Post-transplant immunosuppression and COVID-19: From a double whammy to a mixed blessing
Ashwin Rammohan
Ashwin Rammohan, The Institute of Liver Disease and Transplantation, Dr. Rela Institute and Medical Centre, Chennai 600044, India
Abstract The coronavirus pandemic (COVID-19) has had an unprecedented effect on various disease processes and their management. COVID-19 is likely to have a complex pathophysiological interplay with the post-transplant patients; one affecting the clinical course and outcome of the other. In the absence of validated data from trials, there is strong dependence on experience based on previous similar epidemics (SARS/MERS), and from consensus based on expert opinions. Despite the fact that our knowledge is rapidly evolving with time, there still is relatively limited objective data on the effect of COVID-19 on the human body. Numerous questions remain unanswered, one of which involves the management of immunosuppression in the post-transplant recipient during this contagion. The core tenet of which continues to be that of establishing an equipoise between infection and rejection. This review summarises the current knowledge on immune interactions of the virus, the immunomodulatory effects that may be at play, and its relation to the art of immunosuppression.
Key Words: COVID-19; Post-transplant immunosuppression; Immunity; Review
INTRODUCTION
Notwithstanding the social isolation and restrictions, the coronavirus pandemic (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), continues to spread globally. The pandemic has had an unprecedented effect on various disease processes and their management[1]. Especially in the field of transplantation, many questions remain with regard to COVID-19 that need to be addressed. As with other diseases, COVID-19 is likely to have a complex pathophysiological interplay with the post-transplant patients; one affecting the clinical course and outcome of the other[1]. These fragile subset of patients, with their immunomodulated state are likely be affected in numerous ways which may not be limited to just a more rapid progression of infection[2]. The need to weigh the benefits of immunosuppression relative to inflammation against its adverse effects also remains. There also remain the pragmatic concerns of donor-derived COVID-19 infection along with the potential for prolonged shedding by immunocompromised hosts leading nosocomial and community transmission of SARS-CoV-2. Besides, only sparse data exist on the biomarkers which define the risk for disease progression, appropriate therapeutic interventions, and graft rejection on a background of COVID-19. With relatively limited objective knowledge of the effect of COVID-19 on the human body, numerous questions remain unanswered. One of which involves the management of immunosuppression in the post-transplant recipient during this contagion; the core tenet of which continues to be that of establishing an equipoise between infection and rejection. This review summarises the current knowledge on immune interactions of the virus, the immunomodulatory effects that may be at play, and its relation to the art of immunosuppression.
CORONAVIRUS-19 AND THE IMMUNE SYSTEM
While there have been important inroads, a full picture of the critical host immune response and its interplay with the virus remains poorly defined. As an initial step in the infection, the virus binds to its target receptor on the host cell. Based on previous work on SARS-CoV, which demonstrated that this virus principally targets cells which express the angiotensin-converting enzyme 2 (airway epithelial cells, alveolar epithelial cells, vascular endothelial cells and macrophages), it has been postulated that the SARS-CoV-2 uses a similar mode of entry[1,3-6]. Viral infection and replication cause high levels of virus-linked pyroptosis (highly inflammatory form of programmed cell death) with associated vascular leakage[4,5,7].
SARS-CoV-2 triggers both the innate and adaptive immune response synergistically, responses which are essential in controlling viral replication and limiting the spread of virus[3-5,8,9]. Local inflammatory cascades lead to increased secretion of the proinflammatory cytokines and chemokines (IL-1β, IL-6, IFNγ, MCP1 and IP-10)[4,5,8,9]. Recruitment of immune cells like monocytes and T lymphocytes from the blood with infiltration of the infected site occurs. This phenomenon contributes to the elevated neutrophil: Lymphocyte ratio and lymphopenia observed in most COVID-19 patients[1,5,10]. In most individuals, this immune response clears the infection and as patients recover, the wave of immune response subsides[1,4,5,8,10].
Nevertheless, sometimes the virus-induced immune response turns dysfunctional, leading to an induction of aberrant production of pro-inflammatory cytokines and an exaggerated recruitment of macrophages and granulocytes. This results in a cytokine storm, an integral component of the macrophage activation syndrome or secondary hemophagocytic lymphohistiocytosis, thus leading to further tissue damage and in severe cases progressing to acute respiratory distress syndrome (ARDS)[1,4,5,11]. Higher plasma levels of IL-2, IL-6, IL-7, IL-8, IL-10, macrophage inflammatory protein-1A, macrophage inflammatory protein-1B, IP-10, MCP1, and tumour necrosis factor have been observed in patients with severe COVID-19 requiring intensive care unit (ICU) admissions[3,5,12]. Patients with severe disease show a significantly higher levels of IL-6, a key cytokine. Higher percentage of CD14+ CD16+ monocytes which secrete inflammatory cytokines, have also been observed in the sicker patients. This immune dysregulation also involves subsets of T cells, and in the severe cases of COVID-19, levels of helper T cells and cytotoxic suppressor T cells, and regulatory T cells (responsible for immune homeostasis by suppression of activation, proliferation, and function of most lymphocytes) were noted to be significantly lower[5,13,14]. Further immune dysregulation is evident by the disruption of equilibrium between naïve T cells and memory T cells. There is therefore a T cell exhaustion, with a poor effector function, and an increased expression of inhibitory receptors on the cells, the magnitude of which worsens in those who are admitted in the ICU[13-15]. Altogether, this dysfunctional immune response induced by the virus results in further tissue damage. In a small subset of patients, this local inflammatory cascade may become systemic, leading on to organ-system damage and multisystem organ dysfunction syndrome[1,3,11,16].
It is nonetheless important to understand that a simple correlation does not extrapolate to causation. It is equally likely that this cytokine storm is not a straightforward case of an inappropriate host inflammatory response that requires correction, instead is due to an increased viral titre (secondary to failure of the immune response to control infection) which drives the inflammation and its consequent severity. Hence, the decision to pharmacologically immunosuppress a patient with COVID-19 remains a difficult one. The deleterious effects of an impaired immune response must be carefully weighed against the likely benefits of reducing inflammation.
IMMUNOSUPPRESSION AND CORONAVIRUS-19: THE EVIDENCE
A systemic review based of 16 articles on immunosuppressed patients (various causes: Cancer, transplant) showed a milder COVID-19 disease and an overall better outcome as compared to other comorbidities[17]. Two out of 200 heart transplant recipients from China developed COVID-19 infection. While one had a mild disease, the other had a more severe course and required a more intensive care management with high dose corticosteroids and immunoglobulins. Both patients recovered without graft loss and their respective courses were not dissimilar from other immunocompetent patients with COVID-19 in the province. The authors however concluded that immunosuppression may have masked the clinical signs of the infection, and may have delayed their presentation[18].
In a series of 200 transplant recipients from Italy, none of the patients developed COVID-19 pneumonia. There was no increased risk of severe disease or mortality in these patients. This led the author to believe that instead of amplifying the risk of recipients to COVID-19, immunosuppression may actually be protective by dampening the amplified immune response[19]. In a report from China, of 1099 patients with confirmed COVID-19, two were immunosuppressed. Both had an uneventful recovery following a mild disease[19]. Similar such reports of post-transplants patients having a course of COVID-19 not dissimilar from the general population has been reported from across the world[20-22]. Of 1590 patients with COVID-19 included in a series to analyse the influence of comorbidities on the clinical course of the COVID-19, 21 were classified as immunosuppressed. Outcomes analysed included ICU admission, invasive ventilation and mortality. While patients with diabetes, hypertension, co-existing lung disease or malignancy were shown to have a more adverse outcome, immunosuppressed patients met similar endpoints as those of the general population[23]. With the airway being the most common route of entry for the SARS-CoV-2, lung transplantation piques one’s interest. Concerns, apart from outcomes would include higher rates and different sources of infection (recipient derived, donor derived or nosocomial) and diagnosis, especially in the early posttransplant period. Non-COVID-19 lung infections or graft dysfunction may have presentations similar to those of a COVID-19 disease, confounding the diagnosis. Nevertheless, anecdotal reports from lung transplant centres across the world suggest a disease presentation and outcome similar to the general population[20,24-26].
Conventionally, immunosuppressants affect humoral immunity and neutrophil action, leading to a higher susceptibility and increased severity of viral infections, often with prolonged shedding. Contrary to other viral illnesses like influenza A and H1N1, SARS-CoV2 does not appear to have a higher predilection towards immunosuppressed hosts[2,17,19]. Immunosuppressed patients may actually have a potential protective effect afforded by a weaker immune response against the pathogen, resulting in a milder course of disease.
IMMUNOSUPPRESSANTS AND CORONAVIRUS-19
The commonly used immunosuppressants in the post-transplant setting include corticosteroids, calcineurin inhibitors, anti-metabolites and biological agents.
Corticosteroids
The use of systemic corticosteroids in COVID-19 remains controversial. On one hand it may worsen viremia by diminishing the immune response and prolong the viral shedding time, on the other with its broad spectrum actions, corticosteroids may suppress the exuberant immune response, and maintain a systemic anti-inflammatory state that can minimize the precipitation of severe pneumonia and ARDS[5,8,16,27].
Studies from the SARS and MERS epidemics have shown deleterious effects of corticosteroids. Apart from a delayed viral clearance, increased rates of secondary infections, steroid related complications and higher mortality rates were observed[3,8,27,28]. Data from two other studies suggested a prolonged SARS-CoV2 shedding and an increased mortality rate with the use of high dose corticosteroids[29,30]. On the contrary, there is some compelling evidence for the use of corticosteroids. Improved outcomes by the suppression of inflammation have been demonstrated, especially in the later stages of ARDS[16,29,30]. Nevertheless, at this point, the potential role of corticosteroids in preventing mild COVID-19 from developing into severe pneumonia remains controversial. While there are recommendations like those from WHO which recommend avoiding the use of corticosteroid, certain other guidelines allow for their usage when there is rapid disease progression on a background of severe inflammation[1,29,31]. There is also no consensus on the dosing of corticosteroids, and these must be individualised for each patient.
Calcineurin inhibitors
Several guidelines variably suggest withdrawal/dose reduction of Calcineurin Inhibitors (CNIs) in transplant patients with severe COVID-19. With evolving data and robust evidence lacking, these guidelines are being updated frequently.
Certain unique features of CNIs are worth mentioning. CNIs are known to inhibit certain viral replication by inhibiting immunophilin pathways[32,33]. Experience with Hepatitis C virus and several coronaviruses suggest that CNIs, especially Cyclosporine can inhibit their replicationin vitroindependent of its immunosuppressive effect. Analyses of virus-host interactions, have shown the SARS-CoV use the host’s cyclophilin family of proteins for interaction.In vitrotests with tacrolimus and FK506 binding protein knock downs have shown to inhibit viral replication[32,34]. Hence, in principle, CNIs could have the potential to inhibit SARS-CoV-2. Based solely on these studies in related viruses, it would however be imprudent to use CNIs for their purported antiviral properties. Also a withdrawal of CNIs is likely to result in an increased dosing of corticosteroids, which as evidence would suggest may well have deleterious effects[27,35]. Due to their inhibitory action on IL-2 gene transcription, and cell proliferation, CNIs notably Cyclosporine, has been used in the treatment of HLH[16,32,36]. Albeit there is little evidence that they would be capable of attenuating the SARS-CoV-2 CRS, this does suggest that CNIs may not be harmful in the dysregulated immune environment of COVID-19. Hence, current evidence suggests that CNIs remain the preferred maintenance immunosuppressant in post-transplant patients with COVID-19.
Mammalian target of rapamycin inhibitors
As a central regulator of cell metabolism and proliferation, mammalian target of rapamycin (mTOR) pathway affects diverse cellular processes across organisms[37]. Apart being an immunosuppressant, due to its interaction with viral proteases,in vitrostudies have shown mTOR inhibitors to have a strong antiviral effect on SARS and MERS viruses[13,38,39]. Nonetheless, using these class of drugs purely for their anti-viral properties would be ill-advised. Adverse effects of mTOR inhibitors include interstitial lung disease and mucosal ulcers which could potentially worsen the course of SARSCoV-2 infection. CNIs and mTOR inhibitors are metabolisedviathe cytochrome P450 enzyme systems (CYP3A4, CYP3A5). These cytochromes are inhibited by anti-viral medication commonly used for COVID-19 pneumonia leading to fluctuations in the levels of both CNIs and mTOR inhibitors. This inhibition is however more intense and unpredictable with mTOR inhibitors[34,37,39-41]. Put together, the recommendations include either stop the drug or reduce to micro-doses in severe cases of COVID-19.
Antimetabolites (mycophenolate mofetil and azathioprine)
Mycophenolate mofetil (MMF) is an inhibitor of inosine-5′-monophosphate, and causes intense immunosuppression by preferentially inhibiting B-cell and T-cell function. In addition to its immunosuppressive properties, several invitrostudies have demonstrated its antimicrobial effects against various viruses including vaccinia virus, herpes simplex virus, Coxsackie virus, hepatitis C and influenza virus[42-46]. On the other hand, MMF causes lymphopenia, and is likely to compound the harmful effect of the virus. Hence, despite a potential anti-viral effect, MMF with its powerful suppression of the immune system is likely to be deleterious than beneficial. Another antimetabolite commonly used for immune suppression, especially in renal transplantation is Azathioprine. Its actions are similar to those of MMF, and is also associated with intense lymphopenia. Evidence is lacking as to the true effect of continuing MMF or Azathioprine in post-transplant COVID-19 patients, it is but intuitive to withhold these drugs during severe infection[39,47,48].
Intravenous immunoglobulin
Consisting of pooled polyclonal immunoglobulin G, the exact mechanism of Intravenous immunoglobulin immunomodulatory action is unknown. Proposed mechanisms include apoptosis, expression of pro-inflammatory cytokines, expansion of regulatory T cell population, phagocytosis, antibody dependent cellular cytotoxicity, immune cell differentiation and maturation, and antigen-presentation[8,10]. All of these responses are integral to viral clearance from the body. High dose Intravenous immunoglobulin has been reportedly used successfully in the treatment of severe COVID-19[49-51]. There are several ongoing trials on its application in COVID-19, but its high cost and limited supply is likely to restrict its general use.
Biological agents
There is very little literature evidence regarding the interactions of routinely used biologic agents in the post-transplant setting like Basiliximab and COVID-19. It is nevertheless wise to use them judiciously during this pandemic[47]. Numerous other biological agents acting at various levels of the cytokine cascade are being tested as treatment options for COVID-19[3,5,8,47].
IL-6 is the master-switch of the body’s immune response. It acts on various cascades simultaneously and IL-6 receptors are universally expressed on immune cells[52]. Rapid elevations of IL-6 levels are noted in various inflammatory conditions including COVID-19 related cytokine storm. Direct correlations between serum levels of IL-6 and SARS-CoV-2 RNAaemia in severe disease suggest that blocking IL-6 or its receptors could potentially attenuate the dysfunctional immune response induced by the contagion[5,11,30,53]. Several therapeutic agents acting a various stages of the signalling pathway have been developed, these include inhibition of IL-6, inhibition of IL-6 receptors, and/or its postreceptor downstream signalling pathways (JAK/STAT)[3,5,8,41,52]. Under trial IL-6 antagonists include sarilumab, tocilizumab and siltuximab, each with different pharmacologic properties. It is however sobering to note that IL-6 antagonists increase the risk of opportunistic infections, therefore must be used in seriously unwell patients, along with antiviral treatments to reduce the viral load[3,5,8,41,52].
CORONAVIRUS-19 TREATMENT DRUG INTERACTIONS
Remdesivir is a NUC/viral RNA polymerase inhibitor which inhibits SARS-CoV-2 invitro, and there are case reports of its efficacy in COVID-19[54-56]. No relevant druginteractions with immunosuppressive agents are known and liver toxicity though possible, is rare[54,56]. With contradictory data on their efficacy, treatment of COVID-19 with Chloroquine/Hydroxychloroquine ± Azithromycin has been a subject of intense debate[54-58]. It is remarkable to note that these agents can significantly alter the drug levels of immunosuppressive agents, and a close monitoring of drug levels is required for CNIs and mTOR inhibitors[39,47,54]. It is imperative to exclude G6PD deciency before starting choloroquine therapy. Despite it being liver-safe, there are reports of clinically apparent acute liver injury[54,57,58]. Lopinavir/ritonavir are approved for Human immunodeficiency virus and have been used in patients with severe acute respiratory syndrome[55,59,60]. Reports of their value in the treatment of COVID-19 exist. They have well known drug-interactions with immunosuppressive drugs and mTOR inhibitors should not be co-administered[54,59,60]. Lopinavir/ritonavir is also a potent inhibitor of CYP3A4 and close monitoring of drug levels are required for CNIs.
CONSENSUS AND RECOMMENDATIONS
The impact of an immunosuppressed state on COVID-19 and vice-versa continues to be unclear, and recommendations extrapolated from the SARS/MERS epidemic remain un-validated. Scientific evidence remains scarce and strategies can only be based on expert opinion. In these uncertain times, based on available evidence, various transplant societies across the world have come up with their recommendations[22,26,39,47,61-68]. Although the management of post-transplant immunosuppression in COVID-19 is largely anecdotal, information from the transplant societies have a high degree of consensus. A summary of their recommendations on post-transplantation immunosuppression during this pandemic include: (1) There is concern that reducing or discontinuing immunosuppressants may cause acute graft rejection, hence dose adjustment of immunosuppressive drugs in transplant recipients without COVID-19 is not warranted; (2) For patients with mild to moderate COVID-19, the current immunosuppressant dosage should be maintained. The patient’s condition nevertheless, should be monitored closely; (3) A close watch on drug interactions that may cause large oscillations in plasma CNI concentrations is imperative. Any such fluctuations should be avoided, and such medications should be prescribed only if the benefits greatly outweigh the risks; (4) As low lymphocyte counts in COVID-19 patients is associated with a more severe course of disease, critical reconsideration and a judicious use of lymphocyte depleting therapies must be done; (5) Transplant recipients with severe or rapidly progressive COVID-19 will need a staged approach with reduction of immunosuppression. Stopping of antimetabolites in the early phase and dose reduction of corticosteroids in the late phase, keeping at least a low dose to avoid adrenal insufficiency is recommended; and (6) Corticosteroids or other immunosuppressive therapies should be re-initiated with caution when their potential benefits outweigh the risks of discontinuation.
Unit protocol
Based on the internationally accepted classification of COVID-19, we classify SARSCoV-2 test (reverse transcription polymerase chain reaction) positive patients into asymptomatic, mild, moderate, severe and critical disease[69-71]. Current immunosuppression is maintained for patients with asymptomatic or mild infections, they are however followed-up closely. Antimetabolites are stopped for those with moderate disease, and their lymphocyte count is monitored. Other immunosuppressants are continued at the usual doses (blood trough Tacrolimus levels 6-8 ng/mL). A low threshold is kept for reducing their immunosuppression, should their clinical condition worsen. For severe and critical disease, immunosuppression is lowered to a bare minimum. CNIs are maintained at low doses and stopped if the patient’s condition becomes critical. Low doses of corticosteroids are given to avoid adrenal insufficiency. Drug levels and interactions with anti-viral medication are monitored.
CONCLUSION
In the absence of validated data from trials, there is strong dependence on experience based on previous similar epidemics (SARS/MERS), and from consensus based on expert opinions. However, with time our knowledge is rapidly evolving and this pandemic does come with the silver lining of worldwide collaboration in clinical care and biomedical research. In an endeavour to return to the familiar domain of evidence based medicine, high quality research and accurate documentation remains the need of the hour. Further studies of the host immune response to SARS-CoV-2, including a detailed investigation of the determinants of healthy versus dysfunctional outcomes, will allow for a more evidence based approach to post-transplant immunosuppression with an improved individualization of care.
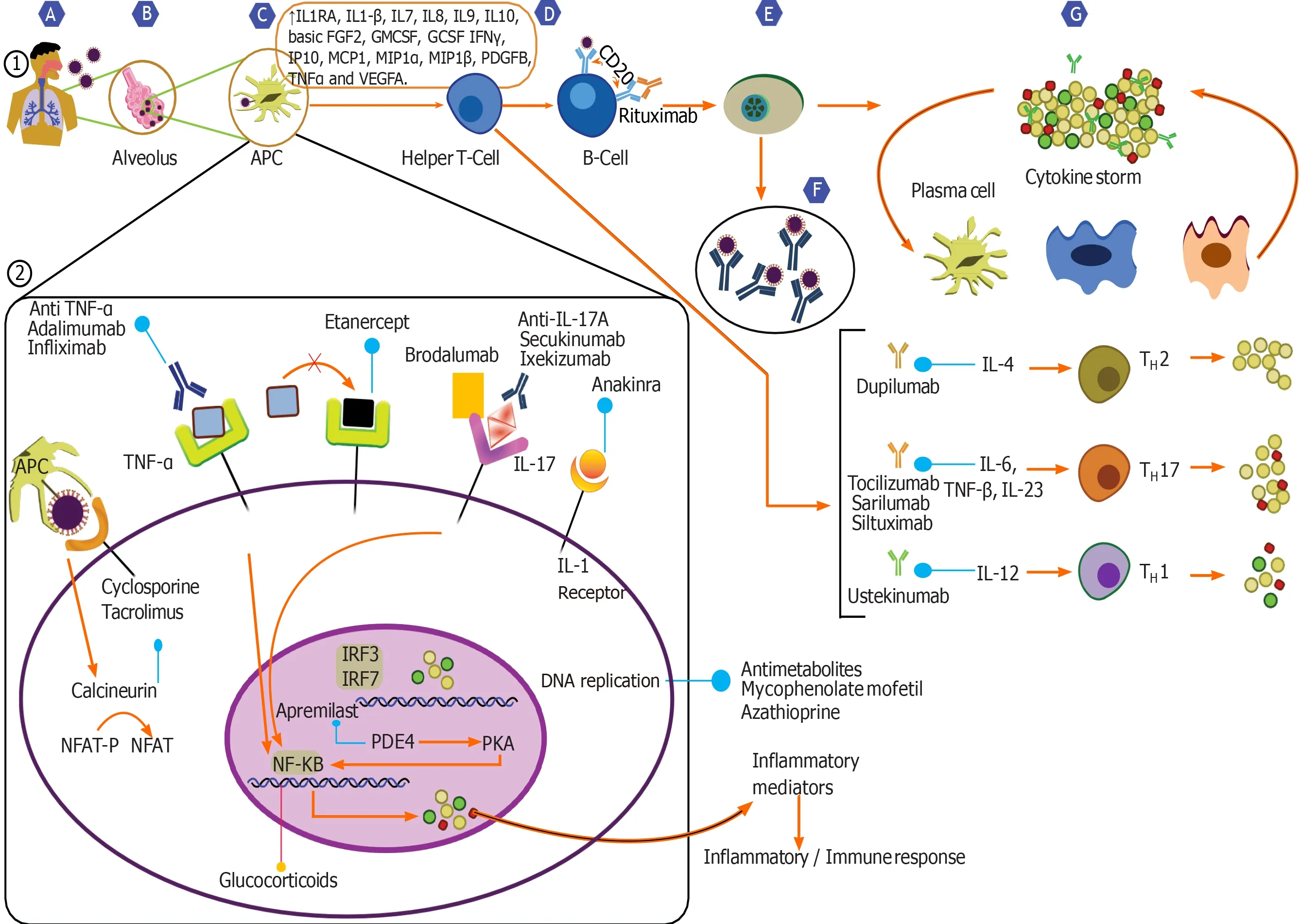
Figure 1 Chronology of events during the severe acute respiratory syndrome coronavirus 2 infection and targets for immunosuppressants and immunomodulators.
 World Journal of Transplantation2020年9期
World Journal of Transplantation2020年9期
- World Journal of Transplantation的其它文章
- Role of novel biomarkers in kidney transplantation
