gufu Town,Xingshan County (Co.),Hubei Province (Prov.).(C)Dorsolateral view of HNNU1507I104 from Mawu Town,Shizhu County (Co.),Chongqing City.(D) Dorsolateral view of HNNU1507I275 from Shuiwei Town,Xuyong County(Co.),Sichuan Province (Prov.).(E,F) Dorsolateral view and ventral view of HNNU1007I063 respectively from Gaojiayan Town,Changyang County (Co.),Hubei Province (Prov.).(G) Dorsolateral view of HNNU1608II239 from Wenquan Town,Kaizhou District,Chongqing City.(H) Ventral view of HNNU1408II162 from Bifengxia Town,Yaan City,Sichuan Province (Prov.).Photographs by X.H.CHEN,L.QIAO,Y.J.ZHU and Q.Q.FENG.
Odorrana ichangensissp.nov.differs fromO.kweichowensis(Liet al.,2018) by the longer snout-vent length of females(O.ichangensissp.nov.72.4-93.0 mm,mean 82.4 mm;O.kweichowensisfemales SVL 62.4-81.1 mm,mean 73.6 mm),having three outer metacarpal tubercles (two outer metacarpal tubercles inO.kweichowensis),the second finger longer than the first (the second finger shorter than the first inO.kweichowensis),lower arm and hand shorter than a half SVL (lower arm and hand longer than a half of SVL inO.kweichowensis),tibiotarsal articulation of adpressed limb reaching nostril level (tibiotarsal articulation of adpressed limb reaching to the level between eye to nostril inO.kweichowensis).

Figure 7 Variation of females in morphology and color patterns of O.ichangensis sp.nov.in life.(A) Dorsolateral view of HNNU1608II147 from Jiucheng Town,Daozhen County(Co.),Guizhou Province (Prov.).(B) Dorsolateral view of HNNU1407II126 from Wenquan Town,Kai County (Co.),Chongqing City.(C) Dorsolateral view of HNNU1507I063 from Gufu Town,Xingshan County (Co.),Hubei Province(Prov.).(D,E) Dorsolateral views of HNNU1507I304 and HNNU1507I276 respectively from Shuiwei Town,Xuyong County (Co.),Sichuan Province (Prov.) (F-H) Dorsolateral views of HNNU1408II133 and HNNU1408II140 and ventral view of HNNU1408II155 respectively from Bifengxia Town,Yaan City,Sichuan Province (Prov.),China.Photographs by X.H.CHEN,L.QIAO,Y.J.ZHU and Q.Q.FENG.
Odorrana ichangensissp.nov.differs fromO.huanggangensis(Chenet al.,2010a) byhaving the dorsum green with small,irregular,dark brown spots (dorsum yellow-green with distinct,round,large brown spots inO.huanggangensis),toe IV webbed to distal subarticular tubercle (all toes webbed to base of disc inO.huanggangensis),females with a larger ratio of ED/SL (O.ichangensissp.nov.,0.58-0.86,mean 0.74;O.huanggangensis,0.52-0.63,mean 0.58),females with a lower ratio of TED/SVL(O.ichangensissp.nov.,0.03-0.05,mean 0.04;O.huanggangensis,mean 0.05),females with pigmentless eggs (eggs beige with black poles inO.huanggangensis) and the absence of stripes on canthus rostralis (narrow brown stripes on canthus rostralis,from tip of snout along margin of upper eyelid to posterior corner of upper eyelid inO.huanggangensis).

Figure 8 Habitats of O.ichangensis sp.nov.(A) Habitats in the type locality,Gaojiayan Town,Changyang County(Co.),Hubei Province (Prov.).(B) Habitats in Gufu Town,Xingshan County (Co.),Hubei Province (Prov.).(C) Habitats in Wenquan Town,Kai County (Co.),Chongqing City.(D) Ha bita ts in Mawu Town,Shizhu County (Co.),Chongqing City.(E)Habitats in Jiucheng Town,Daozhen County (Co.),Guizhou Province (Prov.).(F) Habitats in Shuiwei Town,Xuyong County (Co.),Sichuan Province(Prov.).(G) Habitats in Huangwan Town,Emeishan City,Sichuan Province (Prov.).(H) Habitats in Bifengxia Town,Yaan City (Co.),Sichuan Province (Prov.),China.Photographs by X.H.CHEN,L.QIAO,Y.J.ZHU and Q.Q.FENG.
Odorrana ichangensissp.nov.differs fromO.tianmuii(Chenet al.,2010b) by having the dorsum green with small,irregular,dark brown spots (dorsum yellow-green with distinct,brown spots inO.tianmuii),tibiotarsal articulation of adpressed limb reaching nostril level (tibiotarsal articulation of adpressed limb reaching to the level between eye to nostril inO.tianmuii),females with lower ratio of TD/ED (O.ichangensissp.nov.,0.42-0.64,mean 0.52;O.tianmuii,0.58-0.78,mean 0.63),females with pigmentless eggs (eggs beige with black poles inO.tianmuii)and the absence of stripes on canthus rostralis (narrow brown stripes on canthus rostralis,from tip of snout along margin of upper eyelid to posterior corner of upper eyelid inO.tianmuii).
4.Discussion
4.1.Taxonomic confirmation and species delimitationThe genusOdorranahas a very complicated history of systematics,and this is partly due to insufficient type series or unreliable published host records.For example,Rana nebulosawas erected from a juvenile specimen and the holotype specimen was lost (Hallowell,1861; Bainet al.,2003),O.sinicalacked definite type locality and detailed description (Ahl,1927 “1925”) andO.lividaused to be short of description of male specimens until Rujirawanet al.(2018) published a detailed description(Rujirawanet al.,2018,Chenet al.,2020).Odorranaichangensissp.nov.most closely resemblesO.schmackeri,and they co-distribute withO.hejiangensisat the type locality ofO.schmackeri(Gaojiayan Town,Hubei Province).Therefore,it is essential to reevaluate the species belonging of the SMF6241 before we describe the new species from the same type locality.Here,we uncovered the fine-scale divergence betweenO.ichangensissp.nov.and its sympatric speciesO.schmackeriandO.hejiangensisfrom Gaojiayan with multiple data sets.
Our results revealed marked differentiation in morphology betweenO.ichangensissp.nov.and its sympatric congeners.For example,O.ichangensissp.nov.was clearly separated from the other two species in the CDA.In addition,O.ichangensissp.nov.showed the opposite direction in sexual dimorphism in head shape and forelimb size,when compared to the other two species.Males ofO.ichangensissp.nov.seemed to have a relatively (size-corrected) larger head (e.g.,HL,HW and IND;Table S4,S5) than females,and the size-corrected LAD (i.e.,lower arm diameter) of females was unexpectedly larger than that of males.Bayesian clustering method (STRUCTURE)using microsatellite data further emphasized three different genetic clusters.In addition,O.ichangensissp.nov.also showed species-specific characteristics based on the bioacoustic analyses.As call signals are considered to be an important premating isolation mechanism for frogs (Leary,2001; Wells,2007),and STRUCTURE detected no heterospecific gene flow,we can expect that there is a complete reproductive barrier betweenichangensissp.nov.and its close relatives.Thus,the divergences revealed based on the nuclear gene,bioacoustics and morphology data support the validity of the new speciesO.ichangensissp.nov.This work represents an example to resolve the taxonomy of old museum specimens under historical taxonomic conflicts.It also highlights the need for the integrative framework to address species boundaries,especially when morphological evolution is conservative.
4.2.The association between morphological and ecological featuresOur results support the idea that ecological similarity weighed more with trait convergence than phylogenetic relatedness (Liuet al.,2013; Jianget al.,2018).Previous phylogenetic studies have revealed thatO.ichangensissp.nov.was more closely related toO.hejiangensis(with a Kimura 2-parameter distance of 3.9%) than toO.schmackeri(with a Kimura 2-parameter distance of 6.8%; Chenet al.,2013; Zhu,2016).However,O.ichangensissp.nov.closely resemblesO.schmackerithanO.hejiangensisinmorphometrics and color patterns (Figure 1).The convergent evolution of phenotypes has been identified associated with similar environments in amphibians (Moenet al.,2016) and reptiles (e.g.Anolislizards,Lososet al.,1998 and Mahleret al.,2013,andCryptoblepharuslizards,Blomet al.,2016).Odorrana hejiangensisalways inhabits on mountain rocky substrates such as cliffs and huge rocks near rushes and narrow rivers in closed and moist microhabitats(Deng and Yu,1992),whileO.ichangensissp.nov.andO.schmackeri(Liet al.,2017),showing similar habitat preferences,dwell on barer stones or rocks near slow and wide rivers in open and more arid environments (Figures 1,8).The pattern of interspecific variance in traits may suggest the important roles of divergent selective pressures in driving trait evolution in this group.Previous studies (Mcguiganet al.,2005; Conteet al.,2012; Moenet al.,2016; Laiolo,2017) suggested that similar environmental habitats could lead to phenotypical similarity among independently evolutionary species due to the same selective pressures,while heterogeneous ecological conditions might promote genetic and phenotypic diversification among lineages.It is likely that identical selective pressure caused by the same environmental factors promoted morphological convergence inO.ichangensissp.nov.andO.schmackeri.In contrast,divergent selection pressures from heterogeneous environments might drive morphological divergence inO.hejiangensis.Such environment-driven interspecific phenotypic differentiation has been revealed in Australasian warblers(Acanthizidae; Garcia-Navaset al.,2018) and African drongos(Dicrurus adsimilis; Fuchset al.,2018).
4.3.Ecological adaptation of signalsRecent studies have suggested that signal ecology might play an important role in the diversification of signal traits (Kolmet al.,2012; Snell-Rood,2012; Odendaalet al.,2014; White and Kemp,2015;Maanet al.,2017; Malulekeet al.,2017),through a process termed sensory drive (Endler,1992; Endler and Basolo,1998).Under the scenario of sensory drive,environmental factors(e.g.temperature and humidity) can exert selective pressure on signals as mediated through sound absorption (Fuller and Behavior,2010; Maloneet al.,2014).In particular,signals of lower frequency and longer duration could benefit the animals in humid environments (Snell-Rood,2012; Jianget al.,2015; Malulekeet al.,2017).Here,the revealed species-specific signal features agreed with this hypothesis.In the field survey,O.ichangensissp.nov.andO.schmackeriwere always found inopen (meaning higher temperature) and relatively arid microhabitats,whileO.hejiangensisoccurred in closed (meaning lower temperature) and moister habitats.Correspondingly,O.ichangensissp.nov.andO.schmackeriemitted calls with higher frequency (the average DFs were 3.679 kHz and 3.965 kHz,respectively) and shorter duration (the average note duration was 0.053 s and 0.078 s,respectively),whereasO.hejiangensisproduced callswith lower frequency (DF 2.807 kHz) and longer note duration (0.121 s).In addition,O.hejiangensisshowed a more complicated call structure.For example,calls ofO.hejiangensiswere 3-or 4-pulsed and showed both short calls and long calls(approximately 5 s per call).
On the other hand,the divergence in temporal and spectral properties of male calls might result from ecologically associated traits,such as body size (Ophiret al.,2010; Snell-Rood,2012;Odendaalet al.,2014; Leeet al.,2016; Malulekeet al.,2017).Interestingly,we found significant interspecific differentiation of body size in males but no differences in females.MaleO.hejiangensis(mean SVL 52.7 mm)is obviously larger than maleO.ichangensissp.nov.andO.schmackeri(mean SVL is 42.4 mm and 44.6 mm respectively).As a proxy of environmental quality,body size in relation to many other ecological traits always bears strong selection and,by extension,might drive the divergence in signals by imposing effluence on signal traits(e.g.,dominant frequency and call duration,Stewartet al.,2016;Köhleret al.,2017; Derryberryet al.,2018).In general,the call duration correlated positively with body size (Castellano and Cristina,1998),whereas the dominant frequency correlated negatively (Hoskinet al.,2005; Pfennig and Pfennig,2005; Nali and Prado,2014).In other words,the heterogeneousness of the microenvironment could also indirectly lead to interspecific variation in acoustic features via association with body size.
AcknowledgementsWe would like to thank Liang FEI from the Chengdu Institute of Biology,Chinese Academy of Sciences for providing the morphological data of SMF6241.We are grateful to Youqiang LU,Liang QIAO,Qiqi FENG,Mengyu XU,Can KE,Liming CHANG,Yake WAN for their efforts in sample collection,Mengyu XU and Xinyue YANG for work in measuring samples,and Sen LIU and Guangzhan FANG for suggestions in recording vocalizations.This work was supported by the National Natural Sciences Foundation of China (NSFC-31872220,31572245,31372164).
 Asian Herpetological Research2020年4期
Asian Herpetological Research2020年4期
- Asian Herpetological Research的其它文章
- Characterization of Skin Symbiotic Bacteria of Sympatric Amphibians in Southeastern China
- Taxonomic Revision of Raorchestes menglaensis (Kou,1990) (Amphibia:Anura),with Descriptions of Two New Species from Yunnan,China
- Description of a New Species of the Asian Newt Genus Tylototriton sensu lato(Amphibia:Urodela:Salamandridae) from Southwest China
- Toad-headed Lizard Phrynocephalus forsythii (Squamata,Agamidae) as a Potential Ring Species Inferred from Population Genetic Differentiation
- The Effect of Attendance Patterns on the Lek Mating System in the Omei Treefrog (Rhacophorus omeimontis)
- No Male Preference for Large Females in the Asian Common Toad(Duttaphrynus melanostictus):Effect of the Sex Ratio and Breeding System
