Dynamic changes of behaviors, dentate gyrus neurogenesis and hippocampal miR-124 expression in rats with depression induced by chronic unpredictable mild stress
Yun-Ling Huang , Ning-Xi Zeng , Jie Chen Jie Niu Wu-Long Luo Ping Liu, Can Yan , Li-Li Wu
1 Research Center for Basic Integrative Medicine, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong Province, China
2 Department of Pharmacology, PLA General Hospital, Beijing, China
AbstractThe depression-like behavior phenotype, neurogenesis in the dentate gyrus and miR-124 expression in the hippocampus are the focus of current research on the pathogenesis of depression and antidepressant therapy. The present study aimed to clarify the dynamic changes of depression-like behavior, dentate gyrus neurogenesis and hippocampal miR-124 expression during depression induced by chronic stress to reveal pathological features at different stages of depression and to further provide insight into depression treatment. Chronic unpredictable mild stress depression models were established by exposing Sprague-Dawley rats to various mild stressors, including white noise,thermal swimming, stroboscopic illumination, soiled cages, pairing with three other stressed animals, cold swimming, tail pinch, restraint and water and food deprivation. Chronic unpredictable mild stress model rats underwent dynamic observation from 1 to 8 weeks and were compared with a control group (normal feeding without any stressors). To observe changes in the depression-like behavior phenotype during chronic unpredictable mild stress-induced depression, a sucrose preference test was used to evaluate the degree of anhedonia. An open-field test was used to evaluate locomotor activity and anxiety status. Compared with the control group, chronic unpredictable mild stress rats lost weight but did not have a depression-like behavioral phenotype at 1-4 weeks. Chronic unpredictable mild stress rats presented decreased sucrose preference and locomotor activity at 5-8 weeks. In addition, chronic unpredictable mild stress rats did not have significant anxiety-like behavior during 1-8 weeks of modeling. To observe neurogenesis dysfunctions and changes in neuronal number in the dentate gyrus during chronic unpredictable mild stress-induced depression, markers (DCX and DCX/BrdU) of neural proliferation and differentiation and the neuronal marker NeuN were assessed by immunofluorescence. Compared with the control group, neurogenesis and the neuronal number in the dentate gyrus did not change from 2 to 6 weeks; however, neural proliferation and differentiation in the dentate gyrus decreased, and the number of neurons decreased until the eighth week in the chronic unpredictable mild stress group.Real-time quantitative reverse transcription polymerase chain reaction assays and fluorescence in situ hybridization were used to measure the expression of hippocampal miR-124 during chronic unpredictable mild stress-induced depression. The results showed that the expression of hippocampal miR-124 was unchanged during the first 4 weeks but increased from 5 to 6 weeks and decreased from 7 to 8 weeks compared with the control group. These findings indicate that during chronic unpredictable mild stress-induced depression, the behavioral phenotype, miR-124 expression in the hippocampus, neurogenesis in the dentate gyrus and neuronal numbers showed dynamic changes,which suggested that various pathological changes occur at different stages of depression. All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Guangzhou University of Chinese Medicine of China in March 2015.
Key Words: chronic unpredictable mild stress model; continuous observation; depression; depression-like behavior; dynamic changes; hippocampus;miR-124; neurogenesis dysfunction; neuronal loss
Introduction
As a common mental disease, depression is one of the main causes of psychosocial disorder and dysfunction. Almost 40% of patients do not recover following an antidepressant trial, and 20% of these patients fail to respond to any intervention, at least in part, due to insufficient realization of the stages and features of the pathogenesis of depression (Warden et al., 2007; Brown et al., 2019). Thus, the lack of targeted treatment for different stages and features of depression may be a reason for poor or even ineffective treatment efficacy. In general, a longer disease course, greater functional impairment and more obvious clinical symptoms are key factors for the antidepressant effect (Fekadu et al., 2018; van Diermen et al., 2018). Nevertheless, the pathological features in different stages of depression remain largely unexplored. Therefore,it is urgent to systematically and dynamically observe these features.
Although many animal models of depression exist, such as the single restraint stress model and chronic corticosterone-mediated model, most of these models only simulate a certain pathogenesis or pathological features and cannot completely replicate the onset of human depression. The chronic unpredictable mild stress (CUMS) model, established by Willner (1997), is considered a good simulation of the complete pathogenesis of depression in humans suffering from long-term, low-intensity stress (D’Aquila et al., 1997).Therefore, the CUMS model adopted in this study can better represent the occurrence and development of depression than the formerly described models. However, to our knowledge, previous studies on the pathological changes of the CUMS model were mostly based on observations at certain time points, and the results were not completely consistent(Mi et al., 2017; Gao et al., 2018; Chai et al., 2019; Shen et al.,2019). Therefore, it is important to explore how the pathology dynamically differs in CUMS model rats.
Hippocampal neurogenesis dysfunction is a classic topic and important concern for stress and the development of depression (Dean and Keshavan, 2017; Liu et al., 2019; Park,2019). The hippocampal formation is vulnerable to damage from a variety of psychological stressors (Stein-Behrens et al., 1994). A preclinical study has reported that chronic stress exposure induces spine loss in hippocampal neurons and impairs synaptic transmission concomitant with the emergence of depressive behaviors (Danzer, 2012). It also has been confirmed that the branch number and length of hippocampal neuron dendrites and their nerve regeneration ability are reduced, which indicates that abnormalities and dysfunctions of the hippocampus are strongly associated with depression (Duman and Aghajanian, 2012). Therefore,a close relationship exists between hippocampal abnormalities and dysfunctions in depression. Adult hippocampal neurogenesis is defined as the ability of granular cells in the dentate gyrus of adult mammals to generate new neurons.Hippocampal neurogenesis is a complex process involving the proliferation and differentiation of neural progenitor cells and the survival of new neurons. Multiple lines of evidence indicate that hippocampal neurogenesis dysfunction is one of the main pathogenic causes of depression (Cole et al., 2011; O’Leary and Cryan, 2014). Furthermore, studies have also shown that the anti-proliferation effect on hippocampal precursor cells caused by chronic stress results in a 30-60% reduction in the cell proliferation rate in the dentate gyrus and affects the differentiation and apoptosis of new neurons (Zhang et al., 2018). However, in many published studies, the time points of occurrence of hippocampal neurogenesis dysfunction in the CUMS model are inconsistent. At 4 weeks, CUMS can down-regulate ki67 in the hippocampus and reduce cell proliferation (Ayuob et al., 2017). At 6 weeks, CUMS reduces the number of 5-bromo-2-deoxyuridine (BrdU)/NeuN-positive cells and new neurons in the hippocampus (Zhang et al., 2018). Based on the close relationship between neurogenesis dysfunction and the pathogenesis and development of depression, it is necessary to dynamically observe neurogenesis dysfunction in CUMS models.
MicroRNA (miRNA) can play an extensive and important role in the biological processes of nerve cells such as generation, differentiation, proliferation and apoptosis (Wang et al., 2018). By occupying 25-48% of the miRNA content in the whole brain, miR-124 is the most abundant miRNA in the adult brain (Papagiannakopoulos and Kosik, 2009). Currently, miR-124 has become a focus of depression research.A growing body of evidence suggests that miR-124 can participate in emotional regulation and is strongly associated with stress and depressive behavior (Bahi et al., 2014; Hu et al., 2017; Zeng et al., 2018). Both clinical and rodent studies have demonstrated that miR-124 levels in the prefrontal cortex and peripheral blood of patients with depression change significantly. Therefore, miR-124 has been suggested for use as a biomarker and therapeutic target for depression (Roy et al., 2017). In addition, as an important regulator of adult neurogenesis, miR-124 plays an important role in regulating the proliferation and differentiation of neural stem cells.MiR-124 overexpression can promote differentiation of neural stem cells into premature neurons and the reduction of glial cells, which leads to depletion of neural precursor cells;however, knocking out miR-124 will cause neural precursor cells to remain in a pre-differentiation state and result in a loss of neurons (Silber et al., 2008; Cheng et al., 2009). Regulation of miR-124 in neurogenesis may also be associated with the Notch, REST/SCP1 and other signaling pathways.At present, the results of miR-124 levels in CUMS model tissues are inconsistent. Some reports suggest that CUMS can up-regulate miR-124 levels in different brain regions, such as the prefrontal cortex and hippocampus (Cao et al., 2013;Liu et al., 2018a, b). In contrast, other studies have found that CUMS can down-regulate the expression of miR-124 in the brain (Higuchi et al., 2016; Ma et al., 2019), which is associated with neuroplasticity. Furthermore, the contributions of different animal species, stress intensities, stressors and stages of depression should be considered to determine the pivotal factors affecting miR-124 expression. Therefore,it is necessary to continuously and dynamically observe the changes in hippocampal miR-124 in CUMS models.
Because they are closely linked, the depression-like behavior phenotype, hippocampal miR-124 and neurogenesis in the hippocampal dentate gyrus are the focuses of treatment for depression. Through continuously observing the dynamic changes of depression-like behavior, hippocampal miR-124 and dentate gyrus neurogenesis in CUMS models for 8 consecutive weeks, this study aims to preliminarily reveal various pathological features at different stages of depression and to provide an effective therapeutic strategy for depression.
Materials and Methods
Animals
All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Guangzhou University of Chinese Medicine of China in March 2015. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). A total of 240 male Sprague-Dawley rats weighing 180-220 g and aged 7-8 weeks (License No. SCXK (Yue) 2016-0041) were obtained from the Laboratory Animal Center of Southern Medical University, Guangzhou, China. Rats were housed(one per cage) under a 12-hour light/dark cycle (light from 8:00-20:00) at a controlled temperature of 23 ± 2°C, with food and water available.
Generation of CUMS models of depression
The CUMS animal model established by Willner P was adopted and modified (Willner et al., 1992). The efficacy of model establishment was standardized by a sucrose preference test to measure anhedonia in stressed rats (Yan et al., 2018). All rats were divided into a control group and a CUMS group. Rats in the CUMS group were subjected to CUMS. Rats in the control group underwent normal feeding without any stressors. The stress protocol was as follows:white noise (85 dB, 5 hours); 5 minutes of thermal swimming at 45°C; 5 hours of stroboscopic illumination (300 flashes/minute); 10 hours of exposure to a soiled cage; 10 hours of being paired with three other stressed animals; 5 minutes of cold swimming at 4°C; 3 minutes of tail pinching;12 hours of restraint; water deprivation for 24 hours and food deprivation for 12 or 24 hours. Solitary-housed rats were randomly exposed to 1-2 of these stimuli once a day for 8 weeks. Beginning on first day of CUMS, stressors were stopped in selected rats in the CUMS group, and behavioral tests were performed every 7 days. After the last behavioral test, rats were sacrificed for biochemical experiments.
Sucrose preference test
The procedure was performed according to a previously published protocol by Liu et al. (2018). The sucrose preference test was divided into four stages: sucrose training for 48 hours, baseline testing for 36 hours, food and water deprivation for 24 hours, and sucrose preference testing for 12 hours. Unqualified rats were excluded according to the following baseline test results: low sucrose preference (less than 60%), location preference (preferred to drink liquid from a fixed location), drinking too little (drinking neither sucrose solution nor pure water) and excessive drinking (total liquid consumption more than twice the average total liquid consumption of all rats). In the sucrose preference test stage(1 day after cessation of stress), two bottles of liquid (sucrose solution and pure water) were given to each animal at the same time. After 12 hours of liquid availability (20:00-8:00 the next day), the consumed volume of each solution was recorded and analyzed with the following formula: sucrose preference (%) = sucrose solution consumption/total liquid intake × 100%.
Open-field test
At 12 hours after the sucrose preference test, an open-field test was performed. Before the test, the rats were transferred to the behavioral test room (soundproof darkroom)for habituation to the environment for 1 hour. Each rat was individually placed into the middle of the open-field apparatus and then allowed to explore freely for 5 minutes. After approximately 10 seconds of adaptation, the overall distance and the time spent in the central area were recorded to evaluate locomotor activity and anxiety-like behavior. After each test, the open-field apparatus was cleaned with 75% alcohol and bromo-geramine solution to avoid contamination, and the next rat was tested after the apparatus was allowed to dry.
Real-time qRT-PCR
Rats were sacrificed 24 hours after the last behavioral test,and hippocampal tissues were collected for quantitative reverse transcription polymerase chain reaction (qRT-PCR).Total RNA was extracted using the TRIzol reagent (Life Science, New York, USA). The quality of RNA was evaluated with a spectrophotometer (NanoDrop-1000, Thermo Scientific, Wilmington, DE, USA) by measuring the optical density ratio. Reverse transcription was performed with RNA using a Prime ScriptTMRT Reagent kit (TaKaRa, Tokyo,Japan) according to the manufacturer’s instructions. cDNA was amplified using the CFX96TMReal Time System (BioRad,Hercules, CA, USA) with SYBR®Premix EX TaqTMII (Ta-KaRa) and miR-124-specific primer pairs purchased from Ribobio Company (Guangzhou, China). Expression levels of miR-124 were determined by the 2-ΔΔCtmethod (Zeng et al.,2017) and normalized to those of U6 as a reference (Wang et al., 2019).
Fluorescence in situ hybridization
Rats were sacrificed 24 hours after the last behavioral test.The brain tissue of the rat was dissected, fixed in 4% paraformaldehyde for 24-48 hours at 4°C and then immersed in 30% sucrose in 0.1 M PBS until it sank to the bottom. The hippocampus was trimmed according to the coronary sulcus. Five samples were obtained from each group to prepare frozen slices (40 μm thick) under RNase-free conditions.According to the manufacturer’s instructions, slices were permeabilized with proteinase K solution (BioFrox, Einhausen, Germany). Digoxigenin-labeled probes against miR-124 (Qiagen, Hilden, Germany) were then hybridized to the slices for 1 hour at 55°C. Probe sequence: 5′-CAT TCA CCG CGT GCC TTA-3′. After hybridization, brain sections were stringently and orderly washed in 5× saline-sodium citrate(SSC), 1× SSC and 0.2× SSC at 55°C and 0.2× SSC at room temperature. After 1 hour of blocking (5% goat serum), brain sections were incubated overnight at 4°C with an anti-DIG antibody coupled to AlexaFluor®555 (Roche, Basel, Switzerland). After 4′,6-diamidino-2-phenylindole (DAPI) staining,images were acquired using a laser scanning confocal microscope (LSM800, ZEISS, Oberkochen, Germany). After laser confocal scanning was performed with fixed parameters, the image was converted into the corresponding fluorescence intensity values using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
NeuN and doublecortin (DCX) single labeling
At 24 hours after the last behavioral test, rats were sacrificed,and the brain tissue was dissected and cut into 40-μm-thick frozen slices. The sections were blocked in 5% goat serum(containing 0.03% Triton-X-100) at room temperature for 1 hour and then incubated in rabbit anti-NeuN (1:1000, mAb,Abcam, Cambridge, UK) overnight at 4°C. After rinsing in Tris-buffered saline with 0.01% Tween-20, the sections were incubated with an AlexaFluor®488 goat anti-rabbit antibody (1:500, Abcam) at 37°C for 2 hours. After rinsing with Tris-buffered saline with 0.01% Tween-20 and DAPI staining, images were acquired using a laser scanning confocal microscope (LSM800, ZEISS). NeuN-positive and DAPI-stained cells (total cells) were counted separately using ImageJ software (National Institutes of Health). NeuN expression in the dentate gyrus was determined by the ratio of NeuN-positive cells to the total cells.
In the DCX staining stage, sections were incubated with a rabbit anti-DCX antibody (1:200, pAb, Abcam). The other steps and parameters were consistent with NeuN staining.Similar to NeuN labeling, DCX-positive and DAPI stained cells (total cells) were counted separately using ImageJ soft-ware. Expression of DCX in the dentate gyrus was determined by the ratio of DCX-positive cells to the total cells.
BrdU and DCX double labeling
BrdU (Sigma, St. Louis, MO, USA) was intraperitoneally injected 1 week before the sampling date (three injections,4 hours apart, 200 mg/kg), and rats were sacrificed 7 days after the injection. Sections were treated with 2 N HCL for 20 minutes at 37°C, rinsed in borate buffer (0.1 M, pH 8.4),blocked in 5% goat serum (containing 0.03% Triton-X-100)at room temperature for 1 hour and incubated with rat anti-BrdU antibody (1:150, mAb, Abcam) and rabbit anti-DCX antibodies (1:200, Abcam) overnight at 4°C. After rinsing in Tris-buffered saline with 0.01% Tween-20, the sections were incubated with AlexaFluor®488 goat anti-rabbit (1:500,Abcam) and AlexaFluor®594 goat anti-rat antibodies (1:500,Abcam) at 37°C for 2 hours. After rinsing with Tris-buffered saline with 0.01% Tween-20 and DAPI staining, images were captured using a laser scanning confocal microscope(LSM800, ZEISS, Oberkochen, Germany).
The procedure was performed according to a previous method (Huang and Herbert, 2006). Five samples were obtained from each group, and the whole dentate gyrus volume of the sample was serially sliced at 40 μm. One of each 11 slices was used for detection, and in total, eight slices were obtained for each sample. Laser confocal imaging was conducted after immunofluorescence staining, and BrdU/DCX double-labeled positive cells were counted using the Photoshop CS5 counting tool. Because these eight sections represented one of every 11 slices, the total number of positive cells in these eight sections was multiplied by 11 and used to represent the total number of BrdU/DCX-positive cells in the whole dentate gyrus volume of the hippocampus.
Statistical analysis
The data were statistically analyzed using SPSS 22.0 software(IBM, Armonk, IL, USA). All results are expressed as the mean ± SEM. The data of each group were consistent with a normal distribution (Shapiro-Wilk test). The sucrose preference test results, body weight and immunofluorescence of NeuN exhibited heterogeneity of variance, while the other results exhibited homogeneity of variance. An independent samples t-test was used for comparisons between two groups with homogeneity of variance (qRT-PCR results), and oneway analysis of variance was used for comparisons of three or more groups. In the pairwise comparisons, the least significant difference test was used to assess results exhibiting homogeneity of variance (open-field test results, fluorescence in situ hybridization, immunofluorescence of DCX and DCX/BrdU), and the Games-Howell method was used to assess results with heterogeneity of variance (sucrose preference test results, body weight and immunofluorescence of NeuN).P-values < 0.05 were considered statistically significant.
Results
Dynamic changes in behavior during CUMS modeling
As shown in Figure 1A, from 1 to 2 weeks, CUMS did not induce any reduction of body weight (n = 10-13; 1 week,P = 1.00; 2 weeks, P = 0.58). Body weight was significantly lower in the CUMS group than in the control group from 3 to 8 weeks (n = 10-13, F(15,168)= 54.258, P < 0.05), which is consistent with the observation that depressed individuals generally exhibit body weight reduction. As shown in Figure 1B, the sucrose preference test results demonstrated that compared with the control group, the sucrose preference of the CUMS group did not significantly change during the first 5 weeks of modeling (n = 10-13; 1 week, P = 1.00; 2 weeks,P = 1.00; 3 weeks, P = 1.00; 4 weeks, P = 0.09; 5 weeks, P =0.67) and decreased from 6 to 8 weeks (n = 10-13, F(15,168)=10.280, P < 0.05). A decreased sucrose preference indicates anhedonia, the main symptom of depression, in rats. Locomotor activity in an open-field test is also an important parameter of depression-like behavior (Figure 1C). Compared with the control group, the total distance traveled by the CUMS group did not change during the first 4 weeks of CUMS (n = 10-13; 1 week, P = 0.63; 2 weeks, P = 0.20; 3 weeks, P = 0.58; 4 weeks, P = 0.16), but it decreased from 5 to 8 weeks (n = 10-13, F(15,169)= 2.576, P < 0.05). In addition,the anxiety state of animals can be determined by the time spent in the central area in an open-field test. As shown in Figure 1D, from 1 to 8 weeks of model establishment, rats in the CUMS group showed no significant change in the time spent in the central area compared with the control group(n = 10-13; 1 week, P = 0.74; 2 weeks, P = 0.08; 3 weeks, P =0.06; 4 weeks, P = 0.10; 5 weeks, P = 0.92; 6 weeks, P = 0.77;7 weeks, P = 0.26; 8 weeks, P = 0.55).
Dynamic changes in miR-124 expression in the hippocampus during CUMS model establishment
As shown in Figure 2, qRT-PCR results demonstrated no difference in miR-124 expression in the hippocampus between the CUMS and control groups during the first 4 weeks of modeling (n = 9; 1 week, P = 0.69; 2 weeks, P =0.84; 3 weeks, P = 0.82; 4 weeks, P = 0.44). Compared with the control group, miR-124 expression in the hippocampus of the CUMS group increased from 5 to 6 weeks (5 weeks,n = 9, t(16)= 6.4, P < 0.05; 6 weeks, n = 9, t(16)= 14.069, P <0.05) and decreased from 7 to 8 weeks (7 weeks, n = 9, t(16)= 11.683, P < 0.05; 8 weeks, n = 9, t(16)= 3.154, P < 0.01).Based on the qRT-PCR results, two time points (the sixth and eighth weeks) were selected, and fluorescence in situ hybridization was used to further confirm the change in miR-124 expression in the hippocampus. As shown in Figure 3A and B, compared with the control group, the CUMS group showed up-regulation of hippocampal miR-124 expression at 6 weeks (n = 5, F(3,16)= 49.288, P < 0.01) and down-regulation at 8 weeks (n = 5, F(3,16)= 49.288, P < 0.01). The fluorescence in situ hybridization results were consistent with those of qRT-PCR.
Dynamic changes in neurogenesis and the number of neurons in the dentate gyrus during CUMS model establishment
DCX is a marker of immature neurons and reflects the ability of neural stem cells to differentiate. In DCX single labeling,compared with the control group, the number of DCX-positive cells in the CUMS group showed no change during the first 6 weeks (n = 4-5; 2 weeks, P = 0.86; 4 weeks, P = 0.69; 6 weeks, P = 0.50; Figure 4A and B) and decreased significantly at 8 weeks (n = 5, F(7,32)= 1.245, P < 0.05), which indicated dysfunctional neural stem cell differentiation. BrdU labels newborn cells, and BrdU/DCX double labeling reflects the proliferation and differentiation of neural stem cells. As a neurogenesis marker, the number of BrdU/DCX-positive cells was used to confirm neurogenesis in the dentate gyrus.As shown in Figure 4A and C, compared with the control group, the number of BrdU (red)/DCX (green)-positive cells in the CUMS group did not significantly change from 2 to 6 weeks (n = 4-5; 2 weeks, P = 0.43; 4 weeks, P = 0.11; 6 weeks,P = 0.19), but it was significantly reduced at 8 weeks (n = 4-5,F(9,37)= 18.918, P < 0.05).
NeuN, a neuron-specific marker, was used to reflect the number of neurons in the dentate gyrus. The results showed that, compared with the control group, the number of NeuN(green)-positive cells in the CUMS group did not change during the first 6 weeks of model establishment (n = 4-5; 2 weeks, P = 0.73; 4 weeks, P = 0.99; 6 weeks, P = 0.22; Figure 5A and B) and was significantly decreased at 8 weeks (n =4-5, F(3,15)= 10.676, P < 0.01; Figure 5A and B).
Discussion
Through dynamic observation of CUMS model rats for 8 weeks in this study, various depression-like behavior phenotype changes were observed in different stages of depression. From 1 to 4 weeks of model establishment, the model rats showed a decrease in body weight but no changes in depression-like behaviors, including sucrose preference and total distance traveled in the open field. This suggests the existence of an incubation period in CUMS-induced depression in which the degree of depression is relatively mild.At 5 weeks, the total distance traveled by model rats in the open field significantly decreased, while the sucrose preference did not change. Then, until 6 weeks, the total distance in the open field and the sucrose preference simultaneously decreased, which may indicate that model rats had gradual changes in depression-like behaviors from 5 to 6 weeks.Depression-like behavior occurred at a similar time as previously reported (Li et al., 2017; Wu et al., 2017; Gao et al.,2018). From 7 to 8 weeks, both the sucrose preference and locomotor activity of model rats significantly decreased, suggesting that the depression-like behavior phenotype of rats tends to be stable, and the degree of depression is serious. In addition, depressed individuals may exhibit anxiety. In this study, CUMS rats did not present significant anxiety-like behaviors in the open-field test, which does not indicate a lack of anxiety in CUMS rats.
As previously recognized, miR-124 is involved in mood regulation, and abnormal hippocampal miR-124 expression is strongly associated with the behavioral phenotype of depression and stress (Meerson et al., 2010; Bahi et al., 2014;Hu et al., 2017; Zeng et al., 2018). However, alterations of hippocampal miR-124 expression induced by CUMS are reported inconsistently. For instance, in a study by Liu et al. (2018b), CUMS up-regulated miR-124 expression in the hippocampus of Sprague-Dawley rats. However, in a study by Higuchi et al. (2016), CUMS down-regulated miR-124 expression in the hippocampus of BALB mice. In this study,abnormal hippocampal miR-124 expression was observed.However, the hippocampal miR-124 expression of model rats showed varying changes during CUMS-induced depression, including no significant changes with no depression-like behavior initially (1-4 weeks), then an increase accompanied by depression-like behavior (5-6 weeks) and finally a decrease while depression-like behavior remained(7-8 weeks). The results suggest that the depression-like behaviors caused by CUMS may not be directly related to the alteration of hippocampal miR-124. We found strong support for our results in the existing literature. In a study by Higuchi et al. (2016), because neither viral-mediated hippocampal miR-124 overexpression nor intrahippocampal in-fusion-induced miR-124 inhibition affected depression-like behaviors in non-stressed mice, the author concluded that chronic stress-induced depression-like behaviors may not be directly caused by abnormal expression of hippocampal miR-124. However, hippocampal miR-124 overexpression conferred stress resilience to the CUMS group, which resulted in faster recovery from depression; in contrast, inhibition of hippocampal miR-124 resulted in greater stress susceptibility. Therefore, hippocampal miR-124 is closely associated with stress resilience and susceptibility to depression but not directly associated with depression-like behaviors. In our study, we dynamically observed hippocampal miR-124 at different stages of CUMS-induced depression and found that the differences at various stages of CUMS may be caused by changes in stress resilience or stress susceptibility. From 5 to 6 weeks, the levels of hippocampal miR-124 were elevated,and depression-like behaviors were conferred but not stable,indicating that stress resilience in model rats was relatively strong and that the stress-induced injuries were relatively easier to recover from than those observed at 7 to 8 weeks.However, from 7 to 8 weeks, with the continuous presence of stress, miR-124 began to decline, neurogenesis dysfunctions appeared, the number of neurons was reduced in the dentate gyrus and depression-like behaviors became more stable,suggesting that stress resilience was relatively weak and recovery from stress induced-injuries was relatively more difficult. Moreover, with increasing stress susceptibility, depression is theoretically more difficult to treat. In summary,alterations of hippocampal miR-124 may not be directly associated with depression. However, based on the strong relationship among hippocampal miR-124, stress resilience and susceptibility to depression, hippocampal miR-124 may potentially be associated with depression and may even be closely related to the development of depression, which requires further study.

Figure 1 Depression-like behaviors induced by CUMS in rats.
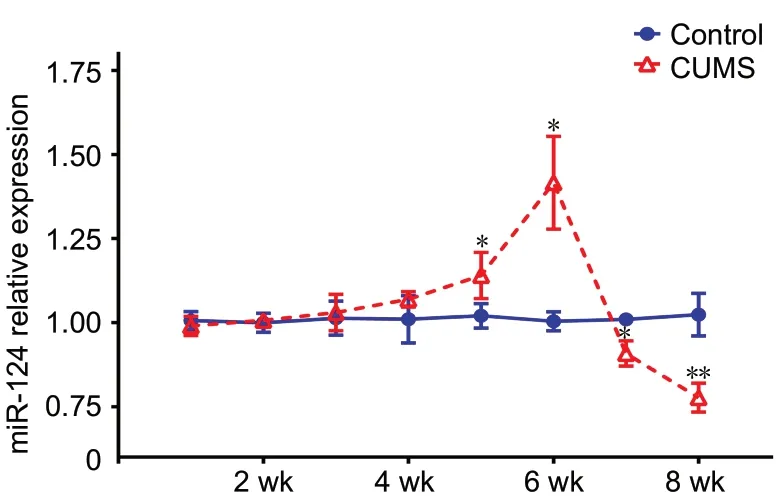
Figure 2 MiR-124 expression levels in the hippocampus as detected by real-time quantitative reverse transcription polymerase chain reaction.

Figure 3 MiR-124 expression levels in the dentate gyrus as detected by fluorescence in situ hybridization.
Neurogenesis dysfunction is strongly associated with depression and can be induced by CUMS (Wang et al., 2011;Sawamoto et al., 2017). This study showed no significant changes in neurogenesis and the number of neurons in the dentate gyrus initially (1-6 weeks); then, neurogenesis dysfunction appeared, and the number of neurons declined(7-8 weeks). These characteristics were consistent over time,indicating that CUMS may not only cause neurogenesis dysfunction, but also induce neural degeneration. In addition to neurogenesis dysfunction, many reasons exist for the decline in the number of neurons in the dentate gyrus induced by chronic stress, such as neuronal apoptosis and necrosis(Ayuob et al., 2017; Shen et al., 2019). Therefore, neurogenesis dysfunction only partly accounts for the reduction in neurons.
Adult neurogenesis is regulated by many factors including migration controlled by precise temporal characteristics,differentiation, integration and maturation of newborn neurons. At the molecular level, miRNAs are attractive candidates for regulating these processes because they have the potential to control large transcriptional networks (Pircs et al., 2018). As a neuron-specific miRNA, miR-124 has crucial functions in the regulation of proliferation and differentiation of neural stem cells (He and Guo, 2018). In this study,from 2 to 6 weeks, the expression of hippocampal miR-124 was unchanged or increased, and the proliferation of neural stem cells and number of neurons in the dentate gyrus did not change. From 7 to 8 weeks, with the decreased expression of hippocampal miR-124, dysfunction in neural stem cell proliferation and changes in the number of neurons were observed. The decreased hippocampal miR-124 levels and neurogenesis dysfunction were consistent in time during CUMS model establishment, which provided insights for further study on the relationship between hippocampal miR-124 and neurogenesis. According to our analysis, from 7 to 8 weeks, the decreased expression of miR-124 resulted in decreased stress resilience, increased susceptibility to stress and obvious damage in the hippocampus in model rats. A previous study demonstrated that changes in hippocampal anatomy, such as hippocampal neurogenesis dysfunction and hippocampal atrophy, are strongly associated with treatment-resistant depression. The hippocampal volume of patients with refractory depression was decreased compared with that in patients without refractory depression and the normal population (Maller et al., 2007). This finding further confirmed that more severe pathological damage caused by depression is more difficult to treat.
During CUMS-induced depression, increases and decreases in hippocampal miR-124 may be regulated by the bidirectional feedback loop between miR-124 and the target mRNA(de la Mata et al., 2015). MiRNA functions by binding to imperfectly complementary sequences present largely in the 3′ UTRs of target mRNAs, causing translational repression of mRNA, deadenylation and degradation. In contrast, remarkable efficacy is exhibited by target RNA-directed miRNA degradation in neurons. Target mRNAs or non-coding RNAs trigger 3′-end “tailing”, (i.e., addition of non-templated nucleotides), 3′-to-5′ trimming and decay of highly complementary miRNAs. This process is called target RNA-directed miRNA degradation. Hence, a competitive inhibition mechanism exists between miRNA and target mRNA, which provides a foundation for the body to maintain a stable state of miRNA.However, the main target mRNA of miRNA may differ at various pathological stages. Glucocorticoid receptor mRNA is a target of miR-124 (Wang et al., 2017). Depression-like behaviors, increased glucocorticoid levels, decreased glucocorticoid receptor mRNA levels, and up-regulated miR-124 expression are found in depression models induced by chronic corticosterone injection (Dwivedi et al., 2015; Yi et al., 2018). Among them, the competitive inhibition of miR-124 and glucocorticoid receptor mRNA may be a possible mechanism to explain the increased miR-124 in the hippocampus. In this study, the elevation of hippocampal miR-124 from 5 to 6 weeks of model establishment may be caused by the competitive inhibition of miR-124 and glucocorticoid receptor mRNA. Additionally, it may be related to other target genes that interact with miR-124. The decreased hippocampal miR-124 and neurogenesis dysfunction observed from 7 to 8 weeks may be associated with the Notch signaling pathway. As a regulatory pathway of neural stem cells, the Notch pathway includes two genes, DLL4 and Sox9, that are target genes of miR-124. Overexpression of miR-124 can significantly reduce the expression of the downstream target,Sox9, in dentate gyrus cells, and downregulation of Sox9 is necessary for neurogenesis. Sox9 is post-transcriptionally regulated by miR-124, and its down-regulation is required for neurogenesis. DLL4 is a direct target of miR-124 in neural stem cells. During neural stem cell differentiation, miR-124 expression is increased, and DLL4 expression is decreased.When neurogenesis dysfunction occurs, the Notch pathway is activated, and DLL4 and Sox9 expression is increased (Cheng et al., 2009; Liu et al., 2011; Jiao et al., 2017). The competitive inhibition mechanism of miR-124 and the target genes DLL4 and Sox9 may be an important basis for the decreased miR-124 levels. In addition, the involvement of other target genes in the decrease in miR-124 cannot be excluded and requires further verification in subsequent experiments.

Table 1 Pathological features at different stages in Sprague-Dawley rats with depression induced by chronic unpredictable mild stress
Based on dynamic observation for 8 weeks, CUMS-induced depression was found to be manifested in three different pathological stages. As listed in Table 1, in the first stage(1-4 weeks), although the CUMS rats exhibited weight loss and withered fur, the depression-like behavior phenotype,hippocampal miR-124, neurogenesis and number of neurons in the dentate gyrus showed no significant changes. In the second stage (5-6 weeks), CUMS rats presented depression-like behaviors, including decreased sucrose preference(anhedonia), reduced locomotor activity and elevated hippocampal miR-124 levels; however, neurogenesis dysfunction and a decrease in the number of neurons in the dentate gyrus were not observed. In the third stage (7-8 weeks),CUMS rats showed a stable and significant change in the depression-like behavioral phenotype, with down-regulation of hippocampal miR-124, neurogenesis disorder and reduction of the neuronal number in the dentate gyrus.
There are some limitations to this study. First, Sprague-Dawley rats were selected to dynamically observe changes in pathological features during the pathogenesis of CUMS-induced depression. Nevertheless, under the same stressors,different species of rodents may not show similar pathological features at the observation stages. Second, in the pathological process of CUMS-induced depression, relationships may exist among hippocampal miR-124, depression-like behavior and neurogenesis in the dentate gyrus, and the mechanisms of this interaction need to be further explored.
In summary, dynamic observation of CUMS model rats for 8 weeks showed that depression-like behaviors, hippocampal miR-124 and neurogenesis in the dentate gyrus exhibited various changes at different stages of depression.These findings could provide insights for further pathogenesis research and clinical treatment of depression. Treatments should be customized because of the differences in features,stress resilience and susceptibility during various stages of depression.
Author contributions:Study conception: LLW; study design: LLW, CY and PL; manuscript writing: YLH and NXZ; manuscript revision: JC;experiment and data analysis: YLH, NXZ, JC, JN, and WLL. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support:This study was supported by the National Natural Science Foundation of China, No. 81573858 (to LLW); the Natural Science Foundation of Guangdong Province of China, No. 2016A030313648 (to CY); and the Major Basic Research Project of Educational Commission of Guangdong Province of China, No. 2017KZDXM020 (to CY). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Guangzhou University of Chinese Medicine of China in March 2015.The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Francesca Antonelli, l’Energia e lo Sviluppo Economico Sostenibile (ENEA), Laboratory of Biomedical Technologies, Italy;Randall L. Davis, Oklahoma State University Center for Health Sciences,USA.
Additional file:Open peer review reports 1 and 2.

Figure 4 Neurogenesis markers subjected to immunofluorescence staining.
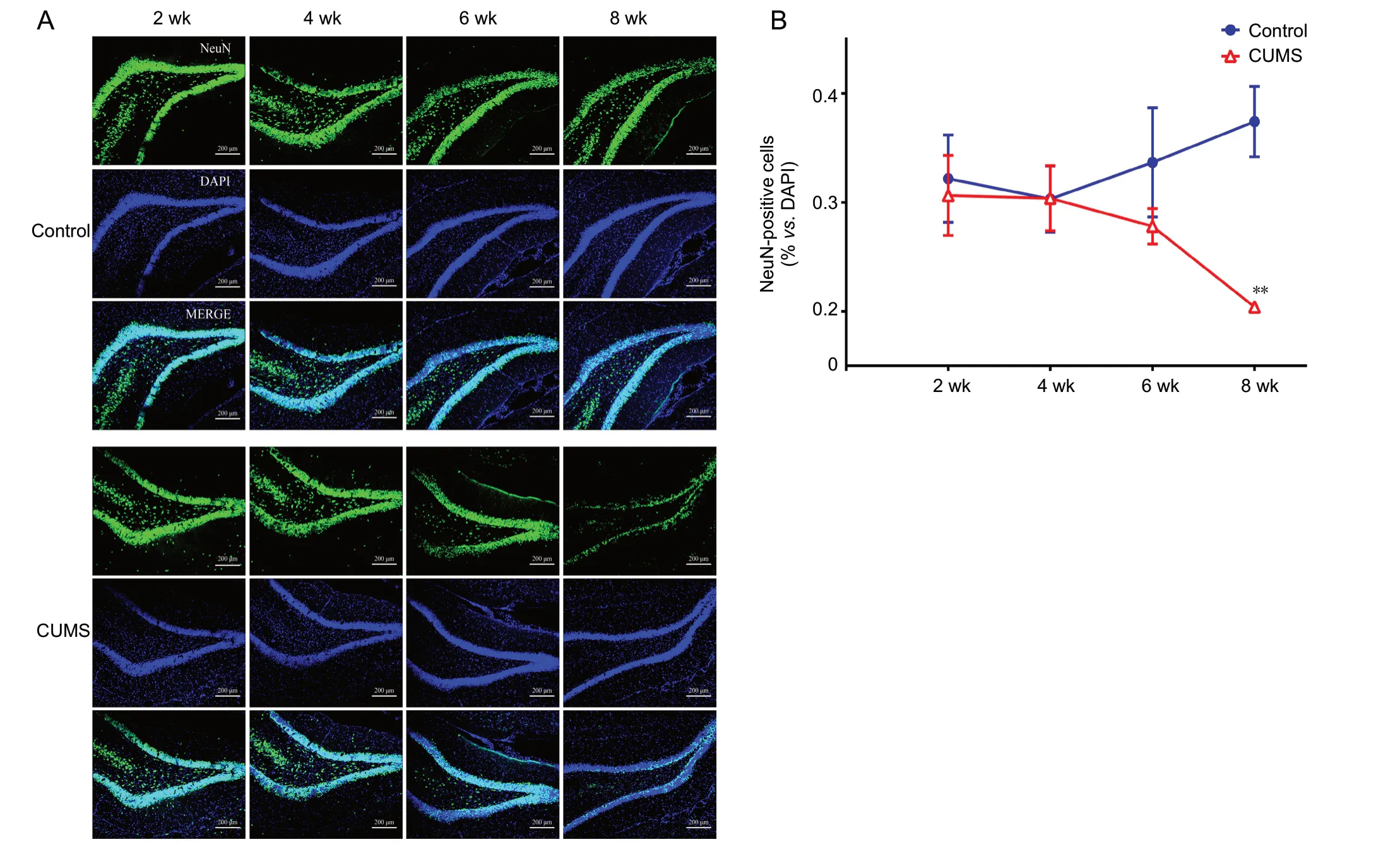
Figure 5 Immunofluorescence staining by the neuron marker NeuN.
- 中国神经再生研究(英文版)的其它文章
- Astrocytic modulation of potassium under seizures
- Type XIX collagen: a promising biomarker from the basement membranes
- Adult neurogenesis from reprogrammed astrocytes
- Heterogeneity in the regenerative abilities of central nervous system axons within species: why do some neurons regenerate better than others?
- Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease
- Stroke gets in your eyes: stroke-induced retinal ischemia and the potential of stem cell therapy